
H
IGHLIGHTS OF PRESCRIBING INFORMATION
Thes
e highlights do not include all the information needed to use
AUVI-Q® sa
fely and effectively. See full prescribing information for
AUVI-Q.
AUVI-Q® (e
pinephrine injection, USP) 0.3 mg, 0.15 mg, 0.1 mg
Auto-Inj
ector, for intramuscular or subcutaneous use
Initia
l U.S. Approval: 1939
--
--------------------
-
------
RECE
NT MAJOR CHANGES
--
---------------------
• Do
s
ag
e and Administration (2)
11/2
017
--
--------------------------
INDI
CATIONS AND USAGE
--
-------------------------
AUVI-Q co
ntains epinephrine, a n
on-selectiv
e alpha and beta
-ad
renergic
rece
ptor agonist, indicated in the emergency treatment of allergic reactions
(T
ype I) including anaphylaxis. (1)
--
--------------------
DO
SAGE AND ADMINISTRATION
--
---------------------
• Patien
ts greater than or equal to 30 kg (66 lbs):
AUVI-Q 0
.3 mg (2)
• Patien
ts 15 to 30 kg (33 to
6
6 lbs):
AUVI-Q 0
.15 mg (2)
• Patien
ts
7.5 to 1
5 kg (16
.5 to
3
3 lbs):
AUV
I
-Q 0
.1 mg (2)
Inject A
UVI
-Q in
tramuscularly or subcutaneously into the anterolateral aspect of the
th
igh, through clothing if necessary.
E
ach device is a single
-u
se injection.
(2
)
--
-------------------
DO
SAGE FORMS AND STRENGTHS
-
---------------------
• Injectio
n, 0.3 mg: 0.3 mg/0.3 mL
ep
inephrine injection, USP, pre
-f
illed
au
to
-in
jector (3)
• Injectio
n, 0.15 mg: 0.15 mg/0.15 mL epinephrine injection, USP, pre
-
f
illed auto
-in
jector (3)
• Injectio
n, 0.1 mg: 0.1 mg/0.1 mL epinephrine injection, USP, pre
-f
illed
au
to
-in
jector (3)
--
------------
--
---------------
C
ONTRAINDICATIONS
-
-----------------------------
No
ne
. (4)
--
---------------------
WARNIN
GS AND PRECAUTIONS
--
----------------------
• In co
njunction with use, seek immediate medical or hospital care.
(5.1
)
• Do
not inject intravenously, into
b
uttock, or into digits, hands, or feet. (5.2)
• To
minimize the risk of injection
-related
injury, instruct caregivers to hold
th
e child’s leg firmly in place and limit movement prior to and during
in
jection when administering to young children
o
r infants
. (5.2
)
• Rare
cases of serious skin and soft tissue infections have been reported
f
ollowing epinephrine injection.
Ad
vise patients to seek medical care if they
d
evelop signs or symptoms of infection at the epinephrine injection site. (5.3)
• The p
resence of a
su
lfite in this product should not deter use. (5.
4)
• Ad
minister with caution in patients with heart disease; may aggravate angina
p
ectoris or produce ventricular arrhythmias. (5.
5)
--
----------------------------
AD
VERSE REACTIONS
-
--------------------------
-
---
Ad
verse reactions to epinephrine include anxiety, apprehensiveness,
restless
ness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea
an
d vomiting, headache, and/or respiratory difficulties. (6)
To
report SUSPECTED ADVERSE REACTIONS, contact kaleo, Inc. at
1-844-8
28
-8
742 or FDA at 1
-800-F
DA
-1
088 or
w
ww.fda.gov/medwatch
.
--
----------------------------
DR
UG INTERACTIONS
-
------------------------------
• Card
iac glycosides or diuretics: observe for development of cardiac
arr
hythmias. (7)
• Tr
icyclic antidepressants, monoamine oxidase inhibitors, levothyroxine
so
dium, and certain antihistamines: potentiate effects of epinephrine. (7)
• Beta-ad
renergic blocking
d
rugs: antagonize cardiostimulating and
b
ronchodilating effects of epinephrine. (7)
• Alp
ha
-ad
renergic blocking drugs: antagonize vasoconstricting and
h
ypertensive effects of epinephrine. (7)
• Er
got alkaloids: may reverse the pressor effects of epinephrine. (
7)
--
----------------------
USE IN
SPECIFIC POPULATIONS
--
---------------
• E
lderly patients may be at greater risk of developing adverse reactions. (5.
5, 8
.5)
See
17 for PATIENT COUNSELING INFORMATION and FDA
-
a
pproved patient labeling
Rev
ised:
11/2017
_
____________________________________________________________________________________________________________________________
_
__
FULL P
RESCRIBING INFORMATION: CONTENTS*
1 INDI
CATIONS AND USAGE
2 DO
SAGE
AND
ADMINISTRATION
3 DO
SAGE FORMS AND STRENGTHS
4 CO
NTRAINDICATIONS
5 WARNIN
GS AND PRECAUTIONS
5
.1
E
mergency Treatment
5
.2
Injectio
n
-related C
omplications
5
.3
Seriou
s Infections at the Injection Site
5.4 Allergic Rea
ctions Associated with Sulfite
5.5 Diseas
e Interactions
6 ADVERSE
REACTIONS
7 DRU
G INTERACTIONS
8 USE IN S
PECIFIC POPULATIONS
8
.1
Pregn
ancy
8
.3 Nursing Mothers
8
.4 Pediatric Use
8
.5 Geriatric Use
10 O
VERDOSAGE
11 DESCRI
PTION
12 CLI
NICAL PHARMACOLOGY
1
2.1
Mechan
ism of Action
1
2.2
Ph
armacodynamics
13 NO
NCLINICAL TOXICOLOGY
1
3.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 H
OW SUPPLIED/STORAGE AND HANDLING
1
6.1
Ho
w Supplied
1
6.2
Sto
rage and Handling
17 PATIE
NT COUNSELING INFORMATION
*
Sections or
su
bsections omitted from the full prescribing information are not
listed
.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
FULL
PRESCRIBING INFORMATION
1 INDICA
TIONS AND USAG
E
AUVI-Q® is i
ndicated in the emergency treatment of allergic reactions (Type I) including
anaphyl
axis to stinging insects (e.g., order Hymeno
pter
a, which include bees, wasps, hornets,
yell
ow jackets and fire ants) and biting insects (e.g., triatoma, mosquitoes), a
llerge
n
imm
unotherapy, foods, drugs, diagnostic testing substances (e.g., radiocontrast media) and other
all
ergens, as well as idiopathic anaphylaxis or ex
e
rcise
-indu
ced anaphylaxis.
AUVI-Q is i
ntended for immediate administration in patients who are deter
m
ined to be at
incr
eased risk for anaphylaxis, including individuals with a history of anaphylactic reactions.
Anaphylac
tic reactions may occur within minutes after exposure and consist of flushing,
appr
ehension, syncope, tachycardia, thready or unobtaina
b
le pulse associated with a fall in blood
pre
ssure, convulsions, vomiting, diarrhea and abdominal cramps, involuntary voiding, wheezing,
dyspnea due to l
aryngeal spasm, pruritus, rashes, urticaria or angioedema.
AUVI-Q is i
ntended for immediate self
-adm
inistration as emergency supportive therapy only
and is
not a substitute for immediate medical care.
2 DOSAG
E AND ADMINISTR
A
TION
Selec
tion of the appropriate dosage strength (
AUVI-Q 0.3
mg
,
AUVI-Q 0.15
mg
,
or
AUVI-Q
0.1 mg) i
s determined according to patient body weight.
• Pati
ents greater than or equal to 30 kg (approximately 66 pounds or more):
AUVI-Q
0.3 m
g
• Pati
ents 15 to 30 kg (33
to
66 pounds):
AUVI-Q 0.15
mg
• Pati
ents
7.5 to 15 kg (16.5 to 33 pounds)
:
AUVI-Q 0.1
mg
Inj
ect
AUVI-Q int
ramuscularly or subcutaneously into the anterolateral aspect of the thigh,
thr
ough clothing if necessary.
Instru
ct
ca
regiver
s of yo
ung children
and inf
ants
who are
pre
scribed
AUVI-Q an
d who may be uncooperative and kick or move during an injection
to hold
the child’s leg firm
ly in place and limit movement
pri
or to and during an injection
[s
ee
WARNING
S AND PRECAUTIONS (5.2)
].
Each AUVI-Q conta
ins a single dose of epinephrine for single
-use i
njection. Since the doses of
epine
phrine delivered from
AUVI-Q are
fixed, consider using other forms of injectable
epine
phrine if doses lower than 0.1 mg are deemed necessary.
The pres
criber should carefully assess each patient to determine the most appropriate dose of
epine
phrine
, re
cognizing the life
-threat
ening nature of the reactions for which this drug is
indi
cated. With severe persistent anaphylaxis, repeat injections wit
h an additi
onal
AUVI-Q m
ay
be nece
ssary. More than two sequential doses of epinephrine should only be administered under
dir
ect medical supervision [
see W
ARNINGS AND PRECAUTIONS (5.1)
].
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
The epi
nephrine solution in the viewing window of
AUVI-Q shoul
d be ins
pect
ed visually for
par
ticulate matter and discoloration. Epinephrine is light sensitive and should be stored in the
outer
case provided to protect it from light [
see ST
ORAGE AND HANDLING (16.2)
].
3 DOSAG
E FORMS AND S
TRE
NGTHS
• Inj
ection, 0.3 mg/0.3 mL epinephrine injection, USP, pre
-fille
d auto
-in
jector
• Inj
ection, 0.15 mg/0.15 mL epinephrine injection, USP, pre
-fille
d auto
-in
jector
• Inj
ection, 0.1 mg/0.1 mL epinephrine injection, USP, pre
-fille
d auto
-in
jector
4 CONTR
AINDICATIONS
None.
5 W
ARNINGS AND PRECA
UTIO
NS
5.1 EMERG
ENCY TREATM
ENT
AUVI-Q is not
intended as a substitute for immediate medical care.
In c
onjunction with the
admi
nistra
tion of epinep
hrine, the
pati
ent should seek immediate medical or hospital care.
Mor
e than two sequential doses of epinephrine should only be administered under direct medical
super
vision [
see I
NDICATIONS AND USAGE (1), DOSAGE AND ADMINISTRATION (2) and
PATIEN
T COUNSELING INFORMATION (17.1)
].
5.2 INJEC
TION
-R
ELATED CO
MPLIC
ATIONS
AUVI-Q shoul
d
ONLY be
injected into the anterolateral aspect of the thigh [
see
DOSAGE AND
AD
MINISTRATION (2) and PATIENT COUNSELING INFORMATION (17.1)
].
• Do
not inject intravenously.
Lar
ge doses or accidental intravenous injection of
epine
phrine may result in cer
ebral
hemorrhage due to sharp rise in blood pressure. Rapidly
act
ing vasodilators can counteract the marked pressor effects of epinephrine if there is such
inadve
rtent administration.
• Do
not inject into buttock.
Injectio
n into the buttock may not provide
eff
ective treatment
of
anaphylaxis. Advise the patient to go immediately to the nearest emergency room for
further
treatment of anaphylaxis.
Additi
onally, injection into the buttock has been
assoc
iated with Clostridial infections (gas gangrene). Cleansin
g
with alcohol does not kill
bact
erial spores, and therefore, does not lower this risk.
• Do
not inject into digits, hands or feet.
Since
epinephrine is a strong vasoconstrictor,
acci
dental injection into the digits, hands or feet may result in loss of blood
flow
to the
aff
ected a
rea.
Advise the patient to go immediately to the nearest emergency room and to
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
inform
the healthcare provider in the emergency room of th
e
location of the accidental
inj
ection. Treatment of such inadvertent administration should cons
ist
of vasodilation, in
addition
to further appropriate treatment of anaphylaxis [
see AD
VERSE REACTIONS
(6)].
• Hol
d
leg fir
mly
during
injection.
T
o minimize the risk of injection
-rel
ated injury
w
hen
adm
inistering
AUVI-Q to young children or inf
ants
, in
struct caregivers to
hold the chil
d’s
leg firm
ly in place and limit movement prior to and
duri
ng injection
.
5.3 SERIO
US INFECTIO
NS
AT THE INJECTION
SI
TE
Rare ca
ses of serious skin and soft tissue infections, includi
n
g necrotizing fasciitis and
m
yonecrosis caused by Clostridia (gas gangrene), have been reported at the injection site
following ep
inephrine injection for anaphylaxis.
Clost
ridium
spor
es can be present on the skin
and introduced int
o the deep tissue with subcutaneous or intramuscular injection. While
cle
ansing with alcohol may reduce
th
e
pre
sence of bacteria on the skin, alcohol cleansing does
not ki
ll
Clos
tridium
spo
res. To decrea
se t
he
po
tential
risk of a r
are, but serious
Clos
tridium
infect
ion, do not inject
A
UVI
-Q int
o the buttock
[see WARNI
NGS AND PRECAUT
IO
NS
(5.2)].
Advise patients t
o seek medical care if they develop signs or symptoms of infection, such as
per
sistent redness,
warmth, swelling,
or tenderness, at the epinephrine injection site.
5.4 ALLERG
IC REACTIONS
A
SSOCIATED WITH SULF
I
TE
Epinephr
ine is the preferred treatment for serious allergic reactions or other emergency situations
even t
hough this product contains sodiu
m
bisulfite, a sulfite that may, in other products, cause
all
ergic
-type reactions in
cluding anaphylactic symptoms or life
-thr
eatening or less severe
ast
hmatic episodes in certain susceptible persons.
The pres
ence of a sulfite
in t
his product should not deter administration of the drug for treatment
of seri
ous allergic or other emergency situations even if the patient is sulfite
-sensiti
ve.
The al
ternatives to using epinephrine in a life
-thre
atening situation may not be satisfa
ctory.
5.5 DISEAS
E INTERACTION
S
So
me patients may be at greater risk for developing adverse reactions after epinephrine
adm
inistration. Despite these concerns, it should be recognized that the presence of these
conditions
is not a contraindication to epin
ephrine
administration in an acute, life
-threa
tening
sit
uation. Therefore, patients with these conditions, and/or any other person who might be in a
posi
tion to administer
AUVI-Q to a pa
tient experiencing anaphylaxis should be carefully
inst
ructed in regar
d
to the circumstances under which epinephrine should be used.
• Pati
ents with Heart Disease
Epinephr
ine should be administered with caution to patients who have heart disease,
incl
uding patients with cardiac arrhythmias, coronary artery or organic heart di
sease,
or
hyper
tension
. In s
uch patients, or in patients who are on drugs that may sensitize the heart to
arr
hythmias
, epine
phrine may precipitate or aggravate angina pectoris as well as produce
ventricul
ar arrhythmias [
see DRUG
INTERACTIONS (7) and ADVERS
E
REACTIONS (6)
].
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
• Other
Patients and Diseases
Epinephr
ine should be administered with caution to patients with hyperthyroidism, diabetes,
elde
rly individuals, and pregnant women. Patients with Parkinson’s disease may notice a
tem
porary worsening of sympto
m
s.
6 ADVER
SE REACTIONS
Due to la
ck of randomized, controlled clinical trials of epinephrine for the treatment of
anaphyl
axis, the true incidence of adverse reactions associated with the systemic use of
epine
phrine is difficult to determine.
Adverse
reactions reported in observational trials, case
rep
orts, and studies are listed below.
Co
mmon adverse
reac
tions to
system
ically administered
ep
inephrine include anxiety;
appr
ehensiveness; restlessness; tremor; weakness; dizziness; sweating; palpit
ati
ons; pallor;
nause
a and vomiting; headache; a
nd/
or respiratory difficulties. These symptoms occur in some
per
sons receiving therapeutic doses of epinephrine, but are more likely to occur in patients with
hyper
tension or hyperthyroidism [
see W
ARNINGS AND
PR
ECAUTIONS (5.
5)].
Arrhyt
hmias, including fatal ventricular fibrillation, have been reported, particularly in patients
with unde
rlying cardiac di
seas
e or those receiving certain drugs [
see W
ARNINGS AND
PREC
AUTIONS (5.
5)
and DRUG INTERACTIONS (7)
].
Rapid r
ises in blood pressure have produced cerebral hemorrhage, particularly in elderly patients
with c
ardiovascular disease [
see W
ARNINGS AND PRECAUTIONS (5.
5)].
Angina m
ay occur in patients with coronary artery disease [
se
e WARNINGS AND
PREC
AUTIONS (5
.5)].
Rare ca
ses of stress cardiomyopathy have been reported in patients treated with epinephrine.
Accident
al injection into the digits, hands or feet may result in loss of blood flow to the affected
are
a [
see W
ARNINGS AND PRECAUTIONS (5.2)
].
Adverse
even
ts e
xperienced as a result of accidental injections may include increased heart rate,
loca
l reactions including injection site pallor, coldness and hypoesthesia or injury at the injection
sit
e resulting in bruising, bleeding, discoloration, erythema or ske
letal injury.
Inj
ection
of
epinephrine
int
o the buttock has resulted in cases of gas gangrene [
see WARN
INGS
AND
PRECAUTIONS (5.2)
].
Rare ca
ses of serious skin and soft tissue infections, including necrotizing fasciitis and
m
yonecrosis cause
d by
Clostridia (gas gangrene), have been reported
at t
he injection site
following ep
inephrine injection
in t
he thigh
[see WARN
INGS AND PRECAUTIONS (5.2)
].
7 DRUG INTER
ACTIONS
Pati
ents who receive epinephrine while concomitantly taking cardiac glycosides,
diu
retics, or
anti-arrhythm
ics should be observed carefully for the development of cardiac arrhythmias [
see
WARNING
S AND PRECAUTIONS (5.
5)].
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
The eff
ects of epinephrine may be potentiated by tricyclic antidepressants, monoamine oxidase
inhi
bitors, levothyroxine sodium, and certain antihistamines, notably chlorpheniramine,
tr
ipelennamine, and diphenhydramine.
The car
diostimulating and bronchodil
ati
ng effects of epinephrine are antagonized by beta
-
adr
energic blocking drugs, such as propranolol.
The vasoc
onstricting and hypertensive effects of epinephrine are antagonized by alpha
-
adr
energic blocking drugs, such as phentolamine.
Ergot
alkaloids may
als
o reverse the pressor effects of epinephrine.
8 USE IN
SPECIFIC PO
P
ULATIONS
8.1 PREG
NANCY
Terat
ogenic Effects: Pregnancy Category C.
There
are no adequate and well controlled studies of the acute effect of epinephrine in pregnant
wom
en.
Epinephr
ine
was ter
atogenic in rabbits, mice and hamsters. Epinephrine should be used during
pre
gnancy only if the potential benefit justifies the potential risk to the fetus (fetal anoxia,
spont
aneous abortion, or both).
Epinephr
ine has been shown to have teratogenic
eff
ects when administered subcutaneously in
rab
bits at approximately 30 times the maximum recommended daily subcutaneous or
int
ramuscular dose (on a mg/m
2
bas
is at a maternal dose of 1.2 mg/kg/day for two to three days),
in m
ice at approximately 7
ti
mes t
he m
aximum daily subcutaneous or intramuscular dose (on a
m
g/m
2
basi
s at a maternal subcutaneous dose of 1 mg/kg/day for 10 days), and in hamsters at
appr
oximately 5 times the maximum recommended daily subcutaneous or intramuscular dose
(on a
mg/m
2
basi
s a
t a mate
rnal subcutaneous dose of 0.5 mg/kg/day for 4 days).
These eff
ects were not seen in mice at approximately 3 times the maximum recommended daily
subcut
aneous or intramuscular dose (on a mg/m
2
basi
s at a subcutaneous maternal dose of 0.5
m
g/kg/day f
or 10 da
ys).
8.3 NURSI
NG MOTHERS
It
is not known whether epinephrine is excreted in human milk. Because many drugs are excreted
in hum
an milk, caution should be exercised when
AUVI-Q is a
dministered to a nursing woman.
8.4 PEDIA
TRIC USE
AUVI-Q m
ay be
adm
inistered to pediatric patients at a dosage appropriate to body weight [
see
DO
SAGE AND ADMINISTRATION (2)
]. Clini
cal experience with the use of epinephrine
sugges
ts that the adverse reactions seen in children are similar in nature and extent to those b
oth
expect
ed and reported in adults. Since the doses of epinephrine delivered from AUVI
-Q are
fixed, consider us
ing other forms of injectable epinephrine if doses lower than 0.1 mg are
deem
ed necessary.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
8.5 GERIA
TRIC USE
Clini
cal studies of AUVI
-Q did not include
sufficient numbers of subjects aged 65 and over to
dete
rmine whether they respond differently from younger subjects. Other reported clinical
exper
ience has not identified differences in responses between the elderly and younger patients.
Epinephri
ne should be administered with caution in elderly individuals, who may be at greater
ri
sk for developing adverse reactions after epinephrine administration [
see WARN
INGS AND
PREC
AUTIONS (5.5), OVERDOSAGE (10)
].
10 OVERD
OSAGE
Overdosag
e
of epinephr
ine may produce extremely elevated arterial pressure, which may result
in ce
rebrovascular hemorrhage, partic
ularly i
n elderly patients. Overdosage may also result in
pulm
onary edema because of peripheral vascular constriction together with card
iac st
imulation.
Treat
ment consists of rapidly acting vasodilators or alpha
-adrenergic blocking drugs
and/or
res
piratory support.
Epinephr
ine overdosage can also cause transient bradycardia followed by tachycardia, and these
m
ay be accompanied by potential
ly fatal
cardiac arrhythmias. Premature ventricular contractions
m
ay appear within one minute after injection and may be followed by multifocal ventricular
tac
hycardia (prefibrillation rhythm). Subsidence of the ventricular effects may be followed by
atrial
tachycardia and occasionally by atrioventricular block. Treatment of arrhythmias consists
of ad
ministration of a beta
-ad
renergic blocking drug such as propranolol.
Overdosag
e sometimes results in extreme pallor and coldness of the skin, metabolic acidos
is, a
nd
kidney fail
ure. Suitable corrective measures must be taken in such situations.
11 DESCRIP
TION
AUVI-Q (ep
inephrine injection, USP) 0.3 mg
, 0.1
5 mg
and 0.1 m
g
is a
n auto
-in
jector and a
com
bination product containing drug and device components.
AUVI-Q incl
udes audible (electronic voice instructions, beeps) and visible (LED lights) cues for
use. The ne
edle automatically retracts after the injection is complete.
Each AUVI-Q 0.3
mg delivers a single dose of 0.3 mg epinephrine from epinephrin
e inj
ection,
US
P (0.3 mL) in a sterile solution.
Each AUVI-Q 0.15
mg delivers a single dose of 0.15 mg epinephrine from epinephrine injection,
US
P (0.15 mL) in a sterile solution
.
Each AUVI-Q 0.1
mg delivers a single dose of 0.1 mg epinephrine from epin
ephrine i
njection,
US
P (0.1 mL) in a sterile solution
.
AUVI-Q 0.3
mg
, AUVI-Q 0.15
mg
a
nd
AUVI-Q 0.1 m
g
each
contain 0.76 mL epinephrine
solut
ion. 0.3 mL
, 0.15
mL
and 0.1 m
L
epine
phrine solution is dispensed for
AUVI-Q 0.3
mg
,
AUVI-Q 0.1
5 mg
and AUVI-Q 0.1 mg, re
spectively, when activated. The remaining solution is
not ava
ilable for future use and should be discarded.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Each 0.3 mL in AUVI-Q 0.3
mg contains 0.3 mg epinephrine, 2.3 mg sodium chloride, 0.5
m
g
sodi
um bisulfite, hydrochloric acid to adjust pH
,
and water for injection. The pH range is 2.2
–
5.0.
Each 0.15 mL in AUVI-Q 0.15
mg contains 0.15 mg epinephrine, 1.2 mg sodium chloride, 0.2
m
g sodium bisulfite, hydrochloric acid to adjust pH, and water for injection. The pH range is
2.2–5.0.
Each 0.1 mL in AUVI-Q 0.1
mg contains 0.1 mg epinephrine,
0.78 m
g sodium chloride,
0.15 m
g
sodi
um bisulfite, hydrochloric acid to adjust pH, and water for injection. The pH range is 2.2
–
5.0.
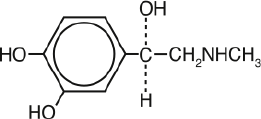
Epinephr
ine is a sympathomimetic catecholamine. Chemically, epinephrine is
(-)-3,4-
Dihydroxy-α-[(m
ethylamino)methyl]benzyl alcohol with the following structure:
Epinephr
ine solution deteriorates rapidly on exposure to air or light, turning pink from oxidation
to adr
enochrome and brown from the formation of melanin.
AUVI-Q is n
ot made with natural rubber latex
.
AUVI-Q inst
ructional and safety systems should be thoroughly reviewed with patients and
car
egivers prior to use [
s
ee PATIENT COUNSELING INFORMATION (17.1)
].
12 CLINIC
AL PHARMACO
LO
GY
12
.1
MECHA
NISM OF AC
TION
Epinephr
ine acts on both alpha and beta
-adr
energic receptors.
12
.2
PHARM
ACODYNAMIC
S
Through i
ts action on alpha
-adrener
gic receptors, epinephrine lessens the vasodilation and
incr
eased vascular permeability that occurs during anaphylaxis, which can lead
to
loss of
int
ravascular fluid volume and hypotension.
Through i
ts action on beta
-ad
renergic receptors, epinephrine causes bronchial smooth muscle
rel
axation and helps alleviate bronchospasm, wheezing and dyspnea that may occur during
anaphyl
axis.
Epinephri
ne also alleviates pruritus, urticaria, and angioedema and may relieve gastrointestinal
and geni
tourinary symptoms associated with anaphylaxis because of its relaxer effects on the
sm
ooth muscle of the stomach, intestine, uterus and urinary bladder.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
W
he
n give
n subcutaneously or intramuscularly, epinephrine has a rapid onset and short duration
of acti
on.
13 NONCL
INICAL TOXIC
OLOGY
13
.1
CARCIN
OGENESIS,
MU
TAGENESIS, IMPAIR
MENT
OF
FERTI
LITY
Long-ter
m studies to evaluate the carcinogenic potential of epinephri
ne h
ave not been conducted.
Epinephr
ine and other catecholamines have been shown to have mutagenic potential
in
vitro
and
to be a
n oxidative mutagen in a
WP
2
bact
erial reverse mutation assay.
Epinephr
ine was positive in the DNA Repair test with
B. sub
til
is (REC
) assay, but was not
m
utagenic in the
Salmo
nella
ba
cterial reverse mutation assay.
The potential f
or epinephrine to impair fertility has not been evaluated.
This s
hould not prevent the use of epinephrine under the conditions noted under
INDI
CATIONS
AND
USAGE (1)
.
16 HOW
SUPPLIED/STORAGE
AND HAN
DLING
16
.1
HOW SU
PPLIED
Cart
on containing two
AUVI-Q (ep
inephrine injection, USP) 0.3 mg auto
-injectors an
d a single
AUVI-Q Tr
ainer
- ND
C
60842-023-01
Cart
on containing two
AUVI-Q (ep
inephrine injection, USP) 0.15 mg auto
-injectors a
nd a single
AUVI-Q Tr
ainer
- ND
C
60842-022-01
Cart
on containing two
AUVI-Q (ep
inephrine injection, USP) 0.1 mg auto
-injectors an
d a single
AUVI-Q Tr
ainer
- ND
C
60842-021-01
Rx only
16
.2
STORAG
E
A
ND HANDLING
Epinephr
ine is light sensitive and should be stored in the outer case provided to protect it from
li
ght. Store at 20°
to 25°
C (68°
to 77°F);
excursions permitted to 15°
to 3
0°C (59°
to 86°F) [S
ee
US
P Controlled Room Temperature]
. Do not re
frigerate. Before using, check to make sure the
solut
ion in the auto
-inj
ector is clear and colorless. Replace the auto
-inj
ector if the solution is
disc
olored, cloudy, or contains particles.
17 P
ATIENT COUNSELIN
G INFO
RMATION
[see FDA-Approved Patient
Labeling (Patient Information and Instructions for Use)
]
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
A healt
hcare provider should review the patient instructions and operation of
AUVI-Q, in de
tail,
with t
he patient or caregiver.
Epinephr
ine is essential for the treatment of anaphylaxis.
Pa
tients who are at risk of or with a
hist
ory of severe allergic reactions (anaphylaxis) to insect stings or bites, foods, drugs, and other
all
ergens, as well as idiopathic and exercise
-indu
ced ana
phyl
axis, should be carefully instructed
about t
he circumst
ances
under which epinephrine should be used.
Ad
ministration and Training
Ins
truct patients
and
/or caregivers in the appropriate use of
AUVI-Q. AUVI-Q shoul
d be injected
int
o the middle of the outer thigh (through clothing, if necessary). Each device is a single
-use
inj
ection. Advise patients to seek immediate medical care in conjunction with administration of
AUVI-Q.
Young chil
dren
or i
nf
an
ts
m
ay be uncoopera
ti
ve and kick or move during an
inj
ection.
Ins
truct
car
egivers to
hold the le
g of young children
or i
nfants
firm
ly in place and limit movement prior
to and durin
g injection. [
see WARN
INGS
AND PREC
AUTIONS
(5.2)]
Co
mplete patient information, including dosage, directions for proper administration and
pre
cautions can be found inside each
AUVI-Q c
arton.
Revi
ew
AUVI-Q’s
instructional and
safety sys
tems with
patients
and/or caregivers
. T
hese
system
s
incl
ud
e the pri
nted label on the
sur
face of
AUVI-Q showing inst
ructions for use and a diagram depicting the injection process
,
an aut
omatic needle retraction system,
visua
l prompts
, ele
ctronic
be
eps, and
voice
instructions
for use. Ins
truct patients and/or caregivers
that
the needle will not be visible after the injection
and the
y may not feel the injection when it occurs. Instruct patients that AUVI
-Q include
s a 2
-
second c
ountdown after it is activated and then the voice instructions will indicate the injection is
com
plete and to seek emergency medical attention. Instruct patients that AUVI
-Q’s bla
ck base
will
lock up onto the device housing and the lights will blink red after the injection is complete.
These post-use i
ndicators help patients and/or caregivers know
that
AUVI
-Q has been
activated
and an epi
nephrine injection administered.
Ins
truct patients
and
/or caregivers to use
and pr
actice with
the T
rainer to familiarize themselves
with t
he use of
AUVI-Q in an a
llergic emergency.
The Trai
ner may be used multiple
tim
es.
Ad
verse Reactions
Epinephr
ine may produce symptoms and signs that include an increase in heart rate, the
sens
ation of a more forceful heartbeat, palpitations, sweating, nausea and vomiting, difficulty
bre
athing, pallor, dizziness, weakness or shaki
n
ess, headache, apprehension, nervousness, or
anxie
ty. These symptoms and signs usually subside rapidly, especially with rest, quiet and
rec
umbency. Patients with hypertension or hyperthyroidism may develop more severe or
per
sistent effects, and patients w
it
h coronary artery disease could experience angina.
Pati
ents
with di
abetes may develop increased blood glucose levels following epinephrine administration.
Pati
ents with Parkinson’s disease may notice a temporary worsening of symptoms [
s
ee
WARNING
S AND P
RE
CAUTIONS (5.
5)].
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Accident
al Injection
Pati
ents should be advised to seek immediate medical care in the case of accidental injection.
Since e
pinephrine is a strong vasoconstrictor when injected into the digits, hands, or feet,
tr
eatment should be
di
rected at vasodilatation if there is such an accidental injection to these
are
as [
see W
ARNINGS AND PRECAUTIONS (5.2)
].
Serious
Infections at the Injection Site
Rare ca
ses of serious skin and soft tissue infections, including necrotizing fasciitis and
myonecr
osis caused by Clostridia (gas gangrene), have been reported at the injection site
following ep
inephrine injection for anaphylaxis. Advise patients to seek medical care if they
devel
op signs or symptoms of infection, such as persistent redness, warmt
h, swelling,
or
tende
rness, at the epinephrine injection site [see WARNINGS AND PRECAUTIONS (5.3)].
Storage
and Handling
Pati
ents should be instructed to inspect the epinephrine solution visually through the viewing
window periodically. AUV
I
-Q should be rep
laced if the epinephrine solution appears discolored
(pi
nkish color or darker than slightly yellow), cloudy, or contains particles. Epinephrine is light
sens
itive and should be stored in the outer case provided to protect it from light. Instruct patient
s
that
AUVI
-Q m
ust be used or properly disposed once the red safety guard is removed [
see
STOR
AGE AND HANDLING (16.2)
].
Co
mplete patient information, including dosage, directions for proper administration and
pre
cautions can be found inside each
AUVI-Q c
arton.
Manufact
ured for:
Kaleo, I
nc.
Richm
ond, VA 23219 USA
This pr
oduct may be covered by one or more U.S. patents or pending patent applications.
See
ww
w.kaleopharma.com/pat
for details.
*For Calif
orn
ia O
nly: This product uses batteries containing Perchlorate Material
–
speci
al handling may apply. See
ww
w.dtsc.ca.gov/hazardouswaste/perchlorate
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
1
PATIENT
INFORMATION
AUVI-Q® (epi
nephrine injection
)
Auto-I
njector
For
allergic emergencies (anaphylaxis)
Read this Pati
ent I
nf
ormation
L
eaflet
be
fore you have to use
A
UVI
-Q and
each ti
me you get a refill.
There may be
new information. You should know
how to
use
AUVI-Q b
efore you
have an
allergic emergency.
Thi
s information
does not
take the place of talking with your healthcare provider about your
medi
cal condition or your t
reat
ment.
W
hat is the most i
mpo
rtant
informa
tion
I s
hould k
now a
bout
AUV
I
-Q?
1. Al
ways carry
AUVI-Q wi
th you because you
may
not know
whe
n a life
-
threateni
ng allergic reaction
(a
naphylactic reaction)
may
happ
en
. Tal
k
to
your doctor if you need additional
uni
ts to keep at work, school, etc.
An anaph
ylactic reaction is a life
-threate
ning allergic reaction that can
happen wi
thin minutes and can be caused by stinging and bit
i
ng
i
nsects (
be
es, wasps, hornets, and mosquitoes), allergy shots, foods,
medi
cines, e
xe
rcise, or other unknown causes.
Fol
low your healthcare
prov
ider’s instructions on when to use
A
UVI
-Q i
f you have the
sy
mptoms of
an
anap
hylactic reaction
, whi
ch may include the
sy
mptoms
l
isted below:
• troubl
e
br
eathing
• wheezi
ng
• hoarsen
ess (changes in
the wa
y your voice
sound
s)
• hi
ves (raised reddened
rash that
may itch)
• sev
ere itching
• swel
ling of your face,
l
ips, mouth or tongue
• sk
in rash, redness, or
swel
ling
• fas
t heartbeat
• weak pu
lse
• fee
ling very anxious
• conf
usion
• stom
ach pain
• l
osing control of urine or
bowel
movements
• di
zziness or fainting
2. Tel
l
your
family members and
other
s where you keep
AU
VI
-Q and h
ow
to
use it before you need it. Y
o
u
may
be
unabl
e to speak in an allergic
emergen
cy.
3. Get medi
cal attention immediately after using
AUVI-Q. I
f you have a
ser
ious allergic reaction, you may need more medicine.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

2
W
hat is
A
UVI
-Q?
AUVI-Q i
s a prescription medicine used to treat life
-t
hreatening allergic
react
ions including anaphylaxis in people who are at risk for or have a history
of seri
ous allergic reactions.
AUVI-Q i
s for immediate self
(o
r caregiver)
admi
nistration and does not take
the pl
ace of
em
ergency medical care.
You
should get emergency medical
hel
p right away after using
AU
VI
-Q.
I
t is not known if
AU
VI
-Q i
s safe and effective in children who weigh less than
16.5 poun
ds (
7.5 kg
).
W
hat should I tell my healthcare provider before using
AUV
I
-Q?
Before
you use
AUV
I
-Q, tell
your healthcare provider if you:
• have
heart problems or high blood pressure
• have
diabetes
• have thyr
oid problems
• have
history of depression
• have Par
kinson’s
di
sease
• have
any other medical conditions
• are
pregnant or plan to become pregnant
. I
t is not known if
AU
VI
-Q
wi
ll harm your unborn baby.
• are
breastfeeding or plan to breastfeed. It is not known if
AU
VI
-Q
passes
into your breast milk.
Te
ll your healthcare
provi
der about
al
l the medicines
you take,
i
ncluding prescription and non
-p
rescription medicines, vitamins, and herbal
suppl
ements.
AUVI-Q an
d other medicines may affect each other
, c
ausing
si
de effects.
AUVI-Q m
ay affect the way other medicines work, and other medicines may
aff
ect how
AUVI-Q wo
rks.
Know
the medicines you take. Keep a list of them to show your healthcare
prov
ider and pharmacist when you get a new medicine.
How s
hould I use
A
UVI
-Q?
• Each A
UVI
-Q contai
ns
onl
y
1 do
se of
medi
cine
.
• AUVI-Q s
hould only be injected into the muscle of your outer thigh. It
can
be injected through your clothing, if ne
eded.
• Read the I
nstructions for Use at the end of th
is Pati
ent Information
Leafl
et
f
or information ab
out
the right way to use
A
UVI
-Q.
• Use AUVI-Q ex
actly as your healthcare provider tells you to use it.
• A
Trainer for
AU
VI
-Q wi
th a
se
parate Trainer Instruction
s for U
se
l
eaflet is included with
AUVI-Q. Addi
tional training resources are
avai
lable at
ww
w.auvi
-q.com.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
3
o Practi
ce with the
Trai
ner for AUVI
-Q befor
e an allergic
emergen
cy happens to make sure you are able to safely use the
real
AUVI-Q i
n an emergency.
o The Trai
ner for AUVI
-Q doe
s not contain a needle or med
i
cine
and c
an be
reus
ed to practice your injection.
W
hat are the possible side effects
of AUV
I
-Q?
AUV
I
-Q may c
ause
se
rious side effects
.
• AUV
I
-Q shoul
d only be injected into your
outer
thigh
. Do
not
i
nject
AU
VI
-Q i
nto
yo
ur:
• vei
ns
• buttocks
• fi
ngers, toes,
hand
s or feet
I
f you accidentally inject
AUVI-Q i
nto any other part of your body, go
to
the nearest
h
ospital
emergen
cy room right away. Tell the healthcare
prov
ider where
on
your body
yo
u received the
a
ccidental
i
njection.
• Rarel
y, patients who use
AUVI-Q ma
y develop infections a
t the
i
njection site within a few days of an injection. Some of these
i
nfections can be serious.
Cal
l your healthcare provider right away if
you
have any of the following at an injection site:
• redness
that does not go away
• swel
lin
g
• tenderne
ss
• the ar
ea feels warm to the touch
• I
f you inject a young child
or
infant
wi
th
A
UVI
-Q, hol
d their leg firmly
i
n place before and during the injection to prevent injuries. Ask your
heal
thcare provider to show you how to properly hold the leg of a
young chi
ld
or
infant
duri
ng an injection.
• If you h
ave certain medical conditions, or take certain
me
dicines, you
r c
ondition
m
ay get
wo
rse or you may have
mo
re or longer lasting side effects when you use
AUV
I
-Q. Talk
to your
healthcare
provi
der about all your medical conditions.
Common
side effects of
A
UVI
-Q i
nclude:
• fast, i
rregular, or ‘pounding’ heart beat
• sweati
ng
• shaki
ness
• headache
• pal
eness
• fee
lings of over excitement,
ner
vousness
, o
r
anxi
ety
• weakne
ss
• di
zziness
• nausea an
d
vo
miting
• breathi
ng
probl
ems
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

4
Tel
l your healthcare provider if you have any side effect that bothers you or
that does
not go away.
These a
re not all of the possible side effects of
AU
VI
-Q. For
more
i
nformation, ask your healthcare provider or pharmacist.
Cal
l your doctor for medical advice about side effects. You may report side
eff
ects to FDA at 1
-8
00
-FDA-1
088.
How s
hould I store
AUV
I
-Q?
• Store AU
VI
-Q at
68°
t
o
7
7°F
(20° to
2
5°C
).
• Do
NOT expose to extreme heat or cold
. F
or example, do NOT store in
your vehi
cle’s glove box
. Do
not store
AUV
I
-Q i
n the refrigerator
or
fr
eeze
.
• Exami
ne contents in the viewing window periodically.
Sol
ution should
be c
lear.
I
f the solution is discolored
(pi
nkish color or darker than
sl
ightly yellow), cloudy
or
contains sol
i
d particles, replace the unit.
• Your AU
VI
-Q ha
s an expiration date
. R
eplace it before the expiration
date.
• Keep A
UVI
-Q i
n the outer case it comes in to protect it from light.
Kee
p
AUV
I
-Q and a
ll medicines out of the reach of children.
General
i
nforma
tion about
t
he safe and effective use of
AUV
I
-Q:
Medi
cines are sometimes prescribed for purposes other than those listed in a
Pati
ent Information Leaflet. Do not use
A
UVI
-Q for a condi
tion for which it
was no
t prescribed. Do not give
AUVI-Q t
o othe
r
people, even if they have
an
al
lergic reaction or
th
e same symptoms that you have
. I
t may harm them.
Thi
s Patient Information L
eafl
et summarizes the most important information
about
AUV
I
-Q.
If you would like more information, talk
to y
our healthcare
provi
der. You can ask your pharmacist or healthcare provider for information
about AUV
I
-Q that i
s written for health professionals.
For more
information and video instructions on the use of
AUV
I
-Q, go t
o
ww
w.auvi
-q.com or c
all 1
-844-828-8
472
.
W
hat are the ingredients in
AUV
I
-Q?
Active
ingredient:
e
pinephrine.
Inac
tive Ingredients:
sodi
um chloride, sodium bisulfite, hydrochloric acid,
and wate
r.
AUVI-Q do
es not contain latex.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
5
Instr
uctions
for U
se
Read these I
nstructions for Use carefully before you need to use your
AU
VI
-
Q. Before
you use
A
UVI
-Q, ma
ke sure your healthcare provider shows you
the r
ight way to use it. If you have any questions, ask your healthcare
prov
ider.
I
f you are administering
AUVI-Q to a you
ng child
or
infant
, hol
d the leg firmly
i
n place
an
d limit movement prior to and
whi
le administering an injection.
Aut
omated Voice Instructions
AUVI-Q c
ontains an electronic voice instruction system to help guide you
through
each step of your injection. If the voice instructions do not work for
any
reason, use
AUVI-Q a
s instructed
i
n these
I
nstructions for Use. It will still
work duri
ng an allergic reac
ti
on emergency.
How to u
se
your AUV
I
-Q
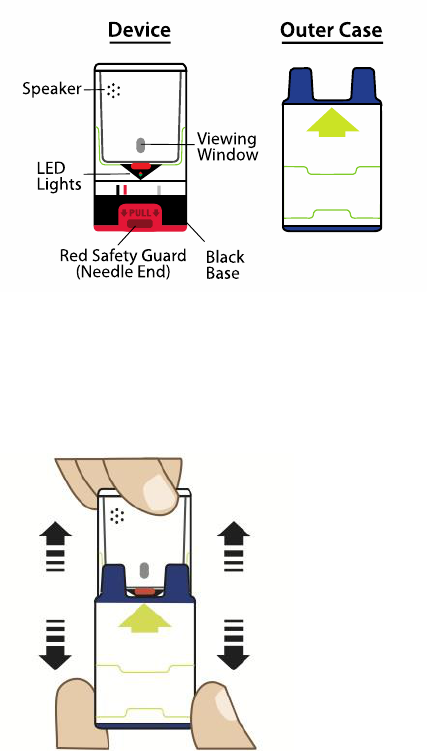
Figure
A
.
1. Pull AUV
I
-Q up from
the outer case
. S
ee Figure B.
Do not go to ste
p 2 until you are ready to use
AUVI-Q. I
f
you
are
not
ready to
use
A
UVI
-Q
,
put i
t back in
the
outer case.
Figure
B.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

6
2. Pull Red safety g
uard
down
and off of
AUV
I
-Q. Se
e Figure C.
To redu
ce the chance of
an a
ccidental injection, do not touch the black
base of
the auto
-i
njector, which is where the needle comes out. If an
acc
idental injection
happens, ge
t
medi
cal help
ri
ght away
.
Note: The red sa
fety guard
i
s made to fit tight
. P
ull firmly to remove.
Figure
C.
3. Place
blac
k
end of AUV
I
-Q a
gainst
the mi
ddle
of th
e outer thigh
(throu
gh
cl
othing, if needed), then
pu
sh
firm
ly
until
you hear a
cl
ick and hiss sound
, and
hold in place for
2 se
conds.
Se
e Figure D.
Onl
y
i
nject into the middle of
t
he
oute
r thigh.
Do
not
i
nject into any
other part
of
th
e
b
ody.
If you are
administering
AUV
I
-Q
to a young child
or infan
t
,
hold
th
e leg firmly in place while
admi
nistering an injection
(S
ee Figure
E
)
.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
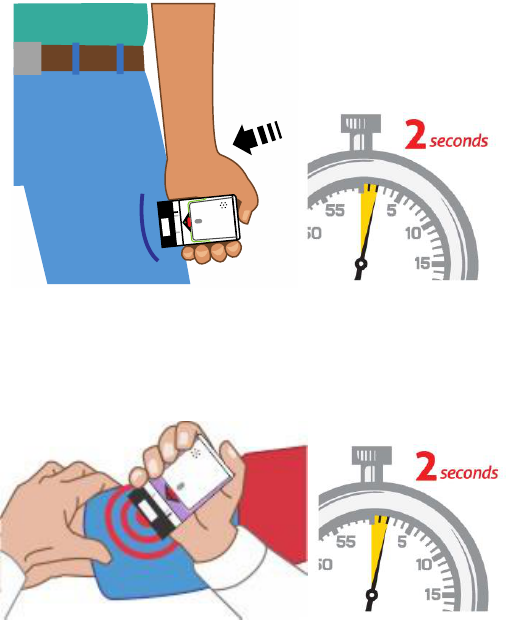
7
Figure
D.
(For AU
VI
-Q
0.3 mg and AUVI
-Q
0.15 mg)
Figure
E.
(For AUV
I
-Q 0.1 mg)
Note:
AUV
I
-Q make
s a distinct sound (click and hiss) wh
en
you
push i
t
fi
rmly
agai
nst
your
o
uter thigh.
Thi
s is normal and indicates
AU
VI
-Q i
s
worki
ng correctly.
Do
not pull
AUVI-Q awa
y from your leg when you hear
the c
lick and hiss sound.
The needl
e automatically retracts after the injection is complete
, so the
needl
e will not be visib
l
e after the
i
njecti
o
n.
AUVI-Q
includes a
2-second
countd
own after it is activated
,
then the voice instruction will indicate the
i
njection is complete
and to
seek emergency medical attention
, AUVI-Q
wi
ll beep, and the lights will blink red.
4. Get em
ergency medical
hel
p
right aw
ay.
Repl
ace the outer case and talk to your healthcare provider about the
right w
ay to throw away your
AUV
I
-Q.
Ask
your healthcare provider for a
n A
UVI
-Q pre
scription refill.
Aft
er the use of
AUV
I
-Q:
• The bl
ack base will lock into place.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

8
• The voi
ce instruction system will
say “seek
emergency medical
attenti
on
”, sa
y
“thi
s
AUVI-Q ha
s been used
…”, and
the lights will
bl
ink red.
• The red
safety guard cannot be replaced.
• The vi
ewing window will no longer be clear.
• I
t is normal for some medicine to remain in your
AU
VI
-Q after
you
have recei
ved your dose of medicine.
• Talk to yo
ur healthcare provider
abou
t the
ri
ght way to throw away
your AUVI-Q.
• AUVI-Q i
s a single
-us
e
auto-i
njector
and c
annot be reused.
Unti
l you
t
hrow away
your
used
AUVI-Q, t
he electronic voice instruction
system
will remind you that it has been used when the outer case is
remo
ved.
I
f you
wi
ll be
ad
ministering
AUV
I
-Q to
a young child
o
r infant
, a
sk your
heal
thcare provider to show you how to
p
roperly hold the leg in place while
admi
nistering a dose.
Thi
s Patient Information has been approved by the U.S. Food and Drug
Admi
nistration.
Rev No
v
2
017
Manufa
ctured for:
Kal
eo, Inc.
Ri
chmond, VA 23219 USA
Thi
s product may be covered by one or more
U.S.
patents or pending patent
appl
ications.
S
ee
ww
w.kaleopharma.com/pat
f
or details.
*For
California Only: This product uses batteries containing
Perc
hlorate Material
– sp
ecial handling may apply. See
www.d
tsc.ca.gov/hazardouswaste/perchlorate
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

1
TRAINER FOR AUVI-Q®
Trainer Instructions for Use
Important:
The TRAINER for AUVI-Q Does Not contain a needle or medicine.
In case of an allergic emergency, use the real AUVI-Q and not the gray Trainer.
Always carry your real AUVI-Q with you in case of an allergic emergency.
Important Information about the TRAINER for AUVI-Q:
Inside your TRAINER for AUVI-Q are:
• batteries
• a speaker that will make a beeping sound and that produces electronic voice
instructions
• red and green blinking lights
The TRAINER for AUVI-Q batteries are made to last long enough for you to practice 1
time each day for 2 years. If your TRAINER for AUVI-Q does not work properly call your
healthcare provider for a new Trainer.
What is the TRAINER for AUVI-Q?
• The TRAINER for AUVI-Q does not contain a needle or medicine and can be reused to
practice your injection.
• Practice with the TRAINER for AUVI-Q before an allergic emergency happens to make
sure you are able to safely use the real AUVI-Q in an emergency.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

2
Your TRAINER for AUVI-Q
Figure A
TRAINER for AUVI-Q
AUVI-Q
AUVI-Q 0.3 mg is orange
AUVI-Q 0.15 mg is blue
AUVI-Q 0.1 mg is white and
lavender
TRAINER for AUVI-Q:
• is inside a gray outer case
• does not have a needle or medicine
inside
• can be reused (the red safety guard can
be placed back on the base of the
Trainer after use)
• has no expiration date
• has embossed “TRAINER” on top of the
device
AUVI-Q:
• is inside an orange (0.3 mg) or blue
(0.15 mg) or white and
lavender (0.1 mg) outer case
• contains a needle and epinephrine
medicine
• cannot be reused (the red safety
guard cannot be placed back on the
base of AUVI-Q after use)
• has a medicine expiration date listed on
the device
In case of an allergic emergency, use the real AUVI-Q and
not the gray Trainer.
Base
Safety Guard
Speaker
Housing
LEDs
Outer Case Device
Top view
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

3
Who should practice using the AUVI-Q Trainer?
Anyone who may need to help you with AUVI-Q in case of an allergic emergency:
• You
• Caregivers
• Family
• Friends
• Co-workers
• Teachers
• Child Care or Day Care Workers
Have them practice using the Trainer and review the Patient Information Leaflet included in
the packaging with each prescription of AUVI-Q.
For more information and video instructions on the use of AUVI-Q, go to www.AUVI-Q.com
or call 1-844-828-8472.
Practicing with the TRAINER for AUVI-Q
Practice with the TRAINER for AUVI-Q before an allergic emergency happens to make sure
you are able to safely use the real AUVI-Q in an emergency.
• You should practice daily for the first week after you receive your TRAINER for AUVI-
Q to help you feel comfortable using AUVI-Q quickly and safely. Even when you are
comfortable using the Trainer, continue to practice using it often.
How to Use the Trainer
How the TRAINER for AUVI-Q works
Although the Trainer does not have a needle and contains no medicine, it works the same
way as the real AUVI-Q.
As with the real AUVI-Q, the TRAINER for AUVI-Q contains an electronic voice instruction
system to help guide you through each step of your injection. If the voice instructions do
not work for the TRAINER for AUVI-Q for any reason, you can still use the TRAINER for
AUVI-Q as instructed in this leaflet to practice.
The TRAINER for AUVI-Q has the same blinking red and green lights as the real AUVI-Q.
As with the real AUVI-Q, if practicing with a young child or infant, hold the leg firmly in
place while using the TRAINER for AUVI-Q. Ask your healthcare provider to show you how to
properly hold the leg to practice so that you will be prepared before an allergic emergency
happens.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
4
Fo
llow These Steps
1. Pull
the T
RAINER for AUVI
-Q f
rom the outer case.
S
ee Figure B.
2. Pull Red s
afety guard
d
own and off of the Trainer
. Se
e Figure C
.
Fig
ure B
Fig
ure C
Note:
The red saf
ety guard
i
s made to fit tight
si
milar to the safety guard on the
real
AUVI
-Q. Pull
firmly to remove.
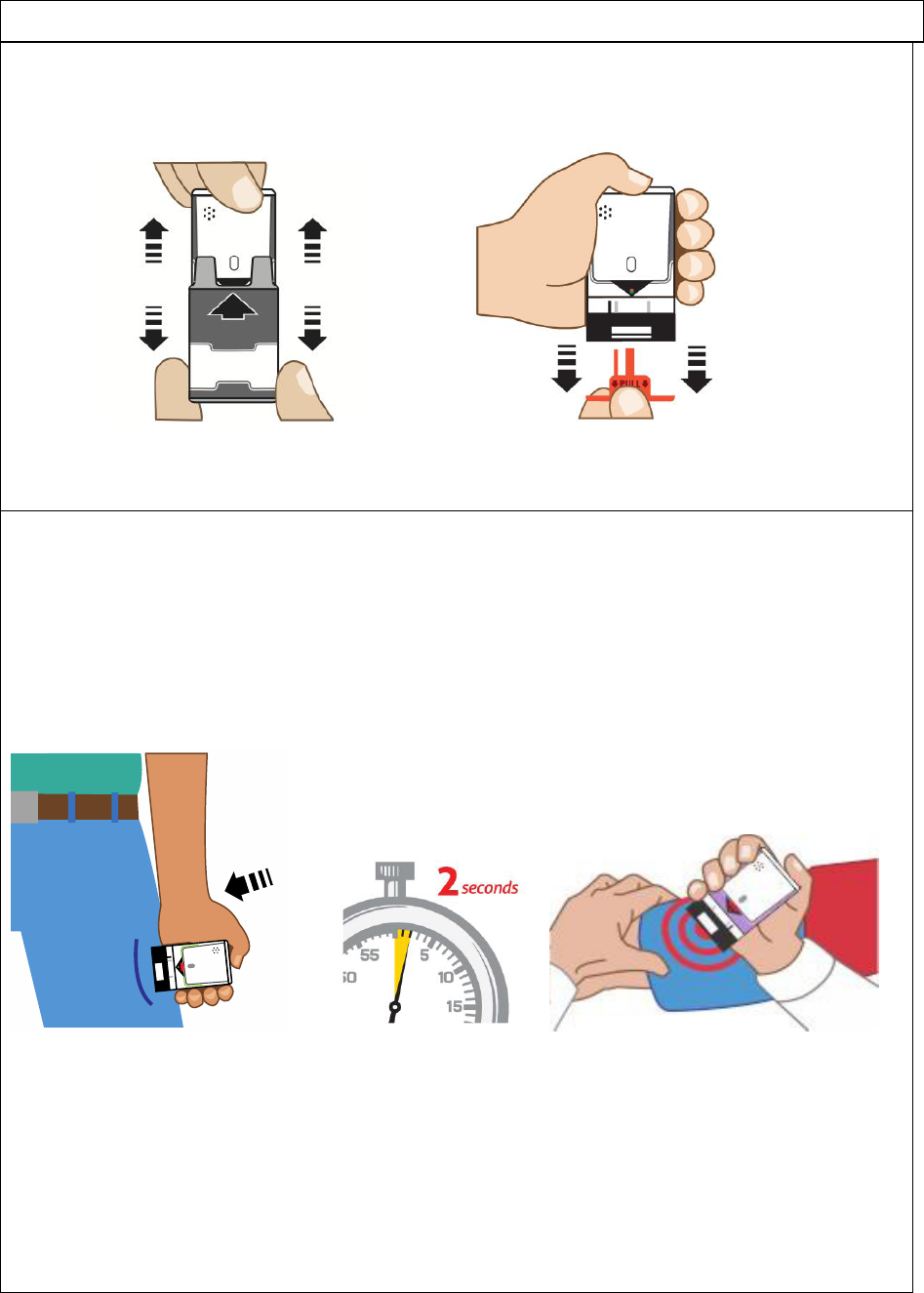
3. Pl
ace black end against the middle of the outer thigh (through clothing, if
needed
), then
p
ush
fi
rmly
until
you hear a click and hiss sound
, and
hold in
pla
ce for
2 secon
ds.
Se
e Figure D.
As
with the real
AUVI-Q, i
f practicing with a young child
o
r infant
, hol
d the leg
f
irmly in place while using
t
he
TRAIN
ER for AUVI
-Q. (Se
e Figure E).
Fig
ure D
Fig
ure E
Note
: In an actual emergency, after the injection you would need to seek medical help
ri
ght away.
Only pra
ctice using the middle of your outer thigh. The outer thigh is where you would
inj
ect with the real
AUVI-Q.
Note
: The
T
RAINER for AUVI
-Q ma
kes a distinct sound (click and hiss) when you
push it
fir
mly
aga
inst your outer thigh. This is the same sound
t
hat is made with the real
AUVI-Q.
This is nor
mal, and indicates
AUVI-Q i
s working correctly. Do not pull
AUVI-Q a
way from
your le
g when you hear the click and hiss sound.
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
5
4. Aft
er practicing, reset the
TRAINE
R for AUVI
-Q:
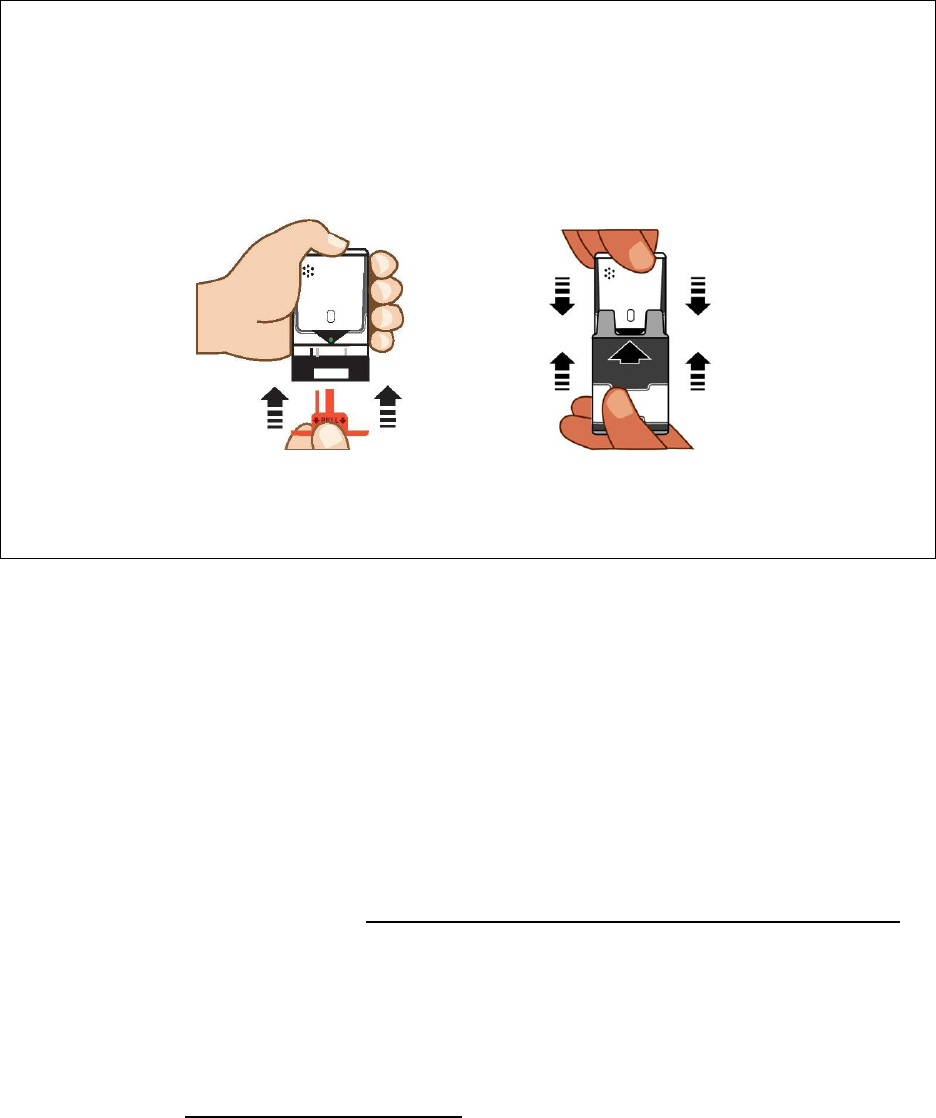
a. Repl
ace the Red safety guard
. Do no
t hold the black base while inserting
the R
ed safety guard. The Black base will drop down into its original
l
ocation during Red safety guard insertion.
See Fi
gure F.
b. Sli
de the
TRAINE
R for AUVI
-Q all
the way back into the gray outer
cas
e to reset the elec
tronic
voice system
. See
Figure G.
Figur
e F
Figur
e G
Note: Leav
e
the
TRAINER
for AUVI
-Q i
n its outer case for
at
least 5 seconds
between
each time you practice to
al
low
the el
ectronic voice system
t
o
r
eset.
Sto
rage
:
• Stor
e the
T
RAINER for AUVI
-Q at r
oom temperature
; t
he
TRAINER
for AUVI
-Q s
hould
not be
used at temperatures
le
ss than
50
ºF
(1
0
ºC) or
greater than
1
04ºF (40ºC).
• Stor
e the
T
RAINER for AUVI
-Q in its outer
case
.
• Kee
p
the
T
RAINER for AUVI
-Q awa
y from dirt, chemicals, and water.
Dispos
al:
The TRAI
NER for AUVI
-Q contai
ns electronics and lithium coin cell batteries, and should be
disp
osed of in the correct manner
. Fol
low your State and local e
nviro
nmental regulations
for
disp
osal
.
Fo
r California Only: This product uses batteries containing Perchlorate Material
-
speci
al handling may apply. See
w
ww.dtsc.ca.gov/hazardouswaste/per
c
hlorate
Man
ufactured for:
Kal
eo, Inc.
Richm
ond, VA 23219 USA
This pro
duct may be covered by one or more U.S. patents or pending patent
appl
ications.
Se
e
www.
kaleopharma.com/pat
f
or details.
Re
v
Nov 20
1
7
DO
NOT HOLD
B
LACK BASE
w
hile inserting Red
S
afety Guard
Reference ID: 4183363
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
