CROP PRODUCTION SCIENCE IN HORTICULTURE SERIES
Series Editor: Jeff Atherton, Professor of Tropical Horticulture,
University of the West Indies, Barbados
This series examines economically important horticultural crops selected from the
major production systems in temperate, subtropical and tropical climatic areas.
Systems represented range from open fi eld and plantation sites to protected plastic
and glass houses, growing rooms and laboratories. Emphasis is placed on the scientifi c
principles underlying crop production practices rather than on providing empirical
recipes for uncritical acceptance. Scientifi c understanding provides the key to both
reasoned choice of practice and the solution of future problems.
Students and staff at universities and colleges throughout the world involved in
courses in horticulture, as well as in agriculture, plant science, food science and
applied biology at degree, diploma or certifi cate level will welcome this series as a
succinct and readable source of information. The books will also be invaluable to
progressive growers, advisers and end-product users requiring an authoritative, but
brief, scientifi c introduction to particular crops or systems. Keen gardeners wishing
to understand the scientifi c basis of recommended practices will also fi nd the series
very useful.
The authors are all internationally renowned experts with extensive experience
of their subjects. Each volume follows a common format, covering all aspects of
production, from background physiology and breeding to propagation and planting,
through husbandry and crop protection to harvesting, handling and storage. Selective
references are included to direct the reader to further information on specifi c topics.
Titles available:
1. Ornamental Bulbs, Corms and Tubers A.R. Rees
2. Citrus F.S. Davies and L.G. Albrigo
3. Onions and Other Vegetable Alliums J.L. Brewster
4. Ornamental Bedding Plants A.M. Armitage
5. Bananas and Plantains J.C. Robinson
6. Cucurbits R.W. Robinson and D.S. Decker-Walters
7. Tropical Fruits H.Y. Nakasone and R.E. Paull
8. Coffee, Cocoa and Tea K.C. Willson
9. Lettuce, Endive and Chicory E.J. Ryder
10. Carrots and Related Vegetable Umbelliferae V.E. Rubatzky, C.F. Quiros and
P.W. Simon
11. Strawberries J.F. Hancock
12. Peppers: Vegetable and Spice Capsicums P.W. Bosland and E.J. Votava
13. Tomatoes E. Heuvelink
14. Vegetable Brassicas and Related Crucifers G. Dixon
15. Onions and Other Vegetable Alliums, 2nd Edition J.L. Brewster
16. Grapes G.L. Creasy and L.L. Creasy
17. Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and
Aroids V. Lebot
18. Olives I. Therios
19. Bananas and Plantains, 2nd Edition J.C. Robinson and V. Galán Saúco
20. Tropical Fruits, 2nd Edition, Volume 1 R.E. Paull and O. Duarte
This page intentionally left blank

T F,
2 E, V 1
Robert E. Paull
Professor of Plant Physiology
College of Tropical Agriculture and Human Resources
University of Hawaii at Manoa
Honolulu, HI, USA
and
Odilo Duarte
Professor and Lead Scientist in Agribusiness
CENTRUM Católica Business School
Pontifi cia Universidad Católica del Perú
Lima, Perú
CABI is a trading name of CAB International
CABI Head O ce
Nosworthy Way
Wallingford
Oxfordshire OX10 8DE
UK
Tel: +44 (0)1491 832111
Fax: +44 (0)1491 833508
E-mail: [email protected]
Website: www.cabi.org
CABI North American O ce
875 Massachusetts Avenue
7th Floor
Cambridge, MA 02139
USA
Tel: +1 617 395 4056
Fax: +1 617 354 6875
E-mail: [email protected]
© CAB International 2011. All rights reserved. No part of this
publication may be reproduced in any form or by any means,
electronically, mechanically, by photocopying, recording or otherwise,
without the prior permission of the copyright owners.
A catalogue record for this book is available from the British Library,
London, UK.
Library of Congress Cataloguing-in-Publication Data
Paull, Robert E.
Tropical fruits / Robert E. Paull and Odilo Duarte. -- 2nd ed.
p. cm. -- (Crop production science in horticulture series ; no. 20)
Includes bibliographical references and index.
ISBN 978-1-84593-672-3 (alk. paper)
1. Tropical fruit. I. Duarte, Odilo. II. C.A.B. International. III. Title. IV. Series: Crop
production science in horticulture ; 20.
SB359.P38 2011
634′.6--dc22
2010016776
ISBN: 978 1 84593 672 3
Commissioning editor: Sarah Hulbert
Production editor: Shankari Wilford
Typeset by Columns Design Ltd, Reading, UK.
Printed and bound in the UK by MPG Books Group.

vii
PREFACE
The monoaxial banana, pineapple and papaya and polyaxial mango are
the most well-known tropical fruits worldwide. Avocado is better known for
production in subtropical areas, but considerably more production occurs in
the tropical zone. Banana, pineapple and avocado are extensively grown by
large companies. Banana, along with plantain, is the largest fruit crop in the
tropics, with only a small fraction entering international commerce. Many
other tropical fruits, already well known in the tropics, are now appearing in
larger temperate city markets.
The fi rst edition of this book was started by Dr Henry Nakasone after he
retired from the University of Hawaii at Manoa in 1981. His work on the book
was prolonged because of his extensive volunteer and consulting activities
from his retirement to 6 months before his death in 1995. The extensive
research carried out by Henry in preparing some draft chapters laid the
foundation for the 1998 fi rst edition. Henry understood the need for a book
that melded equally the genetics, physiology and cultural practices with
postharvest handling of each fruit crop as an interrelated whole.
This second edition has been completely revised and new chapters added.
A colleague, Dr Odilo Duarte, formerly Professor from Escuela Agrícola
Panamericana – El Zamorano, Honduras, and now Professor and Lead
Scientist in Agribusiness, CENTRUM Católica Business School, Pontificia
Universidad Católica del Perú, Lima, Perú, joined me in this revision. It was
decided to make this a general tropical fruit production textbook and only
cover the major tropical crops in Volume 1. The other tropical fruits have been
moved to Volume 2, which should appear next year.
The fi rst fi ve chapters deal with the general aspects of the tropical climate,
fruit production techniques, tree management and postharvest handling.
Subsequent chapters deal with the principal tropical fruit crops that are
common in temperate city markets. The information in each fruit chapter
deals with taxonomy, varieties, propagation and orchard management, biotic
and abiotic problems, variety development and postharvest handling. The
information contained should be of use to all readers and students interested
in an introductory text on tropical fruit production.

viii Preface
Many have contributed to the fi rst edition and to this edition. Encourage-
ment and help to Henry in this passion came from many, and they were
acknowledged in the fi rst edition. Others must be mentioned who provided
help and encouragement since the fi rst edition, including Skip Bittenbender,
Victor Galán Saúco, Ying Kwok Chan, George Wilson, Ken Rohrbach, Duane
Bartholomew, Francis Zee, Ken Love and Chun Ruey Yen. Their numerous
comments and suggestions have been incorporated in most cases. All errors
and omissions are our responsibility. The illustrations of each crop were done
by Susan Monden, and her perseverance and skill were greatly appreciated.
Thanks are also due to the Commissioning Editor, Sarah Hulbert, for her
assistance and patience during the book’s development.
We would greatly appreciate receiving all comments and suggestions on this
text. We can be reached at the addresses given on the title page or via e-mail at
paull@hawaii.edu or odiloduarte@yahoo.com
In closing, we both acknowledge the continued support, assistance and
love of our wives, Nancy and Carla, and our children, which enabled us to
complete this undertaking.
Robert E. Paull
Honolulu
USA
2010
Odilo Duarte
Lima
Perú
2010

© CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1 1
(R.E. Paull and O. Duarte)
1
INTRODUCTION
INTRODUCTION
The tropics, with its warm climate and little temperature variation, occupies
approximately 40% of the earth’s land surface. The region also has half the
world’s population. The majority of the world’s biodiversity is also found in
the tropics, biodiversity being the total of all living organisms on earth. These
endemic animals and plants are adapted to the diverse tropical environments,
which range from wet tropical rainforests to deserts and snow-covered, high
mountains.
The tropics can be divided into three major zones. The zone most
recognized is that with year-round rainfall and lies on the equator (Amazon,
Central America, Central Africa, Indonesia, New Guinea) and is ~8% of
the world’s land surface. As one moves away from the equator, the rainfall
becomes more seasonal, and this zone occupies 16% of the land area (Central
America, north and south Amazon, West Africa, India, South-east Asia,
northern Australia). The last is the dry tropics, which makes up 16% of the
land area and ranges from deserts to large areas with long dry seasons of 9
months or more. Examples would be the Sahara, Bolivian El Chaco lowlands,
central India and northern central Australia.
About half of the plant families are tropical, and the tropical region
contains 15 of the 25 world biodiversity ‘hot spots’ (Crane and Lidgard, 1989;
Meyer et al., 2000). The ‘hot spots’ are regarded as centres for agricultural
origins, and it is thought that crop domestication took place in or near these
‘hot spots’. This domestication refl ects the role of hunter–gatherers and early
farmers, and their dependence on these crops for their daily subsistence. The
abundance of species with di erent life cycles, adaptations and useful products
in these ‘hot spots’ would facilitate their selection by hunter–gatherers and
early farmers. Examples of these centres include half of the southern part
of Mexico and the northern half of Central America, Ecuador, western and
central Brazil, the Indo-Burma region, South-east Asia, the Indonesian and
Philippine archipelagos, the East Melanesian Islands and Pacifi c Micronesia.

2 Chapter 1
TROPICAL FRUITS
Most botanical families have at least one species of tropical fruit (Table 1.1).
In tropical America, more than 1000 fruit species are described, though only
100 are found in local markets. Asia has about 500 tropical fruit species, the
Indian subcontinent about 300, with about 1200 in Africa. Of these fruits
only a few are found in local markets and fewer are exported. Ninety per
cent of the export market is made up of citrus, banana and plantain, mango
and pineapples (Table 1.2). A further 5% is made up of papaya, avocado
and dates. The remainder is made up of more than 20 species, ranging from
breadfruit and litchi to mangosteen, passion fruit and coconut. More than
90–95% of tropical fruits are not exported from the producing country but
are consumed locally.
The most common tropical fruits in trade come from three major areas:
Central and South America (papaya, avocado, pineapple, guava), Asia
(most citrus fruits, litchi), and South and South-east Asia (banana, mango,
mangosteen, durian) (Gepts, 2008). Only one important tropical fruit is native
to Africa and that is the date, though the continent has many other tropical
fruits. Fruit species were selected by man and distributed widely throughout
the world, based upon various factors, which included the crop’s adaptability
to di erent environments, the fruit’s seed storage life, ease of plant propagation
(seed, cuttings, plants), the size and shape of the plant, a multiplicity of uses
other than as a fresh fruit (cloth, medicinal, wood) and having an agreeable
taste. Many tropical seeds are recalcitrant and cannot be dried and must be
transported as cuttings or plants to be introduced to new areas.
As people migrated, often the crops with which they were familiar were
taken along. The spread to areas surrounding that of their origin probably
began early. For example, the mango, a native of the Indo-Burma region,
had spread to all of South-east Asia by the end of the fourth century .
Arabs traders in the Indian Ocean probably took mangoes to the east coast
of Africa around 700 . The orange was also moved, most likely by Arab
traders, to the Mediterranean and southern Europe. Opportunities probably
also existed to move some tropical fruits (e.g. pineapple) around the warmer
areas of Central and South America. The European discovery of America led
to a rapid exchange of tropical fruit crops between the Old and New Worlds.
Bananas were carried to Santo Domingo from the Canary Islands in 1516. The
Portuguese spread tropical fruits from their colony in Brazil around the Cape
of Good Hope to Goa in India, Malacca in Malaysia, China and Japan. The
Spanish had a regular galleon service from Mexico to the Philippines between
1565 and 1815. The Dutch, British and French ships also spread tropical
fruits around the globe.

Introduction 3
Table 1.1. Taxonomy and primary centre of diversity and probable centre of origin of the major tropical fruits (Gepts, 2008).
Order Family (subfamily) Crop(s), taxa Centre of origin
Magnoliid
complex
Laurales Lauraceae
Avocado, Persea americana
Tropical Central America
Magnoliales Annonaceae
Annona spp., cherimoya, ilama, soursop, sweetsop,
atemoya; Rollinia pulchrinervis, biriba
Tropical South America
Monocots Arecales
Coconut, Cocos nucifera
South-east Asia
Date, Phoenix dactylifera
N. Africa, Middle East
Poales Bromeliaceae
Pineapple, Ananas comosus
South America
Zingiberales Musaceae
Banana and plantain, Musa spp.
South-east Asia
Eudicots Caryophyllales Cactaceae Pitaya Tropical America
Oxalidales Oxalidaceae
Carambola, Averrhoa carambola
South-east Asia
Malpighiales Malpighiaceae
Barbados cherry, Malpighia glabra
West Indies, South America
Clusiaceae (Guttiferae)
Mangosteen, Garcinia mangostana
South-east Asia
Passifl oraceae
Passion fruit, Passiflora spp.
Tropical America
Rosales Moraceae Breadfruit, chempedak, jackfruit, etc.,
Artocarpus spp.
Polynesia
Myrtales Myrtaceae
Surinam cherry, Eugenia spp.
Tropical America
Jaboticaba, Myrciaria cauliflora
Brazil
Guava, Psidium guajava
Tropical America
Brassicales Caricaceae
Papaya, Carica papaya
Central America
Malvales Malvaceae
Durian, Durio zibethinus
South-east Asia
Sapindales Sapindaceae
Longan, Dimocarpus longan; litchi, Litchi
chinensis; and rambutan, Nephelium lappaceum
South-east Asia
Rutaceae
Citrus, Citrus spp.
South-east Asia
Anacardiaceae
Cashew, Anacardium occidentale
Tropical America
Mango, Mangifera indica
India, South-east Asia
Hog plum, mombins, Spondias spp.
Tropics
Ericales Sapotaceae
Caimito, Chrysophyllum cainito
South America
Sapodilla, Manilkara zapota
Central America
Mamey sapote, Pouteria sapota
Mexico, Central America

4 Chapter 1
TROPICAL FRUIT CHARACTERISTICS
Tropical fruits are harvested from woody plants (avocado, mango, orange)
but also from herbaceous plants (banana, papaya) and vines (passion fruit).
The evolution of fruit in the early Tertiary period was a major advance that
Table 1.2. World production and acreage of major tropical fruits in 2007, from FAO
Statistics Division (FAO, 2009).
Fruit
Production
(1000 of t)
Acreage
harvested
(1000 × ha) Important producing countries
Avocado 3,569 407 Mexico, United States,
Dominican Republic, Brazil,
Colombia, Chile, South Africa,
Indonesia, Israel, Spain
Banana Dessert 85,856 5,109 Burundi, Nigeria, Costa Rica,
Mexico, Colombia, Ecuador,
Brazil, India, Indonesia,
Philippines, Papua New
Guinea, Spain, Central America
Plantain 33,925 5,375 Colombia, Ecuador, Peru,
Venezuela, Ivory Coast,
Cameroon, Sri Lanka,
Myanmar
Citrus Oranges 7,104 1,071 Brazil, United States, India,
Mexico, Spain, China, Italy,
Egypt, Pakistan, Greece, South
Africa
Tangerines
and
Mandarins
27,865 2,052 Brazil, United States, India,
Mexico, Spain, China, Italy,
Egypt, Pakistan, Greece, South
Africa, Japan
Coconut 61,504 11,106 Indonesia, Philippines, India,
Sri Lanka, Brazil, Thailand,
Mexico, Vietnam, Malaysia,
Papua New Guinea
Mango 33,446 4,610 India, Pakistan, Indonesia,
Philippines, Thailand, Mexico,
Haiti, Brazil, Nigeria
Papaya 7,208 378 Nigeria, Mexico, Brazil, China,
India, Indonesia, Thailand, Sri
Lanka
Pineapple 20,911 2,378 Philippines, Thailand, India,
Indonesia, China, Brazil, United
States, Mexico, Nigeria, Vietnam
Introduction 5
increased the e ciency of angiosperm seed dispersal. Climate change,
radiation of birds and animals, and changes in plant community habitats are
all potential evolutionary forces that led to the appearance of a range of fruit
types. The fl eshy fruits, with their mutually benefi cial interaction of providing
nutrition to animals and improving seed dispersal, have arisen independently
in di erent families, have disappeared and reappeared, are not evolutionarily
conserved, and show no clear association with phylogeny. The fossil and
morphological evidence indicates that multiple fruit types have evolved
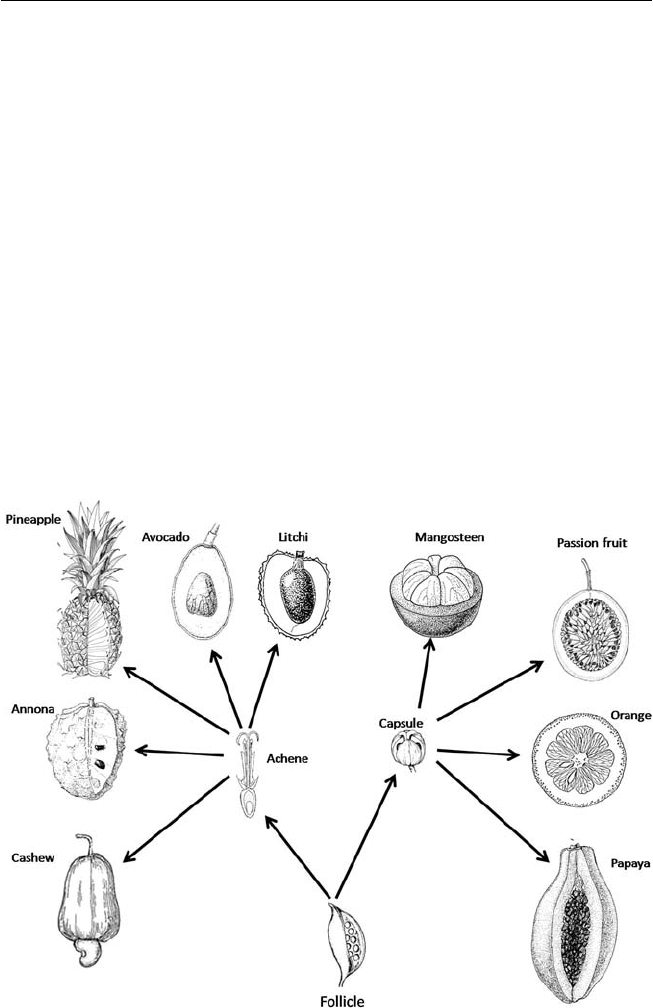
directly from a dry follicle-bearing ancestor (Fig. 1.1).
The follicle is seen as the archetypical progenitor fruit, with a single
fused carpel that splits along a single seam (dehiscent zone). The fused carpel
appeared about 97 million years ago (Mya), in the middle Cretaceous. The
abscission (separation) zones are found much earlier in the fossil record
(400 Mya) in early vascular plants. In fruit, the biochemical processes in the
dehiscence zones and during ripening are thought to have co-opted systems
that evolved for the abscission of sporangia, leaves, petals and stamens. The
fruits that are consumed have soft and juicy arils (rambutan, litchi, longan),
pedicel (cashew), fl oral and accessory tissue (pineapple, annonas), mesocarp
Fig. 1.1. Types and structures of tropical fruits and their evolutionary development
from dehiscent and non-dehiscent dry fruits (redrawn from fi gures in Nakasone and
Paull, 1998).

6 Chapter 1
(papaya, avocado) and endocarp (citrus). A few species are in the magnoliid
complex (annonas, avocado) and monocots (banana, coconut, pineapple);
the most important species are all eudicots. The fl oral parts of the magnoliid
complex occur in whorls of three (trimerous); the pollen has one pore and
they usually have branching-veined leaves and are regarded as basal or more
‘primitive’ angiosperms.
Tropical fruits, in most cases, are sold fresh, and o -grade fruit is
processed. The exception to this would be coconut, which is grown principally
for other products (copra, oil, coir) with a small acreage, often of special
varieties, that are grown for fresh consumption. Cashew is grown mainly
for its nut, with the fl eshy pedicel being eaten fresh, processed and made into
juice. Most tropical fruits are highly perishable, and signifi cant development
has taken place to process selected fruits into dried products, juices and purees.
Bananas such as plantains are also often used as a starch staple in Africa, Asia
and Latin America and not as a dessert fruit.
NUTRITIONAL VALUE
Nutrient contents of tropical fruits found in food composition tables are used
for nutritional assessment, research linking diet to health, nutritional policy,
food labelling, and consumer education. Accurate data are needed in order to
predict dietary energy intake and undernourishment. For tropical fruit, this
is important, as they are often regarded as signifi cant sources of minerals,
vitamins and carbohydrates (Favier et al., 1993).
Natural variation occurs in the nutrient content of fruits. This variability
is due to soil and climatic conditions, variety grown, the stage of maturity at
harvest and physiological state when eaten. Traditionally, food composition
tables for most foods are presented as mean values, ignoring the natural
biological variability. It is probably more useful to know the range of values
found and the standard error or deviation.
Most food composition tables present data as nutrient values per 100 g of
edible food. Tropical fruits have low to moderate energy content and provide
about 200 to 300 kJ (FAO, 2003). Some tropical fruits, such as bananas (380
kJ), avocado (572 kJ) and durians (536 kJ), are higher and others less energy-
dense, such as the carambola (121 kJ). The protein content of most fruits,
including tropical fruits, is low (<1 g/100 g), though avocado (1.8 g) and
durians (2.6 g) are higher. Fat contents are also low, except for avocado (14.2 g)
and durians (2–5 g). The carbohydrate content is presented as monosaccharide
equivalents with fi bre excluded, and contents normally range from 10 to 15 g,
which is the range that most consumers regard as sweet. Higher carbohydrate
contents are found in bananas (~20 g), atemoya (~21 g) and durians (~26 g).
Dietary fi bre is reported to range from 1 to 2 g in tropical fruits, though di erent
analytical methods are used that give di erent values in the same fruit.
Introduction 7
Tropical fruits are low to moderate sources of macronutrients and good
sources of micronutrients. For example, while most fruits have 10–20 mg of
calcium, mango only has ~1.2 mg. Iron ranges from 0.2 to 0.4 mg. Banana
and durian are good sources of potassium, having 100–200 mg. Some fruits
are good sources of folate, and most are high sources of vitamin C (>20 mg).
The beta-carotene in fruit varies widely, depending upon the content of the
di erent carotenes present. The di erent varieties of mangoes can vary in beta-
carotene from 350 to 13,000 mcg. Other components present in tropical fruits
include antioxidants and other phytochemicals that have potential health-
promoting e ects, with various claims being made.
Nutrient and health claims are frequently made for tropical fruits. Codex
Alimentarius (2001) has set standards for health-claim labelling. Using these
standards, some nutrient claims can be made for tropical fruit (Table 1.3). For
example, for a product to be low in fat it must have less than 3 g/100 g; for a
tropical fruit to be a ‘source’ of a particular nutrient it must contain 15% and
a ‘high source’ 30% of the Codex Alimentarius (2001) reference nutrient value.
SIGNIFICANT TRENDS – PRODUCTION AND MARKETING
The production and world trade in fresh tropical fruits is expected to expand
(Sarris, 2003). Most of the production occurs in developing countries (98%),
while developed countries are the major importers (80%). Citrus and bananas
are traded worldwide, followed by mango, pineapple, papaya and avocado.
Table 1.3. Potential nutrient claims that can be made for fresh tropical fruits using
standards from Codex Alimentarius (2001).
Nutrient claim
Fruit Energy Fat Vitamin A Folate Vitamin C
Avocado Source Source
Banana Low Source
Carambola Low Low High
Durian Low High
Guava Low Low High
Lime Low Low High
Litchi Low High High
Longan Low Source High
Mango Low High Source High
Papaya Low Low Source Source High
Passion fruit Low High
Pineapple Low High
Rambutan Low High

8 Chapter 1
Litchi, durian, rambutan, guava and passion fruit are produced and traded in
smaller volumes, with their market shares expanding rapidly in recent years.
The projections made by the FAO assume normal weather patterns and
the continuation of past trends in area planted, yield, income growth and
population for mango, pineapple, papaya and avocado (Table 1.2). World
production is expected to reach 62 million tonnes by 2010, an increase of
15.4 million tonnes over the 1998–2000 period, with developing countries
continuing to account for 98% of the global production. This is a compounded
growth rate of 2.6% per year. The Asia and Pacifi c region accounts for 56%
of production, followed by Latin America and the Caribbean (32%) and Africa
(11%). The production increase has come from additional planted acreage
intended for export. The growth has occurred mainly in Latin America and
the Caribbean region, with their more accessible trade route to the major
importing regions, the United States and Europe.
Demand for fresh tropical fruits has increased and imports are at about
4.3 million tonnes for mango, pineapple, papaya and avocado, with 87%
going to developed country markets. Europe is the world’s largest import
market, followed by the United States, accounting for 70% of import demand.
In Europe, France is a major importer, and the Netherlands is the major trans-
shipment point.
TROPICAL FRUIT AND CONSUMERS
In most markets, consumers are demanding higher quality. This quality is
no longer judged solely by size and appearance; aroma, fl avour and nutrient
value are now increasing in importance. This can be seen in the larger
range of commodities on the retail shelves, the number of varieties of each
commodity now o ered, and reduction in seasonality of supply in developing
country markets. The traditional term, quality, implies excellence or suitability
for use and means di erent things to di erent groups. Suitability for use
includes freedom from microbial and chemical contaminants. Understanding
of consumer behaviour is related to how it will be accepted in the marketplace
(Sabbe et al., 2009).
Consumer satisfaction is related to their view as to what constitutes
quality, and this varies widely in di erent markets and is decided by familiarity,
economic status and marketing. For many minor tropical fruits, familiarity
in many temperate markets is a major limitation to expanding the market for
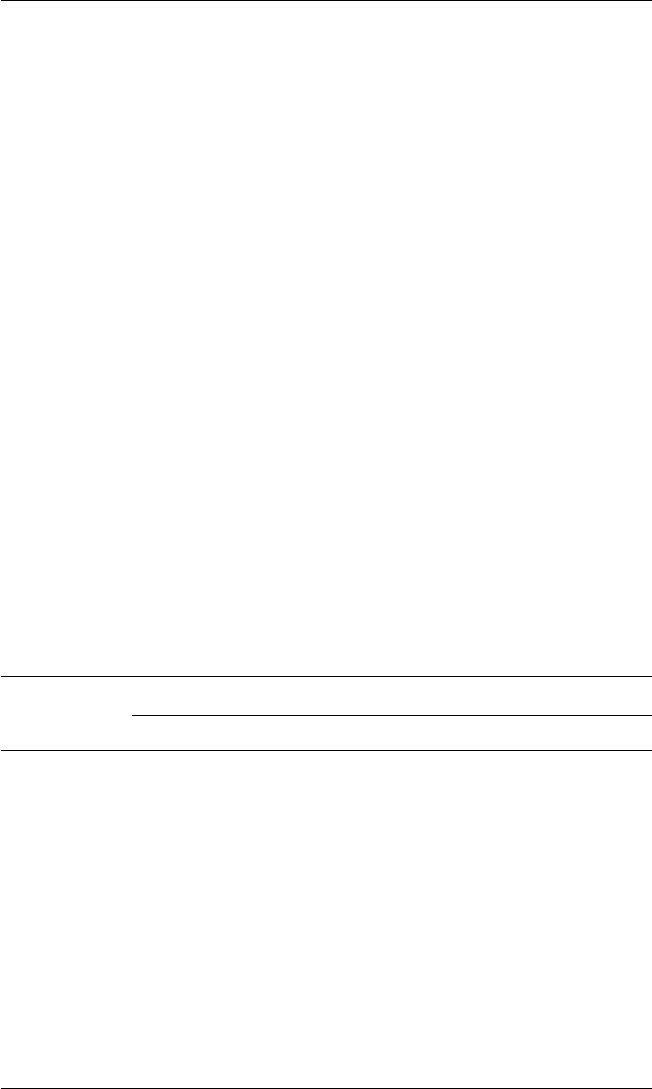
tropical fruits, coupled to a consumer willingness to try new fruits (Fig. 1.2).
INTERNATIONAL FORUMS
Numerous national and international groups are dedicated to specifi c
tropical fruits or groups of closely related fruits. The International Society
Introduction 9
of Horticultural Science (ISHS) has established a Commission of Tropical
and Subtropical Horticulture, with working groups in specifi c tropical and
subtropical fruits. The various working groups meet at regular intervals,
and meeting times and places are posted on the ISHS web site (http://www.
ishs.org/calendar/). The calendar posted at this site is the most extensive
that deals with horticulture conferences. The International Tropical Fruit
Network (TFNet) (http://www.itfnet.org/) is an excellent source of tropical
fruit knowledge. TFNet is an independent global network that serves as a
depository of tropical fruit production, postharvest, processing, marketing
and consumption information. For Latin America, an InterAmerican Society
of Tropical Horticulture (formerly Tropical Region of the American Society
of Horticultural Science) was active until 2006. Annual meetings were
held in di erent Latin American countries. Their web site is at (http://www.
ashs.org/isth/index.html) (accessed 19 January 2010), and this site lists the
many volumes published from 1951 to 2004, which are available in some
libraries and were abstracted in Horticulture Abstracts until 1998, and are now
available by subscription through CAB Direct (http://www.cabdirect.org/).
TROPICAL HORTICULTURE
Tropical agriculture, including fruit production, has a number of limitations.
In the next chapter we will consider the constraints associated with
Fig. 1.2. European consumers’ knowledge of different fresh tropical fruits (redrawn
from Sabbe et al., 2009).

10 Chapter 1
temperature, rainfall amount and distribution, evapotranspiration and soil
moisture. These climate factors have had, and continue to have, a signifi cant
impact on abiotic and biotic factors that a ect fruit production, which will be
discussed in the individual fruit chapters.
Frequently tropical soils are highly leached and acid, with aluminium
toxicity occurring. Nitrogen levels are frequently low, due to high rainfall. The
continual high temperature in the tropics means that organic matter turnover
is high, compounded by low nitrogen availability and poor soil structure.
Leached soils are high in iron and low in phosphorus, and show micronutrient
defi ciencies (Zn, Mn, S).
Pests and diseases are more prolifi c in the tropics, with year-round
development in the absence of a cold winter (no frost, snow or ice) to reduce
inoculum and pest levels. This biotic stress carries over to the postharvest stage
and contributes to high postharvest losses. Integrated pest management (IPM)
is now being widely applied in the tropics, where it can be successful. Ongoing
research will lead to wider application by reducing pest populations below
levels that cause economic injury.
FURTHER READING
Centeno, G. (2005) El mercado de las frutas tropicales exóticas en la Unión Europea.
CIMS, Alajuela, Costa Rica.
Chandler, W.H. (1958) Evergreen Orchards. Lea and Febiger. Philadelphia, Pennsyl vania.
Coronel, R.E. (1983) Promising Fruits of the Philippines. College, Laguna, Philippines,
College of Agriculture, University of the Philippines at Los Banos.
FAO (2003) Tropical fruits – their nutrient values, biodiversity and contribution to
health and nutrition. Intergovernmental group on bananas and tropical fruits,
third session, ftp.fao.org/unfao/bodies/ccp/ba-tf/04/j0715e.pdf (accessed 12
November 2009).
Gepts, P. (2008) Tropical environments, biodiversity, and the origin of crops. In: Moore, P.
and Ming, R. (eds) Genomics of Tropical Crop Plants. Springer, New York, pp. 1–20.
Meyer, N., Mittermeier, R.A., Mittermeier, C.G., da Fonseca, G.A.B. and Kent, J. (2000)
Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
Norman, M.J.T., Pearson, C.J. and Searle, P.G.E. (1995) The Ecology of Tropical Food Crops.
Cambridge University Press, Cambridge, UK.
Popenoe, W. (1974) Manual of Tropical and Subtropical Fruits. Hafner Press, New York.
Facsimile of the original 1920 edition.
Sabbe, S., Verbeke, W. and Van Damme, P. (2009) Familiarity and purchasing intention
of Belgian consumers for fresh and processed products. British Food Journal 110,
805–818.
Sarris, A. (2003) Medium-term prospects for agricultural commodities – projections to
the year 2010. Food and Agriculture Organization of the United Nations, Rome
http://www.fao.org/docrep/006/y5143e/y5143e00.htm#Contents (accessed 24
October 2009).

© CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1 11
(R.E. Paull and O. Duarte)
2
THE TROPICS, ITS SOILS AND
HORTICULTURE
INTRODUCTION
Climate is defi ned as the general temperature and atmospheric conditions of an
area over an extended period of time. Atmospheric conditions include rainfall,
humidity, sunshine, wind and other factors. Climates are subject to modifi cation
by various factors, such as latitude, elevation and whether or not the land
mass is continental, coastal or oceanic, direction of wind and ocean currents,
proximity to large bodies of water and mountain ranges, and cloudiness.
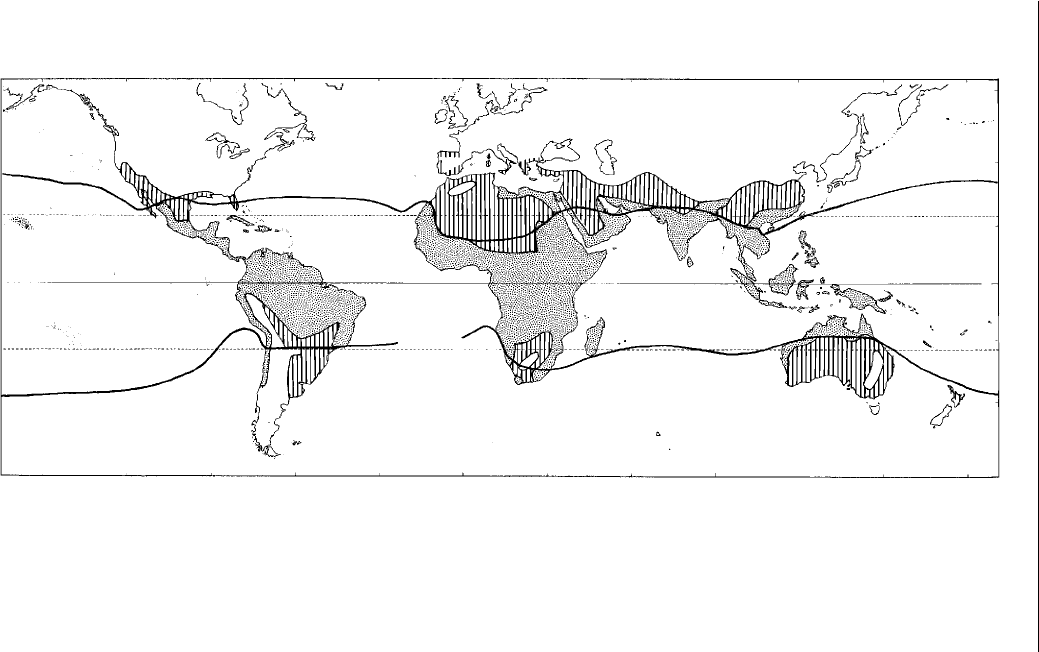
The tropical region is a belt around the earth between the Tropic of
Cancer at 23° 30′ latitude north of the equator and the Tropic of Capricorn
23° 30′ latitude south of the equator (Fig. 2.1). The term ‘tropics’ has its
origins in astronomy and comes from the Greek meaning ‘a turning’. In
astronomy, it defi nes the farthest southern- and northernmost latitudes where
the sun shines overhead. The Tropics of Cancer and Capricorn are rather rigid
boundaries and do not take into consideration the presence of areas that do
not meet the various climatic characteristics generally established to describe
the tropics. Some climatologists have extended the region to 30° N and S of
the equator, based upon surface temperatures and precipitation, or use the
18°C isotherm of the coolest month (Fig. 2.1). This increases the land mass
in the tropics substantially, from ~40% to ~50%, especially on the continents
of Africa, China, South America and India, and would include approximately
two-thirds of Australia’s land mass.
CHARACTERISTICS OF THE TROPICS
The tropical zone is generally described as possessing the following character-
istics:
1. An equable warm temperature throughout the year, having no cold season
at lower elevations. The average annual temperature of the true tropics is
12 Chapter 2
Tropic of Cancer
Equator
Tropic of Capricorn
150 120 90 60 30 0 30 60 90 120 150 180
40
20
0
20
40
60
Fig. 2.1. Distribution of tropical and subtropical regions of the world and the position of the 18°C sea-level isotherm for the
coolest month as the boundary of the tropics. The white area indicates where frost can occur; the vertical hatching indicates the
subtropical areas, while the mottled areas are regarded as tropical.

The Tropics, its Soils and Horticulture 13
generally greater than 25°C, with no month having an average less than
18°C. Others have described the tropics as areas with a mean temperature of
not lower than 21°C and where the mean annual range of temperature equals
the daily range of temperature. The latter boundary is very much infl uenced
by continentality. Another boundary is the isotherm where the mean sea-level
temperature in the coldest months is not below 18°C; though it can include
certain errors, these are relatively small on a world scale and reliable data are
available for its computation (Fig. 2.1). In the tropics, diurnal temperature
variation is greater than the seasonal change.
2. Rainfall is usually abundant, seldom less than the semi-arid 750 mm to as
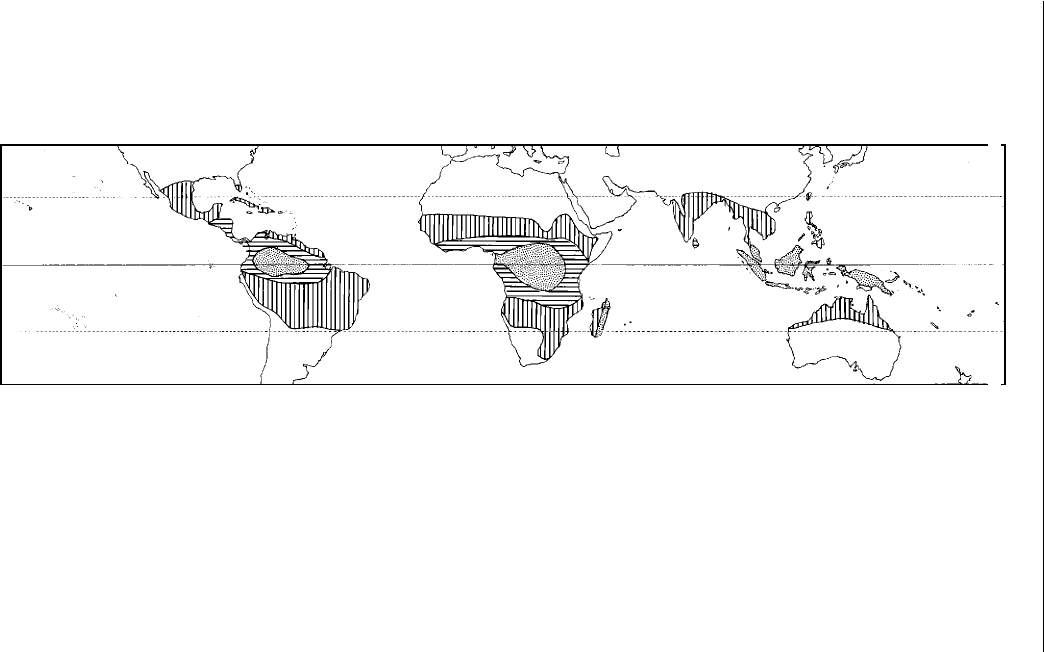
high as 4300 mm, indicating considerable variation (Fig. 2.2). The heaviest
rainfall occurs near the equator. Seasonality in rainfall increases with distance
from the equator. Where rainfall is marginal for agriculture, its variability takes
on greater signifi cance.
3. Photoperiod varies little throughout the year; at the equator day length is
about 12 h (Table 2.1).
4. The position of the sun is more directly overhead, giving a year-round
growing season (Fig. 2.3).
5. Rainfall, temperature, and solar radiation lead to higher potential
evapotranspiration.
These characteristics describe the true tropics, on and near the equator, with
latitudinal changes toward the poles producing a variety of subclimates.
Even near the equator, mountain ranges and other geographical factors
can produce various subclimates. Since temperature, solar radiation and
photoperiod are fairly constant in the tropics, the variety of subclimates and
vegetation are frequently dependent upon rainfall.
A continuous succession of climates starts with a long season of well-
distributed precipitation and a short dry season close to the wet tropics. As
you move away from the equator and the latitude increases, there is a gradual
change to a short season of relatively low rainfall with a long dry season.
Some seasonal variation in mean daily temperature becomes apparent, with
cool temperatures increasing with increasing distance from the equator.
Table 2.1. Day length extremes in hours and minutes at various latitudes in the
tropics and subtropics.
Latitude º 0 10203040
Longest day 12:07 12:35 13:13 13:56 14:51
Shortest day 12:07 11:25 10:47 10:04 9:09
14 Chapter 2
Tropic of Cancer
Tropic of Capricorn
Equator
0
20
20
40
40
Fig. 2.2. Seasonal rainfall distribution in the tropics (after Bluthgen, 1966). The mottled areas are those that receive rain throughout
the year. The horizontal hatching indicates the wet and dry tropics with seasonal rainfall. The vertical hatching indicates the dry
tropics and monsoon areas with long dry periods.
The Tropics, its Soils and Horticulture 15
MAJOR TROPICAL CLIMATE TYPES
Many geographers and climatologists have classifi ed climates into zones by
temperatures (tropical, temperate and frigid zones), by vegetation or crop
requirements, precipitation, altitude, soils, human responses, or by combining
these factors. A well-recognized classifi cation system is the Köppen system,
named after the Austrian botanist and geographer, Wladimir Köppen. This
classifi cation is based on temperature, rainfall, seasonal characteristics and
the region’s natural vegetation and was developed from 1870 to his death in
1940. The newer systems have most often been built upon the 1918 scheme
of the world climactic regions (Table 2.2). Another well-known modifi cation
is that of C.W. Thornthwaite (1948), who based his classifi cation on
distribution of e ective precipitation (P/E (precipitation/evaporation) ratio),
temperature e ciency and evapotranspiration. This led to nine moisture and
nine temperature regions. Numerous other classifi cations have been published
based upon similar criteria (Oliver and Hidore, 1984; Schultz, 2005).
The classifi cation systems of Köppen, Thornthwaite and others are
focused on the major factors (temperature, precipitation and evaporation)
that limit vegetation growth and hence horticultural production. The 18°C
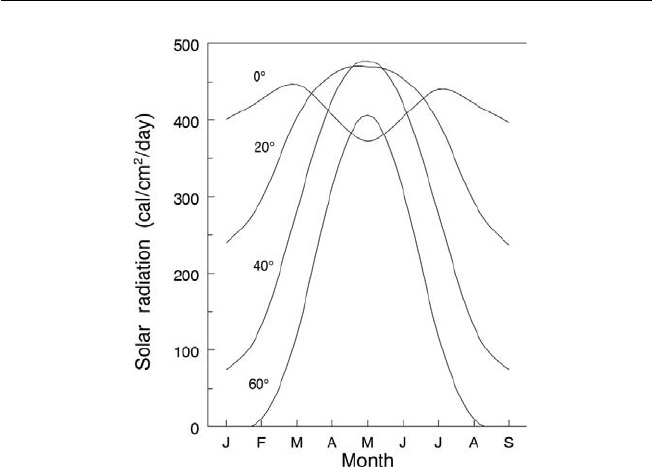
Fig. 2.3. Solar radiation received at the earth surface at different latitudes and an
atmospheric coeffi cient of 0.6 (Gates, 1966). Solar radiation changes signifi cantly
between the summer and winter periods as you move away from the equator. The
data in the fi gure are for the northern hemisphere.

16 Chapter 2
Table 2.2. Köppen’s major climates based upon four major temperature regimes, derived from monthly mean temperatures, monthly
precipitation and mean annual temperature: one tropical, two mid-latitudes and one polar. The second division is based on moisture
availability. The system does not completely agree with natural vegetation and climate, and frequently the boundaries are rigidly
interpreted.
Principal climatic types Temperature (°C) Rainfall
Tropical Rainy Coolest month > 18 >600 mm driest month, range 2000 to 4000 mm per year
Wet and dry
Seasonal rainfall
<600 mm driest month, range 500 to 1500 mm per year
Dry Steppe Evaporation > precipitation, 100 to 500 mm per year
Desert
Mid-latitudes Mediterranean Coolest month < 18 3 × more precipitation in winter than summer
Mild winter Wet and dry > −3 10 × more precipitation in summer than winter
Rainy ≥ 30 mm per month
Mid-latitudes Wet and dry Coolest month < −3
Cold winter Rainy Warmest month > 10
Polar Tundra Average warmest month 0 to 10
Ice cap Average warmest month < 0

The Tropics, its Soils and Horticulture 17
boundary of Köppen recognizes the dramatic slowing of tropical plant growth
and development at lower temperature. At temperatures less than 10–12°C
and above freezing, most plants that evolved in the tropics stop growing and
are injured, depending upon length of exposure and species; this response
is called chilling injury. Precipitation and evaporation signifi cantly impact
natural vegetation and subsistence agriculture, although irrigation does allow
horticultural production to proceed.
Several major tropical climatic types have been described:
1. Wet tropics: The wet equatorial or humid tropics, equatorial zone or tropical
rainforest occurs within 5–10° of the equator. It is characterized by constantly
high rainfall, humidity and heat. Rainfall is well distributed and may range
from 2000 to 5000 mm or more annually. Solar radiation is reduced due to
cloudiness. Vegetation is luxuriant on very weathered soils. Undisturbed, the
soil supports natural vegetation very well, but under cultivation the soils lose
their organic matter and porosity rapidly. Much of the land in the wet tropics
is undeveloped and in some areas unpopulated. Attempts have been made to
improve productivity, but past attempts by private industries and government
agencies have often not succeeded.
This wet tropics climatic type is common in parts of Africa within the 10°
N and S latitudes and includes the Congo Basin, Gulf of Guinea in West Africa,
parts of Kenya and Tanzania in East Africa; in South America the Amazon
Basin of Brazil and countries bordering the basin, such as French Guiana,
Guyana, Surinam and Venezuela; and in South-east Asia most of Malaysia,
Indonesia, Papua New Guinea, the Philippines and some Pacifi c islands.
The wet tropics are not limited to the above areas and are also widespread in
countries bordering the equator.
2. Wet and dry tropics: This is also known as the monsoon rainforest, with
marked seasonal rainfall between 5
and 15° N and S of the equator and as far
north as 25° in parts of tropical Asia (Fig. 2.2). Walter (1973) extended this
zone from about 10°
N and S to about 30° N and S latitudes. Maximum rainfall
occurs in the summer when the sun is directly overhead, with the dry season
in the cooler months. A tropical fruit horticulturist will probably spend most of
their time in this climatic zone.
This wet and dry tropical climate is found in a wide region of Africa, Asia,
the Americas, Australia and the Pacifi c tropics. Many tropical fruit species are
well adapted to wet–dry climatic conditions. For fruit production, some form
of irrigation is necessary, especially in areas where the wet season is relatively
short. A dry period in winter may substitute for cool temperatures in crops
requiring some stress prior to fl owering. However, irrigation is desirable once
fl owering begins and during fruit development.
3. Dry tropics: Also called the tropical savannah, occurs to the north and
south of the monsoon climate zone along the Tropics of Cancer and Capricorn,
between 15°
and 20°
N and S latitudes. It is characterized by hot, dry desert

18 Chapter 2
conditions where crop production cannot succeed without irrigation. This
climate is found in North Africa, bordering the Tropic of Cancer, north-east
India, Australia and parts of the Pacifi c coast of South America. The coast of
Peru has arid plains and foothills and fertile river valleys. For example, average
annual rainfall for the town of Piura on the north-western coast is 51 mm,
and average minimum and maximum temperatures are 17.6°C and 30.5°C,
respectively. Thousands of hectares of land remained unproductive due to lack
of irrigation until large dams were built to produce hydroelectric power as well
as to provide irrigation.
ALTITUDINAL CLIMATES
Climates change with altitude at the same latitude, and the change is related to
temperature. The environment can be divided into three temperature zones at
the equator: the hot zone, from sea level to 1000 m; the temperate zone, from
1000 to 2000 m; and the cool zone, above 2000 m, with frost occurring at
approximately 5000 m at the equator. Temperatures in these zones di er with
changes in latitude, prevailing wind patterns, precipitation and other factors.
In the Selva region of eastern Peru with large rivers, principally the
Amazon, two subclimates are recognized in terms of altitude and rainfall.
The low jungle or humid tropics extends from sea level to 800 m and has
rainfall throughout the year. The high jungle or central Selva, located between
800 and 1500 m above sea level, has a wet and dry climate, with about 6 to
7 months of wet season and the remainder with little or no rain. In the low
jungle, subsistence agriculture prevails, with crops such as co ee, cacao,
banana, mango, papaya, pineapple, soursop, citrus, black pepper, cassava and
poma rosa (Syzygium malaccensis). Under large-scale commercial cultivation,
some of the traditional crops, such as mango, papaya, pineapple and citrus,
would do better at latitudes somewhat removed from the equatorial region,
having better soils and reduced disease problems associated with high rainfall.
The high jungle (wet and dry tropics) in the central Selva area of Peru is better
developed, with farms of large commercial size. Citrus (Valencias, mandarins
and limes) and co ee have done well.
SUBTROPICS
Strict separation of tropical, subtropical and temperate climates is not
practical because of the many factors that infl uence climate. Even within
the geographical limits of the tropics, there are areas that are subtropical
and temperate, or even frigid, because of altitude, topography, ocean and air
currents. The subtropics occur between the two tropics and about 40° latitude,
with summers being hotter and winters cooler than in the tropics. Humidity is

The Tropics, its Soils and Horticulture 19
generally lower in this region, and the di erence in day length becomes greater
with higher latitude (Table 2.1). The limit for the subtropics is the isotherm of
10°C average temperature for the coldest month. This 10°C isotherm excludes
the large land masses whose climates are temperate and includes almost half
of China, three-quarters of Japan, all of South Korea, the southern half of the
United States, all of the southern half of Australia, the North Island of New
Zealand and more than half of Argentina and Chile between 23° 30′ and 40°
latitude.
Horticulturists who have spent their professional careers in regions where
the temperatures during the coldest month at sea level are rarely lower than
15–18°C fi nd it di cult to accept the tropical classifi cation of regions with
winter temperatures down to 4–7°C and frost potential.
CLIMACTERIC FACTORS
Day length
The day length at the equator is about 12 h. At low latitudes in the tropics, the
increase in di erence between the longest and shortest days is about 7 min per
degree (Table 2.1), increasing to 28 min per degree at latitudes between 50
and 60°. The di erence in photoperiod (Table 2.1) is associated with the earth
being inclined on its axis by approximately 23° 30′; hence the solar equator
moves about 47° as the earth moves around the sun. The extremes are the
Tropic of Cancer (23°
30′ N) to the Tropic of Capricorn (23°
30′ S); within
this belt the sun’s rays are perpendicular at some time during the year. At
the spring and autumn equinoxes, the lengths of the day and night are equal
everywhere over the earth.
Fruit trees such as mango, papaya, bananas, the annonas, avocado,
acerola and guava show no response to photoperiod and are capable
of fl owering at any season of the year. In equatorial Colombia, with
approximately 12-h day length, it is common to fi nd mango trees fl owering
during February–March and again in August. In the subtropics, fl owering is
more precise, occurring in the spring as a function of lower temperature and
moisture availability limiting growth. For guava, seedlings grown under 15-h
day length from germination to 140 days and fi eld transplanted produced fruit
within 376 days from sowing; the control seedlings under 10-h day length did
not fl ower. This result with guava refl ects the longer period available each day
for photosynthesis and not photoperiodism. Guava can also be forced to fl ower
by pruning.
Pineapple can fl ower naturally at any time of the year, depending upon the
size of the planting material, though it is a quantitative, but not an obligatory,
short-day plant. Interruption of the dark period by illumination suppresses

20 Chapter 2
fl owering. Though pineapple does not require low temperatures or diurnal
variations in temperature to fl ower, there is an interaction with temperature.
Temperatures lower than 17°C during short days can induce considerable
fl owering, even in small plants. No fl owers are produced on yellow passion
fruit (Passifl ora edulis f. fl avicarpa) vines under artifi cially induced short days
(8 h). Long days promote passion fruit vine growth and fl owering, while short
days promote vine growth only. This observation, however, could be due to the
amount of solar radiation received and not photoperiod.
Radiation
When compared to higher latitudes, the tropical latitudes have small seasonal
variation in solar radiation along with high intensity. The longer summer
day length at the higher latitudes means that these latitudes exceed the
daily amounts of solar radiation received in the tropics. The highest annual
energy input on the earth’s surface, 12 MJ/m
2
/day, occurs in the more cloud-
free subtropical dry belt of 20–30° (Fig. 2.3). In the tropics, solar radiation
received is reduced by clouds and water vapour in the air, through refl ection
and absorption, to a minimum of ~7 MJ/m
2
/day at the equator. Over a large
portion of the tropics the average is 9 MJ/m
2
/day ± 20%.
In the tropics, atmospheric radiation transmissivity varies from 0.4 to
0.7, due largely to clouds and seasonal variation. The maximum recorded
irradiance under cloudless skies at noon in the tropics is 1.1 kW/m
2
, with a
daily total received of from 7 to 12 MJ/m
2
. About 50% of this energy is in the
0.4–0.7 μm waveband, which is known as photosynthetically active radiation
(PAR). In full sunlight, C
3
plants, including all fruit crops discussed in this book
except pineapple, which is a CAM plant, are ‘light’ saturated. This saturation is
due to ambient CO
2
availability limiting the rate of photosynthesis.
High shade (3–5 MJ/m
2
/day) does not infl uence litchi fl owering, though
it does increase early fruit drop. Flowering in passion fruit is reduced once
irradiance falls below full sun. Irradiance is normally not a factor limiting
plant growth in the tropics except during heavy mist and cloud, and in shade
from other vegetation and mountains.
Temperature
Near the earth’s surface, temperature is controlled by incoming and outgoing
radiation. Surface temperatures are modifi ed spatially and temporally
throughout the year by local factors more than radiation. The main factors are
continentality, the presence of large inland waterbodies, elevation modifi ed by
prevailing topography, and cloudiness. Highest diurnal temperatures occur
in dry continental areas, at higher elevations and in cloud-free areas. The

The Tropics, its Soils and Horticulture 21
rate of decrease in temperature with elevation (adiabatic lapse rate) varies
with cloudiness, hence season, and between night and day. The normal rate is
about 5°C per 1000 m under cloudy conditions and can range from 3.1 to 9°C
per 1000 m.
The human sensitivity to temperature is modifi ed by the rate of
evaporation. Evaporation from human skin is primarily infl uenced by
humidity, wind speed and response to sunshine. A human can endure high
temperature if the humidity is low; hence the discomfort felt in the humid
tropics is associated with high temperatures and humidity (>25°C and >80%
R.H.). These conditions are also favorable to growth of microorganisms and
insects. The problem of controlling plant diseases and insect pests in the
tropics is compounded by the absence of a cold winter and aridity to limit their
adult development.
Tropical fruit crops such as mango, guava, acerola, papaya, pineapple,
some annonas and others originated in the warm, lowland tropics. Others
such as the litchi, Mexican and Guatemalan races of avocado, cherimoya
and purple passion fruit are subtropical fruits by virtue of their origin in
the subtropics or at higher elevations in the tropics. Man, in his attempt to
commercialize tropical fruit crops, has extended production into subtropical
regions beyond the Tropics of Cancer and Capricorn and has generated
considerable knowledge on the range of temperature adaptability of these
crops. Data on threshold temperatures and durations of exposure for various
stages of plant development of tropical fruit trees are often unavailable;
minimum temperatures in the coolest month that support survival,
commercial or best production have been approximated (Table 2.3). The
minimum temperature criterion takes into account di erences in elevation and
latitude. In regions subjected to marginal winter temperatures, site selection
becomes a paramount consideration, such as southern-facing slopes in the
northern hemisphere and northern-facing slopes in the southern hemisphere.
Young plant growth may also be inhibited when soil temperature exceeds
35°C, a common condition in the tropics. Leaf temperature can exceed air
temperatures by 20°C; for example, pineapple fruit and leaf temperature have
been recorded in excess of 50°C in the fi eld. Hence, maximum temperatures
in the orchard microclimate need to be considered in site selection. Three
crops (mango, litchi and avocado) illustrate this temperature adaptability and
the varietal and race adaptation at various stages of development. Mature
mango trees have been found to withstand temperatures as low as −16°C for
a few hours with some injury to leaves. Flowers and small fruits may be killed
when temperatures less than 4.5°C occur for a few hours during the night.
Mango varietal di erences for cold resistance have not been observed. Mango
responds to cool night temperatures (10–14°C) with profuse fl owering. Ideally,
day temperatures during this period should be warm (21–27°C). When winter
night temperatures are mild (16–18°C), fl owering is more erratic.
22 Chapter 2
Vegetative and fl owering behaviour of litchi is very similar to mango,
except that it is adapted to even lower minimum temperatures than mango.
The total duration of relatively low temperatures seems to be the determining
factor rather than the frequency or time of occurrence during a critical
period. There are considerable cultivar di erences in the temperature exposure
necessary to induce fl owers, e.g. ‘Brewster’ litchi fl owers better in seasons with
200 or more h of temperature below 7.2°C. Other cultivars fl ower profusely
when night temperatures of 14–15°C occur.
The three races of avocado originated under di erent ecological
conditions. The West Indian race is best adapted to humid, warm climates,
with the optimum around 25–28°C. It is susceptible to frost, and the mini-
mum temperature tolerated by the foliage is recorded at 1.5°C. Mature trees
of the Mexican race have shown tolerance to as low as −4 to −5°C without
damage to foliage, although fl owers are damaged. The Guatemalan race is
adapted to a cool tropical climate but is less tolerant of low temperatures than
cultivars of the Mexican race. The leaves of the Guatemalan cultivars have
Table 2.3. Guidelines for survival, commercial and best production of tropical fruit
based upon mean minimum temperature in the coldest month (after Watson and
Moncur, 1985).
Crop Botanical name
Mean minimum temperature (°C)
for coolest month
Survival Commercial Best
Acerola
Malpighia glabra
10–12 >12 >14
Banana
Musa spp.
6–8 >8 >16
Breadfruit
Artocarpus altilis
14–16 >16 >16
Carambola
Averrhoa carambola
6–8 >8 >14
Chempedak
Artocarpus polyphema
12–14 >14 >16
Cherimoya
Annona cherimoya
4–6? 5–12? 6–10?
Durian
Durio zibethinus
14–16 >18 >18
Duku and langsat
Lansium domesticum
12–14 >14 >18
Guava
Psidium guajava
4–8 >8 >14
Jackfruit
Artocarpus heterophyllus
6–10 >10 >14
Longan
Dimocarpus longan
4–8 8–18 8–14
Litchi
Litchi chinensis
4–8 >14 >16
Mango
Mangifera indica
6–8 >8 >12
Mangosteen
Garcinia mangostana
10–14 >14 >16
Papaya
Carica papaya
6–8 >8 >14
Pineapple
Ananas comosus
6–8 >8 >10
Rambutan
Nephelium lappaceum
8–12 >12 >14
Sapodilla
Manilkara zapota
6–10 >10 >14
Soursop
Annona muricata
6–10 >10 >16

The Tropics, its Soils and Horticulture 23
shown tolerance of light frost down to −2°C, with fl ower damage by even light
frost. The Mexican–Guatemalan hybrids, such as ‘Fuerte’ have shown wider
tolerance of cold than the Guatemalan cultivars. Temperatures of 12–13°C
during fl owering can prevent growth of pollen tubes and embryos, leading to
production of unfertilized, underdeveloped fruits.
Rainfall
Temperature determines agricultural activity in the temperate regions of the
mid-latitudes, while rainfall is the crucial factor in the tropics. The seasonal
and diurnal distribution, intensity, duration and frequency of rainy days
vary widely in the tropics, both in space and in time (Fig. 2.2). The maximum
rainfall occurs near the equator, with no dry season. Surrounding this
equatorial zone in Africa and South America are areas with two rainy seasons
alternating with two dry seasons; rarely are the seasons of the same duration
or intensity. Further from the equator is a region of minimum rainfall at
20–30° latitude, associated with the subtropical high pressure area, with one
rainy season, frequently due to the monsoons. Topography can signifi cantly
modify the generalized rainfall pattern; examples include the western coast
of India and Borneo, and the coastal areas of Sierra Leone, where monsoonal
winds are forced to rise because of mountain ranges. Trade winds can bring
considerable rainfall and are subjected to the forced rise by topographical
features. Other factors infl uencing rainfall include changing and slowing
down of wind speed as it approaches the equator and continentality, such as
in south-west and central Asia. The above factors lead to complicated rainfall
patterns, with broad generalization possible while remembering that there is
considerable variation (Fig. 2.2).
Tropical fruit production is normally limited by available soil moisture. The
stage of growth or development at which water stress occurs greatly a ects the
fi nal yield and quality. Many factors infl uence the amount of rainfall available
to plants, including evaporation and transpiration rates, surface runo , soil
water-holding capacity and percolation through the soil profi le beyond the
rooting area. Using the average tropical daily net radiation of 9 MJ/m
2
and
the latent heat of vaporization for water (2.45 MJ/kg), an evaporation rate of
ca 4 mm per day can be calculated. This evaporation rate is similar to that in
a temperate summer. Higher rates of 10–15 mm per day occur for irrigated
crops in the semi-arid tropics due to the advection of hot, dry air. Rainfall and
irrigation need to make up this evaporative loss, and a mean monthly rainfall
of 120 mm (~4 mm per day) would be required.
Excessive rainfall causes major problems with fl owering, pests, diseases and
fruit quality. Many trees, such as mango and litchi, require a dry (or cold) period
to stop vegetative growth and induce fl owering. Mango and litchi originated
in areas with a monsoon climate that provides distinct wet and dry seasons.

24 Chapter 2
Dry conditions, preferably accompanied by cool temperatures during the pre-
fl owering period, promote fl owering, while cool, wet conditions reduce fl owering
in both crops. When mango fl ower buds begin to emerge, some soil moisture is
needed, preferably from irrigation rather than from rainfall. Light rain during
mango fl owering leads to severe anthracnose (Colletotrichum spp.), which can
destroy most of the infl orescences. Too much rain during litchi anthesis also
reduces fl ower opening and/or the insect activities needed for pollination.
Total rainfall is frequently less important than its distribution throughout
the year. In Loma Bonita and Acayucan, Mexico, two large pineapple-
producing areas, mean rainfall over a 5-year period was 1600 mm and 1500
mm, respectively. These approximate the upper limits of the optimal range for
pineapple; however, periods of serious drought are encountered, as 89 and
82%, respectively, of the rainfall occurs from June to November. In a tropical
rainforest, 85% of a 1 mm shower may be intercepted by plants, but only 12%
of a 20 mm rainfall, indicating that fall intensity, duration and frequency are
signifi cant factors. The interception by plants is signifi cantly infl uenced by
species; pineapple with its upright leaves funnels water to the centre. Plant
density similarly a ects rainfall interception.
Orchards located on fl at lowland areas can experience fl ooding during the
rainy season, particularly if drainage is poor. This is important for avocado,
papaya, litchi and pineapple, for which waterlogging causes severe root-rot
problems. Mango is slightly fl ood-tolerant, as indicated by reductions in leaf
gas exchange, vegetative growth and variations in tree mortality. Mangosteen,
by contrast, grows well under conditions of fl ooding and high water table.
A high water table may prevent mangosteen trees from experiencing the
moisture stress needed to induce fl owering.
Water defi cit, and therefore irrigation demand, is determined by
evaluating rainfall, evaporation and soil water storage. The two most common
approaches are the water balance (rainfall, evapotranspiration, water
storage, change in the root range, surface runo ) and actual soil moisture
measurement. The crop needs, along with the soil type, determine frequency
of irrigation; this determination should be made on at least a weekly basis for
fruit crops. The use of drip (trickle) and micro-sprinkler irrigation enables a
grower to match the needs of the crop to irrigation needs at di erent stages of
growth and development, avoiding the need to rely on rainfall. These irrigation
methods allow precise placement of the water, reduce surface evaporation and
seepage, and increase water-use e ciency. Other irrigation methods used are
basin, furrow, overhead sprinklers and cannons.
Strong winds, frost and hail
In the equatorial zone, strong winds are associated with localized thunder-
storms (diameter <25 km) having greater intensity than those in the middle
and upper latitudes and lasting from 1 to 2 h. Most occur outside the 0–10°

The Tropics, its Soils and Horticulture 25
latitude zone (Fig. 2.4) and are convectional in origin and associated with
intense solar heating. Other strong winds can be due to sea or land breezes and
unstable warm and humid air masses. Hail occurs rarely in the tropics except
in the highlands, though it is known to damage tea in Kenya and tobacco in
Zimbabwe.
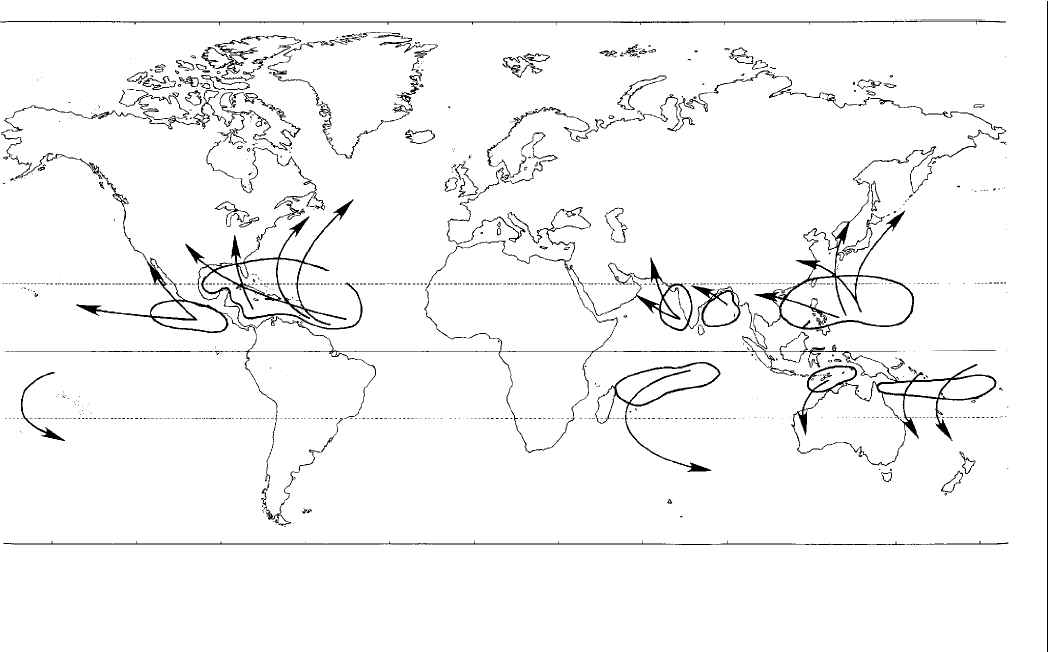
Tropical cyclones (hurricanes, typhoons) are an almost circular storm
system, ranging in diameter from 160 to 650 km and winds from 120 to
200 km/h, originating over water in the warm summer season. Most develop
within latitudes 20° N and S of the equatorial belt and may turn north-east
in the northern hemisphere or south-west in the southern hemisphere to 30–
35° latitude (Fig. 2.4). These systems bring violent winds and heavy rains. The
Philippines are very prone to such systems. Crop damage, especially to trees,
can be very severe due to the high winds.
Monsoon depression is a less intense weather phenomenon. It brings 80%
or more of the precipitation to the Indian subcontinent, with considerable
year-to-year variation. It occurs when there is at least a 120° directional shift
in prevailing wind direction between January and July. It is a characteristic
of the wet and dry tropics and spreads from Asia to Africa. The intensity of
rainfall can lead to considerable fl ooding.
In the subtropics, frost is a major limiting factor to tropical horticultural
production. In isolated tropical high mountainous areas, frosts can occur
frequently. Frost in the subtropics is associated with incursions of cold air
masses (advection frost), while on tropical mountains it is mainly due to rapid
cooling on clear nights (radiation frost).
Trees have inherent di erences in the degree of resistance to winds, but all
fruit species benefi t from wind protection. Mango, acerola and guava exhibit
greater resistance than other tropical tree crops such as banana, to the extent
that they survive strong gusts of wind without losing limbs or being blown
down. Leaves, fl owers and fruits are often completely blown away. Pineapple,
by virtue of being low growing, gives the appearance of resistance, but wind
can damage leaves, and during the fruiting period the peduncles may be
broken, resulting in loss of fruit. However, windbreaks are almost never found
on pineapple plantations. The annona species, avocado and litchi are known
for their brittle branches and show limb splitting even under moderate gusts
of 65–80 km/h. Limb braces are occasionally used to prevent splitting of
large limbs, forming ‘Y’ crotches on litchi trees. Guava trees propagated by
grafting have tap roots that provide substantial anchorage. However, guava
trees propagated by rooting cuttings or by air-layering are subject to uprooting
during the fi rst 3 years, probably due to faster top growth than root growth.
Papaya plants and passion fruit vines are vulnerable to even moderate winds.
Papaya trees are easily blown over, especially if the soil is softened by heavy
rains. Passion fruit vines on trellises can be tangled and broken, or the entire
trellis may be blown down. Developing carambola fruit is easily bruised and
marked by rubbing on branches and adjacent fruits due to wind, reducing fruit
26 Chapter 2
Tropic of Cancer
Tropic of Capricorn
150 120 90 60 30 0 30 60 90 120 150 180
180
60
40
20
0
20
40
Equator
Fig. 2.4. Areas of tropical storm development, which can signifi cantly infl uence fruit production in the tropics (after Gray, 1968).
Tropical storms can uproot trees, break limbs and snap the top of the trees. For plants with large leaves, such as papaya and
banana, the leaves can be shredded, with signifi cant loss in photosynthetic capacity.

The Tropics, its Soils and Horticulture 27
appearance and grade. Mangosteen trees may also require protection from full
sun during early establishment as well as from wind. Windbreaks for these
crops are standard practice (see Chapter 3).
Soils
Using the US classifi cation system, soils are separated into ten groups, based
on parent material, soil age and the climatic and vegetative regime during
formation. Tropical soils are diverse, having formed from di erent parent
material and climatic conditions (Fig. 2.5). These soils have formed in areas
where the soil temperature at 50 cm di ers less than 5°C between the warm
and cool season. The parent rock materials are as di erent in the temperate
zone as in tropics; erosion and deposition are similar; soil formation can have
been from recent volcanic or alluvial fl ood plains to 1 million years old. The
di erences in temperate regions lie in soil-forming factors such as glaciation
and movement of loess, which have not occurred in the tropics (Sanchez and
Buol, 1975).
The majority of tropical soils are found in US soil orders oxisols, aridisols,
alfi sols, ultisols and vertisols and are spread widely throughout the tropics (Fig.
2.5). The soil orders are separated on the presence or absence of diagnostic
horizons or features that indicate the degree and kind of the dominant soil-
forming process. It is very di cult to make generalizations about tropical soils
other than they have less silt than temperate soils and that surface erosion
and deposition have been more signifi cant. There are greater volcanic deposits
in the tropics and a larger proportion of younger soils than in the temperate
region. Only a small proportion (2–15%) of the tropics has so-called lateritic
soils (oxisols and ultisols), defi ned as soils that have high sesquioxide content
and harden on exposure (Table 2.4). The red colour of tropical soils does not
mean that they have low organic matter. For example, the average organic
carbon content in the top 1 m of a black North American mollisol is 1.11%,
while red, highly weathered tropical oxisols may have 1.05%, the reddish
temperate ultisols 0.4% and tropical ultisols 0.66%.
The more intensively farmed, fertile soils of the tropics cover about 18% of
the area and are alfi sols, vertisols, mollisols, and some entisols and inceptisols
(Table 2.4). These soils generally developed from alluvium and sediment and
are high in calcium, magnesium and potassium (Table 2.5). This gives them
a high base status with no acidity problem. Phosphorus defi ciency can be
readily corrected. Larger groups of tropical soils (oxisols, ultisols and others)
are of low base status, highly leached and cover 51% of the tropics (Table
2.4). Phosphorus defi ciency can be signifi cant as it is fi xed by the iron and
aluminium oxide in these soils, which also often have aluminium toxicity
problems, with sulfur and micronutrient defi ciencies (Zn, B, Mo, S). However,
they have good physical properties. The high-base soils (aridisols) in tropical
28 Chapter 2
Tropic of Cancer
S
S
S
D
D
V
I
I
I
I
I
I
I
I
I
D
D
D
D
D
D
D
D
D
D
X
D
V
V
V
D
I
I
I
I
I
I
I
I
I
I
I
I
I
I
H
H
D
H
Tropic of Capricorn
Equator
150 120 90 60 30 0 30 60 90 120 150 180
40
20
0
20
40
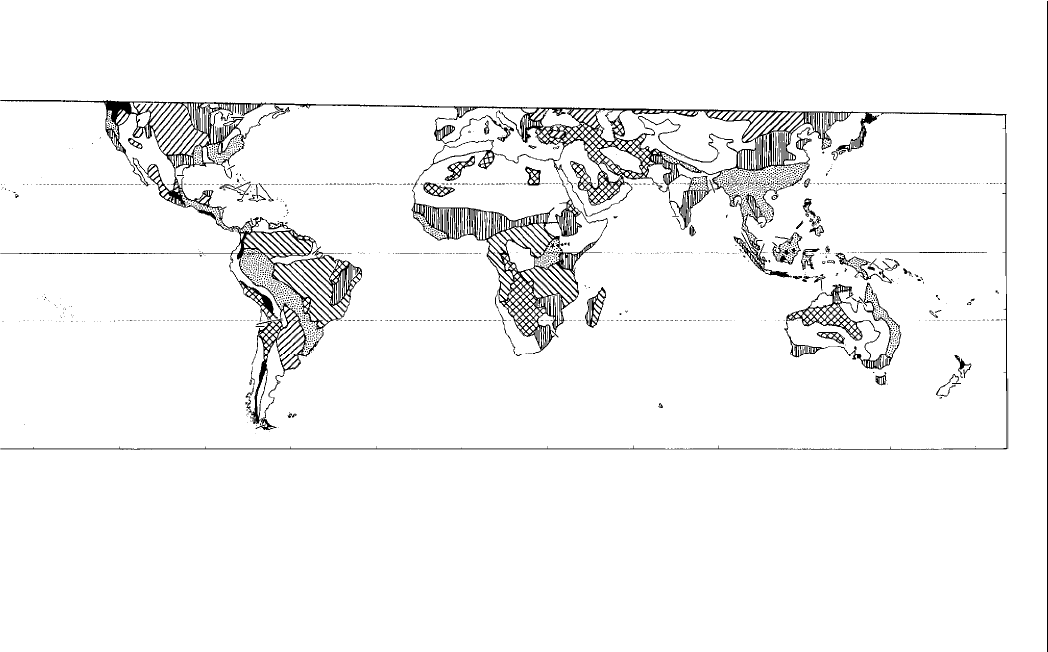
Fig. 2.5. Soil-type distribution in the tropics (after Kalpage, 1976; Sanchez, 1976). Tropical soils are diverse, having formed from
different parent material and under different climatic conditions. Key: vertical lines – aridisols; cross-hatched – entisols; rising
diagonal lines – mollisols; declining diagonal lines – oxisols; and speckled – ultisols. D – aridisol; I – inceptisol; S – spodosol; V –
vertisols and X – mountain areas.

The Tropics, its Soils and Horticulture 29
deserts (14%) can be very productive with irrigation. Nitrogen defi ciency
and sometimes salinity can be problems. The 17% covered by dry sands
and shallow soils are greatly limited in agricultural productivity. The most
important aspect is the development of management strategies for the di erent
tropical soils, taking into consideration their unique properties. Management
practices developed in temperate regions may not be directly transferrable.
Soil physical characteristics are of primary concern for tropical fruit
production, with soil nutrients being secondary because they can normally be
readily corrected. Soil texture and structure, soil water storage and drainage
are crucial. Defi ciencies in these characteristics are major constraints to
production because they are di cult and expensive to correct. Under natural
conditions, most soils considered for fruit crops in the tropics have a good
topsoil structure. This includes the highly weathered oxisols and ultisols (Table
2.5). Loss of organic matter may lead to loss of structure and crusting of these
soils after heavy rains. Some soils, however, do not favour root development
due to a dense subsoil layer that needs to be broken during soil preparation to
avoid shallow root systems. Heavy machinery may also cause the formation
of a compact subsurface layer in medium-textured oxisols with low iron and
in fi ne-textured oxisols. Low calcium and phosphorus and high aluminium
contents in the subsoils can also restrict root growth.
Tropical fruit crops have shown a wide range of soil adaptability and
have been observed to grow and produce well in a wide variety of soil types,
Table 2.4. Distribution of major soils in the tropics (after Sanchez, 1976; Sanchez
and Salinas, 1981).
Humid
tropics
a
(%)
Seasonal
(%)
Dry and
arid
(%)
Tropics
(%)
Highly weathered, leached, red or yellow
soils – (oxisols, ultisols, alfi sols)
36 61 2 51
Dry sands and shallow soils – (entisol–
psamments and lithic group)
10 33 58 17
Light-coloured, base-rich soils – (aridisols
and aridic groups)
0158514
Alluvial soils – (inceptisol–aquepts,
entisol–fl uvents)
40 52 8 8
Dark-coloured, base-rich soils – (vertisols,
mollisols)
860326
Moderately weathered and leached soils –
(inceptisol–andepts and tropepts)
759344
% of tropics 24 49 27 100
a
Classifi ed on number of rainy months: humid tropics, 9.5–12 months; seasonal, 4.5–9.5
months; dry and arid, 0–4.5 months.

30 Chapter 2
provided other factors are favourable. In some cases considerable management
skill is required to maintain the crops in good growth and production. Soil pH
can be corrected by liming during fi eld preparation, with most trees preferring
pH 5.5–6.5. Papaya is one of the few fruit crops that is adapted to a wide
range of soil pH, growing and producing well in soil pH ranging from 5.0
to well into the alkaline range. Defi ciencies in phosphorus associated with
adsorption and excess aluminium need to be addressed in the oxisols, ultisols
and some inceptisols. Soil organic matter can be maintained by use of manure,
ground cover crops and mulches to preserve soil moisture and structure, and
improve the rhizosphere. Magnesium, zinc and boron defi ciencies may also be
encountered in some tropical soils, but these are relatively easy to correct in a
management programme. Saline and alkaline soils, along with deep peat soils,
should be avoided for fruit production because of their di cult nature. Acid
Table 2.5. Soil classifi cation and main characteristics of tropical soils and orders.
Order Characteristic Agricultural productivity
Entisol Recent alluvium, also on barren
sands and near deserts. Lacks
signifi cant profi le
Some very productive alluvial soils
Vertisol Swelling clay. Cracks in dry
weather
Productive, diffi cult to till
Inceptisol Horizons forming, little accumu-
lation of Al or Fe oxides. Tropic
with long, wet season,
acid
Very prone to erosion, good if well
drained
Aridisol Deserts, little organic matter,
CaCO
3
, CaSO
4
and soluble
salts
Need irrigation to be agriculturally
productive
Mollisols Granular or crumbly soils. High
in silt and organic matter
Most productive worldwide
Alfi sol Humid regions soil with grey or
brown upper horizon and silicate
clay below
Good soils, prone to erosion, easily
compacted
Ultisol Highly weathered, acidic in moist,
warm tropical areas, moderate
fertility
Very productive, easily compacted,
prone to erosion, needs fertilizer
Oxisol Highly weathered, in hot, heavy
rainfall areas, deep clay of
hydrous oxides of Al and Fe, can
be very acidic, leached
Needs nutrients to be productive,
susceptible to erosion if left bare
Andisols Surface deposits of volcanic ash,
free draining, low bulk density
Erosion serious problem, high P
fi xation, high Al and Fe fi xation,
excellent structure, productive

The Tropics, its Soils and Horticulture 31
sulfate soils require specialized management strategies, such as raised beds, to
be productive.
A prime soil requirement for all crops is good drainage to prevent
waterlogging, which leads to root diseases. Drainage is crucial for crops that
are susceptible to Phytophthora root rot, such as avocado, papaya, passion
fruit and pineapple. Mango and avocado have been observed to show branch
dieback in parts of fi elds in western Mexico with a water table around 50–60
cm below the soil surface.
CLIMATE CHANGE
Climate change is regarded as the most important threat facing the environ-
ment that will have a signifi cant e ect on society and agriculture. For the
tropics, climate change is predicted to expand the tropical region, with
signifi cant changes in temperature (2–5°C increase) and rainfall (more
variable). The predictions are made for large regions and the expectation is
that there will be less rainfall near the equator and more rainfall, with greater
variability, further away from the equator, in the current drier tropical zones
(Easterling and Apps, 2005; Cerri et al., 2007; Ingram et al., 2008).
The predicted direct environmental changes include freshwater avail-
ability, carbon and nitrogen cycling, land cover and soils. All these changes
will directly impact biodiversity and agricultural, including horticultural,
productivity. It is expected that the induced biodiversity changes will lead to
new pest and disease pressures and greater weed problems (Sutherst et al.,
2007). For example, aphids are expected to become an increasing pest because
of their low development temperature threshold, short generation time and
high dispersal ability. Pathogens may have higher growth rates and more
generations per growth season. Weeds, with their more rapid growth and
development, may be more favoured in comparison to crops.
SUMMARY
Whereas temperature is the major limitation to plant growth in temperate
areas, in the tropics rainfall plays that role. There is considerable variation
in tropical climates due to altitude, continentality and the presence of large
bodies of water. Disease and insect problems are more severe in high-rainfall
areas. Year-round plant growth is generally only limited in the tropics by
moisture availability. Good soils are available in the tropics, and the high
value of horticultural crops means that they can command the use of more
favoured areas.

32 Chapter 2
FURTHER READING
Ayoade, J.O. (1983) Introduction to Climatology for the Tropics. John Wiley and Sons, New
York.
Gepts, P. (2008) Tropical environments, biodiversity, and the origin of crops. In: Moore, P.
and Ming, R. (eds) Genomics of Tropical Crop Plants. Springer, New York, pp. 1–20.
Norman, M.J.T., Pearson, C.J. and Searle, P.G.E. (1995) The Ecology of Tropical Food Crops.
Cambridge University Press, Cambridge, UK.
Sanchez, P.A. and Buol, S.W. (1975) Soils of the tropics and the world food crisis. Science
188, 598–603.
Schultz, J. (2005) The Ecozones of the World: the Ecological Divisions of the Geosphere, 2nd
edition. Springer, Berlin.

© CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1 33
(R.E. Paull and O. Duarte)
3
CULTIVATION
Growing tropical fruit trees is a long-term investment, with harvesting not
beginning for several years. The exceptions are the herbaceous fruit crops,
banana, plantain and papaya, which will start producing marketable yields
in less than 2 years. Perennial and herbaceous fruit crops involve large initial
investment costs for site selection, land preparation, varietal selection, planting
establishment, fertilization, irrigation, pest control, pruning and your own
management costs. For perennial trees these costs will continue for several
years before the trees come into full production. After the trees start to produce
marketable volumes of fruit, the largest cost is for labour, and this is during
harvesting. It is therefore very important to provide the best management
possible so that the trees start fruiting as soon as possible and are in the best
possible condition to maximize yield and fruit quality at the lowest costs.
MARKET ANALYSIS
Market analysis is the crucial fi rst step in establishing a new orchard. This
analysis will determine where the demand is for the products you plan to
grow, how big the demand is and who are your competitors, and can the
market be expanded. This analysis will enable you to decide if you will be able
to sell the product at prices that will leave you with a profi t. The most e cient
production methods and the best varieties will not make up for an inability to
sell your product. This detailed market analysis has to be done before venturing
into this activity. Additional components in this analysis include knowledge
regarding the best time of year for fruits to arrive in the markets for higher
prices and what the market desires with regard to varieties and fruit quality.
New or di erent varieties that have di erent quality characteristics (colour,
texture, aroma, taste) can increase demand for a fruit and expand the current
export markets and should be considered. Export markets are very competitive
and the quality, including fruit safety requirements, is much more rigorous
than for most local markets. Export windows when a fruit may command a

34 Chapter 3
premium price are becoming narrower. For many products, export markets
have variety-specifi c quality requirements, with only one or a few varieties
being in demand. Export markets will also require you to meet certifi cation
requirements with respect to how a product is grown and managed, working
conditions and environmental stewardship. An example of a certifi cation
system is the European GlobalGap. All of these factors have to be carefully
analysed before starting this business.
LAND SELECTION
The ideal land for a fruit orchard should normally be fairly fl at with a deep soil
of medium texture and good water percolation to enhance root growth. Soils
with poor drainage are prone to be waterlogged during heavy rains, which can
limit root growth and increase root diseases. Other soil chemical and physical
properties must also be evaluated before starting an orchard. Investing a large
amount of money in land that is not suited for the species to be grown will
normally mean reduced yields and increased production costs. Many times
the savings in purchasing cheap land with poor soil quality will be o set by the
negative economic returns. For an annual crop, if a mistake is made in land
selection and the wrong soil type is chosen, this will become obvious at the end
of the fi rst cycle, while for perennial fruit tree crops with a long juvenile stage
it may take a number of years before the mistake is apparent. This mistake will
be felt every year, resulting in large investment of money spent, not returned,
or both. Careful land selection is therefore essential, and if you are not familiar
with the area, the opinion of an expert will help. A number of key questions
need to be answered (Fig. 3.1) before making a selection. A simple decision
tree (Fig. 3.1) ensures that you evaluate the more important factors that can
potentially impact production and economic return.
In many instances there is a tendency by farmers to plant fruit crops in
the worst areas of their property. Producing fruit is normally a long-term
investment and has large upfront costs of land acquisition, preparation,
planting, establishment and management, and the expectation is for high
returns. The best land available should be used for fruit crops in order to
obtain the best possible economic return. In some cases marginal lands that
are normally only suited for forestry or grazing can be used for fruit trees,
but there is no reason to expect optimal profi ts. Irrigation systems using
drip and micro-sprinkler with fertigation can provide water and nutrients to
the plants, reducing the di erences in orchard productivity between good
and marginal soil types. The soil, in some situations, has become a place for
the roots to establish themselves and the plants get water and fertilizers from
the irrigation system, but these production technologies cannot correct the
problems associated with poor soils that are saline, have poor structure and
water percolation, frequently are waterlogged or have toxic levels of nutrients.

Cultivation 35
Land too steep Yes
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Ye s
Make sure no negative effects for
new crop, otherwise discard
m Discard
No
No
No
No
n
Soil extremely heavy m Discard
n
Irrigation impossible, long dry season m Discard
n
Irrigation possible, long dry season m Irrigate if economic
n
Soil too shallow m Discard or prepare mounds
n
Very poor soil structure m Discard
n
Poor soil drainage m Drainage or subsoil if economic
n
Very high water table m Drainage if economic
n
Too many salts in soil m Leach if economic
n
Soil too acid m Raise pH with lime
n
Soil very alkaline m Lower pH with sulfur
n
Saline sodic soil m Normally discard
n
Hardpan or plow pan m Subsoil adequately
n
Soil poor in macronutrients m Add macronutrient before planting
n
Soil poor in organic matter m Add organic matter, green manure
n
Previous crop was the same m
n
Previous crop heavily root diseased m Avoid susceptible species, otherwise
discard
n
Persistent weeds m Choose clean land if possible
n
Heavy nematode infestation m
Add organic matter heavily, use non-
host crop or discard
n
USE LAND
No
No
No
No
No
No
No
No
No
No
No
No
No
No
No
Fig. 3.1. The major steps in deciding whether land is suitable for a tropical fruit
orchard are set out as a decision tree. Going through the questions allows a
decision to be made as to suitability.
Hardpan or plough pan

36 Chapter 3
Soil evaluation
The soil on the selected site should be appropriate for the species you plan to
grow to improve the chances of success. Soil preparation and management,
including liming to change the pH, provide some fl exibility with a soil that has
a good structure. If the soil is improperly treated it will not provide the best
conditions for plant growth and production will not be the ideal.
The results from the physical analysis of the soil will determine its
texture and structure, water retention and drainage. These characteristics, if
unfavourable, can be very serious production constraints and are di cult and
expensive to modify. Most soils considered for tropical fruit crops have good
topsoil and subsoil structures. Since fruit trees often have deep root systems,
poor subsoil conditions, such as a perched water table, will not allow root
growth below it. Other subsoil problems, in addition to a high water table, are
associated with the existence of rocks or hard soil layers. The presence of a
heavy soil layer above a sandy layer or the opposite can lead to di culties for
water drainage. An easy way to determine this is to dig three or four trenches
to 2–3 m scattered over the planned site to evaluate the soil profi le.
Soil chemical analysis will tell you the soil’s nutrient content, pH, salinity
and alkalinity levels, organic matter content and the presence of elements that
could be toxic. Soils with undesirably low pH (<4.5) are frequent in the humid
tropics. This acidity is corrected by incorporating lime during land preparation
and often during the life cycle of the crop. Liming should raise the pH to
adequate levels of around 5.5–6.5. Some crops can do well in a wide range of
pH values while others need a narrower range. A frequent problem in tropical
soils is low calcium and phosphorus levels and high aluminium availability,
in addition to magnesium, zinc and boron defi ciencies. These defi ciencies
can be corrected by an appropriate fertilization programme and liming. In
arid tropical zones, soils with high pH and low phosphorus availability, with
defi ciencies in iron, copper, manganese and zinc due to the nutrient being in
a non-soluble, unavailable form, are common and most can be corrected by
fertilization. Soils with high salinity or alkalinity should be avoided since they
can be very costly to correct. The soil organic matter content should also be
adequate and be maintained or increased if necessary by the incorporation of
manure or green manure crops. Soil organic matter improves moisture and
nutrient retention, maintains soil structure and enhances biological life in the
root zone (see Chapter 2 for more information).
Irrigation water
The potential irrigation water’s quality needs to be determined. The salinity
and sodium level in relation to calcium and magnesium (sodium adsorption
ratio or SAR) are evaluated, as these parameters can cause problems with

Cultivation 37
perennial crops in arid zones (Table 3.1). The data will enable a decision to be
made on usage, as species and varieties vary in their salt tolerance, with some
crops being very susceptible to salt injury (Table 3.2).
Previous land usage
A replant problem can exist with some fruit crops. Peach trees are probably
one of the best examples in a deciduous fruit crop; replanting into old peach
orchard areas often leads to failure due to chemical substances left by the
initial crop that can a ect the growth of the replanted crop (allelopathy) (Rice,
1974). This is sometimes referred to as soil fatigue. Another cause of replant
problems can be due to fungi, bacteria or nematodes that were hosted by the
former crop. Plants of the Solanaceae family are very prone to being infected by
root fungal diseases, bacteria and nematodes, and therefore it is recommended
not to plant a species of this family right after another. Citrus are also known
to show this problem. In many cases, the only solution to this soil fatigue is
crop rotation, which can last as much as 10 years using di erent species,
including grasses, before planting the same crop again. The alternative is to
plant a crop that will not be a ected by the above factors.
Planning
The collection of all the above data before land preparation allows you to plan
and cost out the project before starting. Potential problems can be avoided by
in-depth planning and making decisions on species and variety, rootstock,
soil management practices, irrigation system and fertilization practices. The
analysis could also lead to the possibility of rejecting a particular fi eld or farm
because the soil or water is not suitable for the fruit species to be grown. If the
grower lacks the proper background knowledge about these matters, a soil
specialist should be consulted to assist in making the right decisions to avoid
unnecessary expenses and future problems. Most tropical fruit crops have a
Table 3.1. Irrigation water quality for tropical fruit trees.
Water quality
Conductivity
(dS/m)
TDS (total dissolved
salts) ppm
No detrimental effects 0.25 175
Sensitive crops could be affected 0.25–0.75 175–525
May have adverse effects 0.75–2.0 525–1400
Salt-tolerant plants only 2.0–3.0 1400–2100
Unsuitable >3.0 >2100

38 Chapter 3
fairly broad range of adaptability and are grown in a wide variety of soil types,
provided other factors are favourable. Ideally the best soils and irrigation
waters should be used and, as with other crops, proper management is always
important to obtain an e cient and sustainable production.
It is normally assumed that fruit trees need a soil profi le that is at least
0.5 m, and ideally more than 1 m, deep, so that roots can grow down to that
depth. This is not always the case since many tropical fruit trees are shallow-
rooted. In instances where the soils are much shallower, either because parent
rock is near the surface or because the water table is close to the surface, good
profi table production can be obtained. Fields with a shallow water table can be
drained or fragile parent rock can be cracked by ripping. Soils with a ‘caliche’,
a natural hard, crusted layer of calcium carbonate found in arid zones, can be
ripped or a hole bored through to allow root penetration.
Table 3.2. Salt tolerance of different fruiting trees. Adapted from Campbell and
Goldweber (1985) and Maxwell and Maxwell (1985).
Good Moderate Fair Poor
Carissa (Natal
plum)
Coconut
Tamarind
Date palm
Akee
Bignay
Black sapote
Canistel (egg fruit)
Cattley (strawberry)
Guava
Fig
Governor’s plum
Imbu (Spondias
tuberosa)
Indian jujube
Jackfruit
Jelly palm
Key lime
Loquat
Mayan breadnut
Monstera
Pineapple guava
Pomegranate
Prickly pear
Pummelo
Purple mombin
Rose apple
Sapodilla
Spanish lime
Tangerine/mandarin
Wax jambu
Atemoya
Barbados cherry
(Acerola)
Cherimoya
Cherry of Rio Grande
Citrus (rootstock
dependent)
Custard apple
Grumichama
Illama
Imbe
Kei apple
Kumquat
Mamey sapote
Miracle fruit
Mulberry
Otaheite gooseberry
Persimmon
Pineapple
Pitomba
Soursop
Sugar apple
Surinam cherry
Wampi
White sapote
Ambarella
Avocado
Banana
Caimito (star apple)
Carambola
Cashew
Jaboticaba
Longan
Lychee
Macadamia
Mango
Papaya
Passion fruit
Strawberry tree

Cultivation 39
Weeds in some areas can be very di cult to control. Areas heavily infested
with aggressive and persistent weeds like nut sedge (Cyperus spp.), Johnson
grass (Sorghum halepensis), Bermuda grass (Cynodon dactylon), star grass
(Cynodon nlemfuensis) and others are best avoided. They can be eliminated but
it will take time, e ort and money.
Mountainous land
Fruit orchards are commonly located on fairly fl at land, as steep and
mountainous land is more expensive to prepare and carry out fi eld
management. The advantage of mountainous land is that it is often cheaper
and usually has not been used for any fruit crops previously and is more
likely to be free of many parasites, weeds and chemical residues common
in other areas. Mountain soils need to have good soil texture and depth to
allow for good root establishment and growth. Erosion can be a problem on
steep hillsides, so care has to be taken not to eliminate the low vegetation
and to plant on the contour to minimize erosion. Terraces built along the
contours often have deep soils, though these terraces are expensive to build
and maintain. Individual terraces for each tree are also used. Drainage will
normally not be a problem under these conditions.
Irrigation of steep hillsides can be di cult, especially in the arid tropics
or tropics with a long dry season. An expensive irrigation infrastructure is
needed, which includes tanks to store water at the highest elevation and
pumps to fi ll the tanks. In older orchards, contour irrigation systems are seen,
with newer orchards using drip irrigation and micro-sprinklers. The fl exibility
of drip and sprinkler systems is that they are not limited to fl at land and can be
used very easily on steep hillsides and on stony or poorer soils. Once the water
is pumped to the tank at the highest part of the orchard, gravity can be used
with pressure compensation drippers. Pressure-compensated drippers emit
equal amounts of liquid along the system, with a range of supply pressure.
LAND PREPARATION
In virgin areas, land preparation for fruit crops can be as simple as just
removing the trees and other vegetation before digging a planting hole. This
approach was used by the companies for banana plantations in Central
America. In mountainous areas, land preparation is normally by hand or with
small motorized equipment; larger machinery normally cannot be used. Large
trees and bushes are removed with the low vegetation left, and individual
holes are dug for each tree. This could include making a small terrace with the
planting hole at the centre.
40 Chapter 3
A hard layer or hardpan can be present in previously cultivated land (Fig.
3.2). These layers can develop in soils that have been cultivated with heavy
equipment when the soil was too wet or where the plough passed many times
at the same depth (plough pan). This hard layer (Fig. 3.2b) is eliminated by
subsoiling or ripping of the soil deeper than the hard layer (Fig. 3.2c). This
disruption of the hard layer (Fig. 3.2a and b) will allow better root penetration
into the subsoil. Subsoiling involves using a heavy tractor or bulldozer pulling
subsoiling hooks or rippers that are set to rip to 50–200 cm (Fig. 3.2c), with
a second pass done at a 45° angle to the fi rst pass (Fig. 3.2d). Some other
subsoiling techniques use a torpedo-shaped device that is pulled with a cable
and leaves an underground passage that improves water and air circulation.
This operation should normally be done when the soil is dry in order to be
e ective.
Subsoiling and ploughing can be used to incorporate any fertilizer or
amendment to the soil. Typical amendments include manure to increase
organic matter, sulfur to lower the pH or lime to raise it, or any element that will
be in big demand can be incorporated at this time, when it is easier than after
planting. Phosphorus and other elements can also be incorporated deeper into
the soil and will be more e ective, since mobility when applied on the surface
is very poor. If there are di erent soil layers, then subsoiling will also help to
mix and homogenize the layers, with large pieces of roots from the previous
crop normally being removed. When a very hard underground ‘caliche’ layer
is present, subsoiling is not normally practised but a hole is made through the
layer using an auger or pointed steel rods to allow roots to penetrate further
Extensive root growth
down the soil profile
Restricted root
growth
(a) (b) (c)
(d)
Hardpan
Little root growth
Foot with wings
Pan
Loosened soil
Pan broken up
Leg
Fig. 3.2. Root development of tree crops mainly occurs in the top metre of the
soil profi le (a); a hardpan can restrict root growth (b) and the hardpan needs to be
broken by a subsoiler (c). The tine of the subsoiler rips through the hardpan and
allows roots to penetrate. Subsoiling should be done with two passes of the fi eld at
about 45° to each other.

Cultivation 41
down the soil profi le and for water to drain to the subsoil. Dynamite has been
used to crack superfi cial rocky soils, especially if the rocks are of coralline
origin or calcareous; this was done in large areas in Cuba. A small hole the size
of the dynamite piece is bored into the rock and then exploded to crack the rock
and leave a passageway for water and roots to penetrate.
Stones can be another problem during land preparation, though not as
much as with annual crops. Ideally all rocks and stones should be removed,
especially from the soil surface, in order to ensure that the machinery will
have no problems in moving around and will not be damaged. In very stony
areas, removal is uneconomic and only large and medium stones on the
surface are taken from the fi eld so vehicles can move freely in the orchard and
mowing can be carried out. Stone removal should preferably be done after
subsoiling and ploughing, so that most large stones will be on the surface.
Often the stones are used to build boundary fences. Alternatively, the stones
are used to fi ll depressions, holes or ditches in the fi eld or throughout the farm.
The next step is ploughing the whole fi eld, sometimes in two directions, with
large stones again being removed. Ploughing is followed by discing to break
up the larger clods and make the fi eld ready for planting. Land levelling is
sometimes performed, especially if furrow irrigation is to be used; with drip or
micro-sprinklers this will not be necessary. If surface fl ood or furrow irrigation
is used, the furrows need to be cut at this time, along with soil fumigation, if
required, and planting holes made.
DRAINAGE
Drainage is needed for most orchards, especially when the land is fl at and the
soil has a low water percolation rate. Failure to provide drainage can mean
waterlogging and standing water remains in the fi eld for more than a few
hours. Standing water in fl ooded fi elds means that the soil atmosphere will
soon become depleted of oxygen and become anaerobic. Anaerobic conditions
cause stress to most tropical fruit tree roots, limit nutrient uptake and make
the roots less able to resist soil-borne pathogens such as root rots due to
Phytophthora and other fungal pathogens.
Drainage is accomplished with ditches and sometimes with interconnected
subsurface drainage pipes. This can be an expensive investment. On gently
sloped land, plant rows are normally oriented to follow the maximum incline
so that the excess water fl ows downhill. This orientation is possible if the slope
does not exceed 10%. When the slope is greater than 10% transverse ditches
will be needed to slow down the water speed, and these transverse ditches are
connected to the main drainage ditches. In mountainous areas, tree rows
normally follow the contour, so as not to create an erosion problem when it
rains. Swales along the contour or ‘French drains’, which consist of a system
of ditches fi lled sequentially with stones and gravel and sometimes covered

42 Chapter 3
with soil, are used. These drains slow the rate of water fl ow and allow the
water to infi ltrate into the soil. In most cases, swales are left open and they
carry the water to a main ditch or creek. In heavy rains, these shallow swales
are overloaded and can lead to signifi cant erosion.
Raised beds are recommended when root rot-susceptible crops such as
papaya, passion fruit, avocado and pineapple are grown on level land (Fig.
3.3). The deep furrows created between the beds drain away the excess water
(Fig. 3.3a). These furrows can be used for irrigation during the dry months.
In fi elds with depressions or under wet or in heavy soils, planting the tree on
a mound reduces the problems due to waterlogging (Fig. 3.3b). The mound
height depends upon the magnitude of the fl ooding problem, with around
25 cm being common. Taller mounds increase the orchard layout costs and
consideration should be given to fi eld-levelling grading, leaving only a gentle
slope. Levelling or land shaping is practised in some pineapple-growing areas.
The shaping is done with heavy machinery by making a successive series of
wide and not very steep mounds so that the excess rainwater runs into their
lower parts, to be drained from the fi elds. This shaping maybe done in addition
to the use of raised beds that are oriented so that their furrows conduct water
to the lower parts of the fi eld.
The banana industry is very sophisticated in its drainage infrastructure
in the wet tropics. Bananas do not tolerate a high water table or long periods
of soil saturation. A successive series of parallel, open drains 1.2–2.5 m
deep every 40–60 m, referred to as tertiary drains, are dug. These drains are
designed to keep the water table at the desired depth and also to collect runo
that is not absorbed by the soil. Shallow drains (quaternary drains) are dug
(a) (b)
Fig. 3.3. Drainage is a major concern for tropical tree fruit production in areas with
high water tables or heavy rains. Trees are grown on raised beds (a) and planted on
mounds (b).

Cultivation 43
with shovels to the tertiary drains after a heavy rain to drain areas where
water accumulates in the fi eld, so that the excess fl ows into the tertiary drains.
Tertiary drains conduct the water to 2.5–4.0-m-deep secondary drains, which
in turn will drain the water into a river or creek or a primary drain that can be
2.5–6.0 m deep.
PLANTATION LAYOUT
A contour or ‘Google’ map of the farmland is ideal to start layout planning.
The map should ideally include the location of buildings, farm boundaries,
roads, fences, irrigation water supply and drainage ditches. Decisions are then
made on the direction and length of the tree rows, the number of plants, the
need for additional interior roads for transporting materials or products to
and from the fi eld, and location of additional structures such as storage and
machinery sheds and packing facilities.
The length of the rows will depend on the type of soil and irrigation; if
irrigation is by furrows or basin, rows that are about 60–80 m long in light
soils and 120–180 m in heavy soils would be a starting point. Rows that are
too short are not convenient for e cient machinery movement. The direction
of the rows should take into account the water movement from both irrigation
and runo .
Using the layout map the easiest way to start in the fi eld is to confi rm that
the direction chosen for the tree rows on the layout map is the best and make
appropriate corrections in the fi eld. Use a long string or rope stretched to follow
the direction chosen and then stake each end. These stakes serve to establish
a 90° angle at each end to start the geometric layout pattern. At each of the
ends use a measuring tape with the 0 m mark at the stake and mark the point
on the rope 3 m from the stake, then mark another point with a stake 4 m in
a perpendicular direction to the rope, adjust the position of this stake so it is 4
m from the end stake and 5 m from the 3 m mark on the rope. The 5 m line is
the hypotenuse of your triangle and a 90°
angle is formed at the stake. At the
other end of the rope repeat the same procedure, so you have two parallel lines.
Measure on both parallel lines an equal distance to confi rm that the distance
between these two points is the same as the distance between the fi rst two
stakes. If the same, then you have parallel lines, if not you will need to recheck
the two triangles so that a square or rectangle is formed. Then all four corners
are staked and new stakes are put in or the spots marked with gypsum or lime
at the distances the trees or rows will be and repeat on the other side. Then a
rope or wire is pulled between the matching stakes of the opposite sides and at
the perpendicular lines the same is done. Where the ropes or wires cross a stake
or a lime mark is left, which will indicate the location to dig a planting hole.
Orchards are usually planted following geometrical arrangements (Avilán
et al., 1989), depending on the land condition, the species, variety, plant

44 Chapter 3
shape, planned pruning and need for machinery. Planting layouts between
trees can be square (Fig. 3.4a), rectangular (Fig. 3.4b), an equilateral triangle
or hexagonal system (Fig. 3.4c) or some other combination, including extra
or temporary plants to increase returns during the initial juvenile years (Fig.
3.4d, e and f). If a rectangle layout is used (Fig. 3.4b), the trees will form this
geometric fi gure; the longer side will correspond with the distance between
tree rows and the shorter side will be the distance between trees in the row. In
the square arrangement (Fig. 3.4a), the distances in both directions will be the
same, and in the equilateral triangle normally a plant will be planted in each
angle, forming triangles or a hexagon that has a plant in its centre (Fig 3.4c),
and this gives 15% more plants per area than the square system while leaving
the same distance between trees.
The rectangular layout is used when bulky machinery has to enter the
fi eld, although in the square layout this can also be done in the initial years
also. Sometimes a square pattern can be used and every few rows a wider
row is included in the layout. The wider row is left to allow movement of
machinery. The location of the wider row will depend upon the length of hoses
or how far the sprayers can reach and the distance you need to move harvest
bins or boxes to facilitate fruit collection. Between-plant distances are shorter
for plantains, bananas and papayas, and in hilly conditions where heavy or
bulky machinery will not be used, normally a square or, better, a triangular
arrangement should be used. The distance between trees will be the same in
the square and equilateral triangle arrangement, with the latter resulting in
more plants per unit area.
To maximize early income, the quincunx (Fig. 3.4d) and other systems
are used. The quincunx consists of a square arrangement with a temporary
or extra plant at the centre of each square. This arrangement almost doubles
the number of plants per unit area and allows higher production during the
initial years. Another intensive initial planting system consists of planting a
temporary plant in the space between the permanent plants in the row (Fig.
3.4e); this will duplicate the initial number of plants. An even more intensive
initial planting system consists of planting three temporary plants for each
permanent plant. This intensive planting has an extra row of temporary plants
between two permanent plant rows and an extra temporary plant in all the
spaces between the plants in the rows, even in the temporary rows (Fig. 3.4f).
As the trees start maturing and yield increases, the extra trees are pruned and
later removed. This system is not recommended for all crops or all growers.
PLANTING DISTANCES
Planting distances will be determined by many factors, the most important
being the species. Tree size and shape can vary from large trees like mango
to small plants like papaya or banana. The variety can play a signifi cant role,
Cultivation 45
a=b
Square
n years
Double density
X = Temporary plants
(a) (b) (c) (d)
(e) (f )
Fourfold density
n years n years
Quincunx
a>b
Rectangular
a = b, h < a or b
Hexagonal
(Equilateral triangle)
aa
a
b
b
b
h
Fig. 3.4. Field layouts can be as a square (a), rectangle (b), hexagonal (c) or quincunx (d). In denser planting for the early
orchard years, a double planting (e) or a fourfold density (f) can be used – the plants indicated by ‘X’ being removed after a
number of years to avoid overcrowding. On steep land the layout may need to be modifi ed with trees planted on the contour.

46 Chapter 3
with di erent growth vigour and habits. Varieties can be compact compared
to the traditional size, or their canopy shape can vary from wide to narrow.
Rootstocks will also play a vital role in tree vigour, varying from very vigorous
to dwarfi ng rootstocks. Sexually propagated plants are normally larger than
their asexually propagated counterparts. Another factor that can modify
distances is how large the tree will be allowed to grow and the pruning and
shaping carried out during initial growth. For example, a tree pruned into
a vase shape will be wider than a tree left with a central leader, while a tree
trained in a palmette system will be wide only in one direction. The severity of
pruning also plays a role.
Soil quality infl uences fi nal tree size, with trees grown in poorer soils
being smaller. Rainfall pattern and its amount in non-irrigated areas can also
reduce tree size. Sometimes the harvest practices, irrigation or the spraying
system can modify certain distances or at least make it necessary to leave some
broader spaces among certain tree rows. The exact distance will be di cult
to establish if the crop has never been grown in that area. An approximate
distance can be established, sometimes with the help of an expert (Table 3.3).
In the middle of the last century, orchards were planted at densities of
less than 250 trees per hectare; today, tree density ranges from 200 to 2000
plus trees per hectare. In the past, tree spaces were based upon the distances
required for the adult trees; this meant a lot of wasted space before the ground
area was covered by the canopies. This practice led to a higher cost of weed
control and spraying and increased harvest cost, as tall trees (>3 m) are more
expensive to collect fruit, requiring ladders and mechanical platforms. This
adult-form concept has now changed, with a more pressing need to recover
your initial investment as soon as possible and reduce your overall management
cost associated with large, tall trees. One system being used is to plant at much
shorter distances, and as the trees become larger, the so-called temporary
or fi ller trees are pruned so as to reduce interference with the growth of the
permanent trees. Finally, the temporary trees are removed completely to avoid
further competition. A second thinning of the remaining trees is sometimes
performed once they have grown larger, in order to leave the fi nal number of
trees per area. This system will require larger early investments in trees and
maintenance, which will need to be balanced against the larger returns and
income during this early orchard development period. In some situations, this
practice may not be worth the extra cost and alternatives such as short-cycle
vegetable crops or even widely spaced banana or papaya may be alternatives.
PLANTING
In a good soil with irrigation and which has been properly prepared, a planting
hole slightly larger than the container holding the root-ball can be used. This is
normally a 30 by 30 cm or a 50 by 50 cm hole made using shovels or augers.

Cultivation 47
A large hole may be used in poor-quality soils or in good-quality soils with no
supplementary irrigation that depend only on seasonal rainfall. A hole of 80
by 80 cm or 100 by 100 cm is fi lled with a good substrate so that during the
initial years the plant will grow vigorously before the roots reach the poor or
dry soil area.
When the hole is dug, keep the topsoil in one mound and the subsoil in a
separated mound since they normally di er in quality, with the topsoil being
better. Before planting, mix the topsoil with organic matter such as matured
moist manure or decomposed co ee pulp or any composted product and fi ll to
the 40–50 cm mark of the 80–100 cm depth, then topsoil is added to about
30 cm from the soil surface and compacted by stepping on the materials in
the hole. The topsoil without manure is placed next to the root-ball since
fresh manure can burn the roots as it starts decomposing if in direct contact.
Manure can burn the roots either by the elevated temperature produced by
decomposition or by the salts that it releases. If the manure is well decomposed
Table 3.3. Some examples of recommended between-tree and row spacings in
tropical orchards for some fruit crops.
Crop With fi llers (m) Trees/ha
Permanent spacing
(m) Trees/ha
Acerola 2.7 × 5.5 673 5.5 × 5.5 332
4.6 × 5.5 395
Atemoya 4.5 × 9.0 247 9.0 × 9.0 124
8.0 × 12.0 104
Avocado 4.5 × 6.0 370 9.0 × 12.0 93
6.0 × 7.5 222 12.0 × 15.0 56
Fuerte 7.5 × 7.5 177 15.0 × 15.0 44
Hass 7.0 × 7.0 204 14.0 × 14.0 51
Cherimoya 5.0 × 6.0 333
5.0 × 7.6 263
Durian 10.0 × 10.0 100
Guava 3.1 × 7.6 424 6.2 × 7.6 212
4.6 × 7.6 286
Litchi 5.0 × 5.0 200 10.0 × 10.0 100
6.0 × 12.0 139 12.0 × 12.0 69
Mango 5.0 × 10.0 200 10.0 × 10.0 100
12.0 × 12.0 138 12.0 × 12.0 69
(+ one tree in
centre)
Papaya 2.4 × 3.1 1344
Passion fruit 3.3 × 4.0 1324
Sapodilla 6.6 × 6.6 400
Soursop 3.7 × 7.6 356 7.4 × 7.6 178
6.0 × 9.0 185

48 Chapter 3
or a composted organic matter is used, then it is not necessary to separate the
material and the hole can be fi lled with the mixture of topsoil and decomposed
manure.
The root-ball can be set in place with a 20-cm-wide ring of topsoil around
it. The remaining part of the hole is then fi lled with topsoil, or ideally with
the mixture of topsoil and composted organic matter, and compacted so the
plant stays fi rmly in position, and the root-ball is in close contact with the
surrounding soil medium. This will allow new roots to grow rapidly into the
surrounding soil without problems. If the subsoil is of poor quality, topsoil
from neighbouring areas can be used and then these areas can be backfi lled
with the subsoil from the hole. It should be remembered that most of the
feeder root activity occurs in the upper 50 cm of the soil. Large holes provide
much better growing conditions than a normal hole. When establishment is
dependent upon seasonal rainfall and irrigation is not available, planting
should occur just prior to the wet season. It may be necessary to water the
plants under these circumstances using buckets during the fi rst 2–3 years to
give the plant a much better start.
PLANTING MATERIAL
Often new growers will purchase planting material that is not of the highest
quality or the best variety. This practice leads to poor tree establishment, poor
yield or poor fruit quality. Having to replant an orchard is expensive and it
should be avoided. Plant quality is determined by several criteria.
Plant identity
Most tropical fruit trees are a combination of a varietal scion and a rootstock.
Alternatively, cuttings, air layers or vegetative organs like suckers and
runners are used. For herbaceous fruit trees such as papaya, seeds are used.
It is necessary to ensure the plants are the variety that you want. In grafted
or budded plants the identity of the scion has to be known, and in many cases
it is also very important to know what rootstock was used. Rootstock choice
is crucial for some fruit species, and it has to be a very specifi c variety or type
in order to allow the tree to grow well under determined soil conditions in the
presence of certain plant pests and diseases and to produce good-size plants.
For some species, choice of rootstock is not so crucial, as it serves only to
propagate the desired variety by grafting or budding.

Cultivation 49
Plant health
The plant needs to be free of the most important diseases so that it does
not contaminate the fi elds with new pests (disease, insect or nematode).
Occasionally, new weeds can be introduced via the substrate the plants were
grown in.
Anatomical and physical aspect
It is best to use plants that are anatomically well formed with a straight and
strong main stem (Fig. 3.5a). The plants should be healthy and vigorous and
be inspected to make sure they have a good root system and a straight stem
with a good caliper. There should be no pronounced bends in the ‘neck’ – the
zone that separates the root from the stem – which indicate an improper seed
or embryo position at sowing time. The bends in the neck are due to chance
or bad sowing position plus a lack of proper sorting of the seedlings when
transplanted to the containers, These seedlings should be discarded at the
time of the fi rst transplant in the nursery or at the time of purchase. The bent
condition is more of a problem in seedlings from polyembryonic seeds like
citrus and mangoes of the Indochina–Philippine group. The mangoes of this
group form many embryos, which compete for the space that is normally for
one embryo, resulting in abnormal growth and entanglement and bending.
Plants with bends in the main roots at the bottom of the root-ball or with a
mesh of roots around it indicate they have stayed far too long in the container
(Fig. 3.5b). Ideally plants with these problems should be discarded. If needed
or because of scarcity of planting material they can be used, but the peripheral
roots have to be pruned away and the root mesh disrupted by making several
longitudinal cuts in this layer with a sharp knife. The objective is to ensure
that this abnormal growth pattern does not continue and that new roots
will emerge from the root-ball and grow outwards. When the primary root
at the bottom of the root-ball has a bend, it should be cut above the bend to
eliminate this abnormal growth pattern. This root pruning will result in root
loss and a plant with reduced water and nutrient absorption capacity. The
recommendation is to defoliate the plant to reduce potential transpiration and
balance the root absorption with leaf transpiration. Failure to defoliate can
lead to plant dehydration and death. This defoliation and root pruning will
retard plant growth and therefore all plants with manipulated roots should be
planted together in one area of the fi eld so that they can be given additional
care. Their growth will be initially slightly behind the other transplants that
have intact roots. Similar procedures should be used if the root-ball cracks or
breaks during transplanting, or if too damaged then discard.

50 Chapter 3
ASSOCIATED CROPS OR INTERCROPS
After or before planting the trees, an associate crop can be started, in order
to maximize the use of land and, hopefully, get extra income during the fi rst
years, which are the most di cult economically. This could be any annual
crop, including vegetables or a short-cycle fruit crop such as pineapple, cocona
(Solanum hyporrhodium) and naranjilla (Solanum quitoense). In some areas
intercropping in orchards with vegetables, leguminous crops and cucurbits is a
common practice. In Mexico, fruit orchards have been observed with excellent
intercrops of beans (Phaseolus vulgaris), watermelons (Citrullus vulgaris),
cantaloups (Cucumis melo var. reticulatus) and chilli (Capsicum annuum). Good
weed control is obtained under these intercropping systems.
In the second and third years, intercropping can be repeated, but the
soil should only be worked between rows at an increased distance from the
fruit trees each year. This reduction in cultivated distance is required to
avoid root damage, which can weaken the plants or become an entry point
for harmful organisms. It is normally recommended that after 3 or 4 years
no soil disturbance by heavy machinery should occur except for the pass of
mowers or brush cutters and the equipment for spraying and harvesting. An
alternative is to kill o the ground cover with herbicide and transplant the
intercrop directly without soil disturbance.
(a) (b)
Fig. 3.5. Nursery production of planting material is crucial for the successful
development of an orchard. Black plastic bags are frequently used (a). It is important
to ensure that the plants are not allowed to stay too long in the bag or they become
root-bound (b), with the roots growing around the outside of the medium against
the inside of the bag.

Cultivation 51
Short-lived fruit crops such as yellow passion fruit or papaya (Fig. 3.6) can
be planted between rows, and after 2 or 3 years they will normally be at the
end of their production cycle. Plantain and banana could be also used, making
sure their shade does not a ect the small trees; after a reasonable time they
can be eliminated and the propagating material taken to another location.
Care has to be taken during the fi rst years not to allow any large plants to grow
close to the young fruit trees since they may check their growth and these
trees are your long-term priority. Pasture can be started, and the shadow of
the trees as they increase in size will eventually eliminate the pasture from the
more shaded areas under the canopy. This pasture, generally a legume, will
show little damage from this shade and can be used as a weed control crop.
The other alternative, discussed above under plant spacing, is to increase the
number of trees planted twofold to fourfold to increase early production with
no room left for an associated crop. All of these alternatives help the farmer to
increase his early returns. It is necessary to estimate the extra costs associated
with these alternatives against the extra income that could be expected, which
could be negative.
Ideally the best approach is to start the orchard with an associated crop
almost simultaneously, so that the initial land preparation serves both crops.
This approach can leave the soil undisturbed except for sowing the cover
crop at a later time, if needed. The associated crop should not host enemies
that could attack the fruit trees and should not produce substances that may
inhibit the tree growth. The fruit trees and the associated crop need a degree
of compatibility with respect to water needs or frequency of irrigation. An
associated crop can also complicate fi eld practices such as spraying and hence
are not recommended after the third or fourth year.
(a) (b)
Fig. 3.6. Short-lived crops such as beans planted between rows of young avocado
trees (a) and papaya and banana planted between rows of the future crop of
rambutan (b) provide income during the juvenile period of the main crop.

52 Chapter 3
ORCHARD FLOOR MANAGEMENT
The physical and chemical properties of the orchard’s soil need to be
maintained in a good condition to ensure that root growth occurs without
di culty. However, weeds need to be controlled, people and machinery need
to move around in the orchard, and irrigation needs to reach all of the trees.
No single system can be used for all soil types as all have their advantages
and shortcomings. Rainfall, soil characteristics, irrigation system, water
availability, type of weeds, and the species and age of the trees should be taken
into account when deciding upon orchard management, without forgetting
costs and returns. The main orchard fl oor management systems used are
mechanical cultivation, cover crops, mulching and keeping the soil bare with
herbicides, as used on the coralline sandy soils of Florida.
Cultivation with machinery is very e ective with annual weeds and it helps
break the crust that sometimes forms and allows incorporation of fertilizers
and organic matter. Its shortcomings are that, after the orchard is 3–4 years
old, disturbing the soil between tree rows is normally not recommended due to
the danger of damaging the root system and the entrance of some pathogen
through the wounded roots. The destruction of irrigation ditches or furrows
can be another problem with mechanical cultivation. The presence of pipes
and hoses limits the use of cultivation, and the multiplication of weeds with
underground structures (nutgrass) can be another problem. Shallow and
careful cultivation avoiding under the tree canopy should be done with o set
discs or equipment that scratches the soil. As the trees grow, the soil should be
worked at increasing distances from the tree canopy line (Razeto, 1993).
Temporary or permanent cover crops are an alternative, especially if there
is good moisture in the soil throughout the year (Table 3.4). These crops help
maintain good soil structure, increase water penetration, prevent erosion from
wind and rain, and keep soil temperature lower. In the humid tropics, a legume
such as kudzu or other suited legume species can be used, with the additional
benefi ts that they fi x nitrogen. Kudzu can be an invasive weed because of its
rapid growth and habit of climbing up and over trees. Extra management is
necessary to periodically cut the climbing shoots with a machete and change
the vines’ growth direction with the hands or a long stick.
Growing sod as a cover crop requires ample water and nitrogen in order
not to a ect tree production (Fig. 3.7a). Mowing or grazing is needed with sod,
with the extra advantage of allowing machinery to get into the fi eld sooner
after rain and irrigation. On steep land, the presence of a cover will prevent soil
erosion during heavy rains. Aesthetically, sod is a good approach, but there is
strong competition for water and nutrients with the crop. In areas with a dry
season, the transpiration from the sod can be very high and it will also need
fertilizer, so care must be taken to irrigate and fertilize taking this additional
need into account. In the absence of irrigation, competition for water can be a

Cultivation 53
Table 3.4. Some tropical ground covers and green manures.
Name Main characteristics
Arachis pintoi
Forage or perennial groundnut.
Good coverage. Permanent. Non-climber. Will revegetate after dry
period. Shade tolerant.
Cajanus cajan
Calapogonium
mucunoides
Calapogonium caeruleum
Canavallia ensiformis
Jack bean
Toxic seeds. Green manure and ground cover. Very drought
resistant, shade tolerant and good for poor soils. Does not
tolerate excess water.
Canavallia gladiata
Sword bean
Uncooked seeds are toxic. Drought resistant. Green manure
Centrosema
pubescens
Clitoria ternatea
Butterfl y pea
Green manure. Very drought resistant. Small leaves will not cover
well.
Crotalaria juncea
For fi bre extraction. Fairly toxic to cattle. Some drought tolerance.
Crotalaria ochroleuca
Sunn hemp
Green manure. Non-toxic cattle feed except seeds. Upright. Fairly
drought resistant.
Desmanthus virgatus
Wild tantan
Green manure or fodder. Drought resistant. Bushy plant grows
back quickly.
Desmodium adscendens
Desmodium ovalifolium
Shade tolerant. Climber.
Dolichos lablab or Lablab
purpureus
Lablab bean
Similar to velvet bean but for more fertile soils. Drought resistant.
Green manure and cattle feed.
Indigofera hirsuta
Hairy indigo
Nematode suppressant. Annual, will reseed itself. Ground cover or
green manure. For well-drained soils.
Medicago hispida
Garrotilla
Mucuna pruriens
Velvet bean
Green manure. Very palatable. Aggressive climber. Tolerates
drought and low soil fertility. Bushy growth.
Phaseolus cocineus
Scarlet runner
Psophocarpus
tetragonolobus
Winged bean
Edible. Climbing. Does best in hot, humid areas. Drought and
sandy soil sensitive.
Pueraria phaseoloides
Tropical kudzu
Green manure cover or pasture crop. Tolerates high water levels,
acid soils and dry season. Grows well under trees. Not to be
confused with the weedy Pueraria lobata.
Vigna unguiculata
Forage cowpea
Green manure/cover crop. Drought tolerant but for well-drained
soils. Bushy.

54 Chapter 3
problem. Mulching can be an alternative, but it is normally expensive to spread
out in the orchard. Straw, hay, dried grass, wood shavings or any cheap and
easily obtained organic material can be used (Fig. 3.7a). Care has to be taken
to not let the mulch come in close contact with the trunks to avoid excess
moisture and collar rot occurring. Mulch will prevent weeds from growing and
at the same time will allow roots to grow near the surface, where the soil is of
better quality. One problem with mulch if allowed to dry out is a possible risk
of fi res and it can harbour rodent pests. Plastic mulches can be used, especially
in short-lived crops like papaya or pineapple, and their use will be determined
by the economic convenience (Fig. 3.7b). Normally only the plant rows are
mulched, and sometimes just a piece of black polyethylene about 1 m square is
placed around the tree pole to avoid the initial direct weed competition.
No vegetation on the orchard fl oor fi ts better in orchards located in arid
zones like the Peruvian coast or the Florida coralline sands. In desert areas,
there is very little to do to control weeds if drip irrigation is used. In Florida,
herbicides are used after rains to control weeds (see next section). The no-
vegetation approach means no competition with the crop for water or nutrients.
It also allows easier incorporation of manure or cover crops into the soil.
WEED CONTROL
Weeds, as well as associated crops, compete with the fruit trees for water and
nutrients, and in certain circumstances can be an important factor in lowering
(a) (b)
Fig. 3.7. Weed control can be achieved by placing organic mulch under the
trees (a), or plastic mulch (b) is also used on shorter-cycle crops or during tree
establishment.

Cultivation 55
crop yields. For example, neglect of weeding results in a 20–40% decrease in
pineapple yields. In situations with adequate moisture in the soils, weeds are
not a factor in competing with the crop for water. Some weeds can also cause
sanitary problems that can a ect the crop, this in addition to the negative
aesthetic aspect. Weeds are an important problem in climates where there
are year-round crop-growing temperatures that favour continuous growth.
This weed problem is especially acute in tropical and subtropical climates,
except for subtropics with cooler winters, or the arid tropics and subtropics.
Weed control is particularly troublesome during the fi rst 2–3 years of orchard
establishment, when the trees have not yet developed substantial canopies to
shade weed growth.
In arid areas with drip irrigation and little or no rainfall, weed problems
are not very di cult to solve, since weeds will only be found in wet areas
around a dripper and these can be eliminated mechanically or chemically.
However, if sprinkler, furrow or basin irrigation is used, weeds will be
widespread and control measures will have to be used frequently. Weeds can
be controlled by non-chemical and chemical means. Both approaches have
advantages and disadvantages, but if done properly can produce similar
results. The approaches can also be combined. The non-chemical control
involves either weed elimination or cutting weeds back to reduce competition.
Annual weeds are controlled by the use of hoes, pulling them manually,
fl ooding the land, the use of mulches, and mechanical tilling or discing the
soil. Mechanical cultivation should be avoided, though very e ective, due to
the damage it causes to the superfi cial feeder roots. Weed height reduction
is achieved by periodically cutting the weeds back with a machete and with
mowers or many types of brush cutters.
The chemical approach relies on herbicides or weed killers, which can
belong to di erent chemical groups. Herbicides can be selective and non-
selective; in both categories some are for foliar application and others for
soil application: The chemicals can work by contact or can be translocated
to other parts of the plant (systemic). Contact herbicides kill only the tissue
that they contact, while the systemic herbicides applied to the soil or the
foliage will be absorbed by the roots or aerial parts and move into the plant,
reaching the places where they will have their lethal e ect. Some herbicides
are applied to the soil before the weed seeds germinate and thus are called pre-
emergence, while the post-emergence herbicides are applied once the plant
has germinated. Pre-emergence application, either before or immediately after
planting, is e ective, the least costly and a desirable management practice.
Some of the techniques used for chemical weed control consist of using
a contact herbicide for annual weeds and a systemic herbicide for perennial
weeds. Sometimes a pre-emergence herbicide can be used before the start of the
rainy season. Several herbicides are on the market and di erent approaches for
their use can be taken. An analysis should be made to be sure about the most
convenient application procedure. Annual weeds should be eliminated when

56 Chapter 3
they are succulent and before they shed their seeds; the same is true for biennial
weeds. With perennial weeds the approach has to be di erent, and normally they
will be much harder to control. Chemicals are applied with spray apparatus,
which ranges from simple knapsack sprayers to the more sophisticated boom-
type sprayers, and some products are incorporated in dry form into the soil.
In young orchards care has to be taken not to use certain herbicidal
products that can check tree growth by being absorbed through the root system.
In other cases, the small trees have to be protected from receiving the chemical
spray or drift, which can be absorbed by the green stem, branches and leaves
or cause severe burns in these young tissues, while in large trees this is not
normally a problem because of their woody bark. For young seedlings and newly
transplanted orchards, glyphosate, a systemic herbicide, may be applied by rope
wick or weed wipers saturated with a more concentrated herbicide solution and
wiped on the weed leaves. These applicators eliminate spray drift and can be
used close to the stem. Application of herbicides through the irrigation system,
known as herbigation, has been shown to be e ective. An important aspect
with many herbicides is to know the time needed after application for them to
be absorbed; in many cases if it rains too soon after application, the product
will not have had a chance to be absorbed and it might be lost. Often morning
applications are done to avoid the afternoon and evening rains.
There are restrictions on herbicide use imposed by manufacturers and
government agencies, and all applicators must know the proper uses. Growers
who intend to export fruit should become acquainted with regulations of the
consuming countries governing the use of registered chemicals, residue levels
and other precautions. In organic production, no chemical herbicides are
normally allowed and only mechanical or cultural practices like cover crops or
mulching can be used.
The use of herbicides can be expensive, so a cost comparison has to
be made between spraying them over the whole surface and the system of
mowing between rows and cutting weeds back in the row with a machete or
the use of a hoe, or the mixed chemical–mechanical approach of applying a
herbicide in the rows and mowing between them. The mixed approach keeps
tree rows free of weeds using mulches or herbicides, or weed growth is kept in
check by hoeing or keeping weeds low with a machete or mowers. In the humid
tropics, the area between rows has either a legume or grass that is mowed
periodically. The approach used is a fi nancial decision and will depend on
the orchard size and fi nancial status of the grower. Weeding is an important
production cost component in orchard maintenance.
WIND BARRIERS
Wind can have several e ects on fruit crops. Gentle winds help ventilate the
orchard and create a healthier atmosphere around the trees. They will also

Cultivation 57
favour wind pollination and fecundation. However, strong winds can produce
mechanical damage and, depending on velocity, can have severe, sometimes
even fatal, e ects on crops, resulting in uprooting the whole tree, broken main
trunks, broken sca old branches or dehydrated and burnt leaves to completely
defoliated trees. Hurricanes or cyclones during fl owering and fruiting periods
can cause a severe fl ower and fruit drop in many species. Bananas and
plantains are some of the most susceptible crops to wind damage. In Central
America, bananas and plantains are sometimes subjected to blow down
caused by gusts of wind that unexpectedly hit certain areas and break all tall
pseudostems, and a whole year’s production is lost until the ratoon pseudostem
grows and harvesting can resume. Windbreaks are especially helpful when
normal prevailing winds become gusty with velocities exceeding 65 km/h.
Occasional high-velocity winds are damaging to orchard trees, but equally
harmful in the long run are the mild prevailing winds of 40–50 km/h. Trees
exposed constantly to such prevailing winds gradually develop a deformed,
lopsided shape, with all branches growing away from the winds.
Winds can also a ect sprinkler irrigation and spraying operations, causing
uneven water distribution of herbicides and other chemicals, which can drift
on to other plants. It is necessary to protect the plantation with a barrier that
should be e ective ideally from planting. Wind barriers will reduce water loss
from the soil and leaves, produce a more uniform temperature in the orchard
and reduce wind erosion (Wilkinson and Elevitch, 2000). The windbreak can
reduce crop evaporation on the leeward side to 40% within twice the height
of the windbreak, being more e ective at higher wind speeds (Fig. 3.8a).
The reduction in evaporation allows the trees in the orchard to develop the
higher humidity in the canopy that is important for crops such as rambutan,
mangosteen and durian. Sometimes a partial solution can be to plant the tree
rows following the same direction of the main winds, so that the fi rst trees in
the rows protect the rest of the row.
Two types of barriers are used: natural (Fig. 3.8b) and artifi cial (Fig.
3.8c). Both should fi lter, not stop, the wind and have about 50% permeability.
The windbreaks are perpendicular to the prevailing wind direction and
extend beyond the width of the orchard so that no wind enters from the
sides. Natural barriers consist of two or three rows of trees. The e ectiveness
of the windbreak depends upon the height and lateral extent of the barrier,
its permeability and the angle of incidence of the wind to the barrier. Wind
velocity is reduced to 35% within a distance of four times the height of the
windbreak (Fig. 3.8a).
Many di erent species are used as windbreaks, which complement each
other in size, growth form and canopy height. Sometimes a single species is
used. All trees used as a windbreak should compete as little as possible with
the crop; therefore deep-rooted and well-anchored, fl exible trees are preferred.
The trees should be adapted to the zone and should not harbour enemies of
the crop (Table 3.5). Ideally the barrier should be planted before the crop so
58 Chapter 3
that it starts protecting it from the beginning. Barriers have to be maintained,
and yearly pruning is usually performed to avoid too much compactness; also
subsoiling hook should be passed 3 m away from the barrier to avoid excessive
root competition with the crop. Barriers of trees that produce fruit instead of
timber or fi rewood can be used. The main disadvantages of natural barriers
are that they take time to be e ective, use up land and compete with the crop
for light, water and nutrients. They also need maintenance and can harbour
crop enemies.
Few windbreak trees can provide crop protection under hurricane
(typhoon) wind velocities. One of the most substantial windbreak trees is
Garcinia spicata, called ‘Fukugi’ in Okinawa, Japan. Windbreaks composed of
this species have been observed to withstand hurricane winds of approximately
210 km/h in northern Okinawa, providing crop protection without itself being
damaged. This species is related to mangosteen and grows well in subtropical
and tropical climates. Its only drawback is the very slow growth rate.
Artifi cial barriers are usually plastic fabric with 30–70% permeability to
wind (Fig. 3.8c). They should be resistant to ultraviolet rays as well as to sun
and rain, and resist deformation. Good barrier materials should last 10–15
years. The main advantage is that they can be installed and start functioning
immediately. Additional advantages include that the permeability can be
chosen and it will remain the same over the lifetime; they do not use much
space or compete with the crop and do not harbour plant enemies; they
can be moved to another location if necessary, and they do not need any
maintenance. Their main disadvantage is the high initial installation cost.
100
(a) (b)
(c)
80
60
40
Wind
direction
20
Wind velocity (%)
0
–10 –5 0 5
Distance – multiple of windbreak height
10 15 20 25 30
Fig. 3.8. Windbreaks reduce tree damage by reducing the wind velocity on both
sides of the barrier (a), with a signifi cant reduction on the leeward side up to ten
times the height of the windbreak. Trees can be hedged (b), as in this kiwi orchard,
or an artifi cial barrier can be made with plastic netting (c), as in this carambola
orchard.

Cultivation 59
Table 3.5. Some trees for windbreaks. Adapted from Wilkinson and Elevitch (2000).
< 6 m
Annona muricata Acacia koaia Averrhoa carambola
Bixa orellana Cassia spectabilis Casuarina equisetifolia
Citrus reticulata Coccoloba uvifera Eugenia uniflora
Gliricidia sepium Leucaena leucocephala Morus nigra
Myrciaria cauliflora Parkinsonia aculeata Pimenta dioica
Psidium guajava Psidium cattleyanum Sesbania sesban
Thepesia populnea
6–15 m
Anacardium occidentale Acacia confusa Albizzia lebbeck
Artocarpus integer Azadirachta indica Bambusa oldhamii
Casimiroa edulis Cassia (Senna) siamea Casuarina cuninghamiana
Cedrella odorata Chrysophyllum cainito Cojoba arborea
Cordia subcordata Cordia egalantha Dalbergia glomerata
Dimocarpus longan Erythrina berteroana Erythrina poeppingiana
Grevillea robusta Ilex tectonica Intsia bijuga
Khaya ivorensis Macadamia integrifolia Mammea americana
Manilkara zapota Melia azedarach Musa balbisiana
Pandanus odoratissimus Pithecellobium dulce Pouteria campechiana
Schinus molle Syzygium aromaticum Syzygium malaccense
Tabebuia pentaphylla Tamarindus indica Terminalia superba
>16 m
Acacia auriculiformis Acacia koa Acacia mangium
Acrocarpus fraxinifolius Aleurites moluccana Artocarpus heterophyllus
Artocarpus altilis Azadirachta excelsa Calophyllum inophyllum
Cocos nucifera Dendrocalamus asper Eucalyptus dunnii
Eucalyptus microcorys Guadua angustifolia Litchi chinensis
Mangifera indica Pinus caribaea Prosopis pallida
Pterocarpus indicus Sandoricum koetjape Swietenia macrophylla
Syzygium jambos Terminalia catappa Tristania conferta

60 Chapter 3
PEST BARRIERS
In some tropical areas, birds and fruit bats can cause extensive losses of fruit
near harvest. Numerous bird species, from solitary species to large fl ocks
of parrots, will eat fruit. Various approaches are used to reduce these losses,
including propane-fi red cannons to scare the birds and permanent net
enclosures (Fig. 3.9). The propane-fi red cannons produce loud, unexpected
blasts at random intervals, which are generally not less than 3 min apart.
The noise keeps the birds nervous, while they will become accustomed to
cannons that are always located in the same spot in a fi eld, if the fi rings occur
at regular intervals or fi re very rapidly. Other electronic devices produce
electronic synthetic sounds to repel birds by reproducing distress calls that
mimic individual bird species. Visual repellents, such as balloons, streamers
and fl ashing lights, are not as e ective as acoustical devices. Bird netting is
available that is lightweight and draped over the tree and, once over, harvest-
ing is possible, to heavier, ultraviolet-resistant protective materials fastened
to an overhead structure that totally encloses the orchard. The overhead
structure has a very high initial cost. Netting comes as rigid or stretch
materials in di erent widths and mesh sizes. The material chosen depends
upon the crop and layout, expected material life and the equipment available
for installation and removal.
In Asia, structures are built and covered with fi ne nylon mesh to exclude
aphids that carry viral diseases. An example would be a mesh structure in
which papaya is grown to protect it from ringspot virus. These structures
cover hectares of land and are rather light in structure and frequently su er
considerable damage from hurricanes (typhoons) during the warm season.
(a) (b)
Fig. 3.9. Birds and fruit bats cause signifi cant damage to fruit and may require the
use of a protected shelter to protect the crop (a). Protective shelters are also used
to protect papaya and other crops from insect-borne viruses (b); these lightweight
shelters are made of very fi ne mesh nylon.

Cultivation 61
FURTHER READING
Chandler, W.H. (1958) Evergreen Orchards. Lea and Febiger, Philadelphia, Pennsyl-
vania.
Choudhury, J.K. (1963) Early bearing of guava by photoperiodic induction. Science and
Culture 29, 213–214.
Coronel, R.E. (1983) Promising Fruits of the Philippines. College of Agriculture, University
of the Philippines at Los Baños, Laguna, Philippines.
Morton, J.F. (1987) Fruits of Warm Climates. J. Morton, Miami, Florida.
Popenoe, W. (1974) Manual of Tropical and Subtropical Fruits. Facsimile of the original
1920 edition, Hafner Press, New York.
Samson, J.A. (1986) Tropical Fruits, 2nd edition. Longman, New York.
Tomlinson, P.B. (1987) Architecture of tropical plants. Annual Review of Ecology
Systematics 18, 1–21.

62 © CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1
(R.E. Paull and O. Duarte)
4
TREE MANAGEMENT
INTRODUCTION
Tree management plays a key role in the economic success of an orchard. The
soil and climate conditions chosen for the species and the variety to be grown
set the foundation for an orchard. The infrastructure and associated logistics,
such as packing and storage facilities, coupled to adequate transportation,
energy availability, and water quality and supply, will aid in continued success.
Tree management can compensate for less than ideal soil or climate conditions
and the opposite is also true; improper tree management can render the best
soil and climate conditions of little value.
Tree management includes, fi rstly, the choice of propagation material that
is of the highest quality. Other components of tree management are irrigation,
pruning, fertilization, pest and disease control, pollination, fl ower and fruit
thinning, fruit bagging and the use of plant growth regulators. All these
components together will result in a proper-functioning tree that will produce
good yields and fruit quality.
A frequent problem is that some growers will attempt to apply very
sophisticated management practices without being aware of their advantages
and limitations. Some management practices apply only to certain varieties
or only under specifi c conditions. Other growers will use plant growth
regulators without having adequate irrigation or fertilization procedures. It
is crucial to solve these basic management practices before attempting more
refi ned practices. We have seen a number of cases when prospective growers
will spend a lot of money in preparing the land and installing an expensive
irrigation system and then buy poor-quality plants for their orchard. Other
problems are experienced with the purchase of a bad piece of land just because
it is cheap and then believing that you will get maximum yields, or hiring
poorly trained fi eld supervisors and/or failing to provide adequate supervision
and training of labour while sophisticated practices are being applied. These
decisions are short-sighted and frequently lead to poor fruit yields and quality.

Tree Management 63
PROPAGATION
Probably most fruits sold and consumed a few centuries ago would have
been rated as poor in quality by present standards. Fruit size, colour, fl avour,
appearance and postharvest handling have dramatically improved, while the
levels of defects, disease and pest damage have declined, especially in the last
half-century. Commercial production a few centuries ago, when most people
lived in rural areas, was based upon backyard or small orchard production,
frequently of plant material that had received limited selection. Initially, the
seed from a selection of the best type was planted, which frequently did not
ensure the maintenance of the desired selected characters, though it is an
easy approach. Large orchards cannot rely on highly variable plant material if
they want high yield and consistent fruit quality. Higher quality was achieved
by selecting superior wild trees or chance seedlings, and these genotypes were
propagated, often as vegetative materials. The use of vegetative material meant
that the genetic characteristics of these desired plants were retained and led
to the creation of varieties or cultivars. At the same time, especially in the
temperate zones, growers, nurserymen and hobbyists were fi nding new types
of plants that arose either as chance seedlings or as mutations, which they
selected and evaluated. Later, fruit-growing specialists and entire institutions
started breeding programmes that initially consisted of sowing numerous
seeds to see if some of the seedlings were better than their parents. In many
cases, nurseries or growers found mutants that were superior or had a special
characteristic. Superior plants known to exist elsewhere were asexually
propagated. Examples include the ‘Fuerte’ avocado, which was brought to
California as scions from Mexico; the ‘Washington’ navel orange, discovered
as a mutation in Brazil and its buds taken to the USA, where it was popularized
and later spread all over the world; the ‘Smooth Cayenne’ pineapple, discovered
in South America and taken to Hawaii; and the ‘Solo’ type of papaya,
discovered in Barbados and also taken to Hawaii. Many tropical fruit breeding
programmes that use modern techniques, such as mutation induction and
hybridization, to obtain superior varieties exist around the world.
Propagation of new selected cultivars is more sophisticated and relies
mainly on asexually propagating certifi ed varieties. The pineapple canning
industry relies especially on selection out of the ‘Smooth Cayenne’ variety,
while the pineapple fresh fruit export industry is dominated by the ‘Smooth
Cayenne’ low-acid hybrids. Five or six major mango varieties are the backbone
of the export trade, and the avocado varieties in international trade are ‘Hass’
and ‘Fuerte’. The international trade in bananas is based on one variety,
‘Grand Nain’, in the Cavendish group.
Many fruit trees need a rootstock that is adapted to adverse soil conditions
and has resistance to certain diseases, nematodes and insects. The rootstock
can also be required to attain a certain plant vigour, to achieve this the variety

64 Chapter 4
will have to be budded or grafted onto the desired type. The rootstock may
have di erent vigour or simply will provide a root for a species or variety that is
di cult to root by cuttings or air layers.
Certifi ed planting material is meant to ensure that the desired variety is
being obtained and it is free of specifi c diseases. Care needs to be taken that
the planting material purchased is free of pests, nematodes and diseases that
could contaminate areas where these problems do not exist.
Main propagation methods
Sexual or seed propagation
Propagation by seed is regarded as the most ‘natural’, but frequently the
new plants are not identical to the mother plant and thus variability occurs.
The only seed-propagated plants that are identical to their mother plants are
those that have been self-fertilized for several generations so that the genetic
arrangement has become uniform (homozygous), and they are called pure
lines, such as rice or wheat varieties. This seldom happens with fruit trees,
which are mostly cross-pollinated and show variability when propagated by
seeds.
In some species, the seed is exactly the same as the mother plant. The
condition is called apomixes, where an embryo develops in the absence of
fertilization by pollen. Apomictic seeds occur in mangosteen, achachairu and
jaboticaba, with vegetative embryos and no sexual embryo being present in
the seed. Mango varieties of the Indochina–Philippine group (see Fig. 10.5)
and most citrus species’ seeds also have a sexual embryo, and several embryos
can arise from the nucellar tissue of the ovary that surrounds the embryo
sac. Where you have embryos from nucellar tissue, often the sexual embryo
is suppressed by these surrounding asexual embryos. These propagation
methods are considered clonal, with seeds being used.
Seeds are used commercially to propagate herbaceous, short-lived crops
such as papaya, cocona, naranjilla, tree tomato, yellow and purple passion
fruit, giant passion fruit, sweet granadilla, banana passion fruit, cape goose-
berry and some others, such as coconut and pejibaye palm (Bactris gassipaes).
Variability is very low since the crops are very homogeneous and the best fruits
are selected to obtain seed. In the case of papaya, hybrids, a cross between two
selected parents or a selected variety like ‘Maradol’ and ‘Kapoho’ are used.
For selected varieties, hermaphrodite plants are self-fertilized to obtain the
same variety.
The other important use of seeds in fruit crop production is to obtain
rootstocks on to which the commercial varieties will be budded or grafted.
Normally seeds for rootstocks are highly variable, leading to the propagation of
rootstocks by cuttings or layers so that they are genetically identical and there
is no variability in the orchard. Rootstocks derived from apomictic embryos are
genetically identical to the mother plants.

Tree Management 65
For many lesser-known tropical fruit trees, seed is used for propagation
because they do not have a high commercial value and little information exists
about asexual propagation. The consequence is that fruits are very variable
in quality and are not easily marketed. If the quality of these fruit is to be
improved, methods to propagate them vegetatively are needed.
Asexual or vegetative propagation
When a new plant is regenerated or propagated from a piece or part of a
mother plant, it is called clonal or vegetative propagation. Plants can be
vegetatively propagated using many di erent tissues from roots (breadfruit)
(Fig. 4.1a), stem cuttings (guava), apices (banana) and buds (citrus) (Table
4.1).
(a) (b)
(c) (d)
Fig. 4.1. Asexual propagation includes the use of root cuttings for breadfruit (a),
tissue culture (b), budding (c) and the use of suckers for pineapple (d).
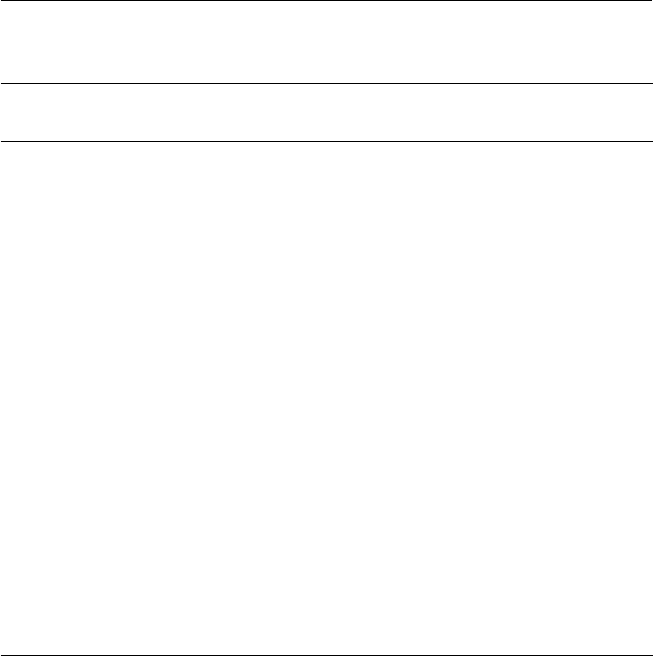
66 Chapter 4
Table 4.1. Propagation methods commonly used for selected tropical fruit crops
(Garner and Chaudhri, 1976).
Crop Seed
Suckers
Stooling
Layering
(Marcotting) Cuttings
Grafting
Budding
Annona Air layers Stem Budded,
grafted
Avocado Grafting
Banana Corm
Breadfruit Root
Carambola Budded
Durian Seed Air layers Stem Budded
Guava Air layers Stem and
root
Budded
Jackfruit Air layers Stem Budded
Lanson, duku,
langsat
Seed Budded
Litchi and longan Air layers Budded
Mango Polyembryony Air layers Stem Budded
Mangosteen Apomixis Stem Grafting
Papaya Seed Stem Cleft
grafting
Passion fruit Seed Air layers Stem
Pineapple Crown and
shoot
Leaf and
bud
Rambutan Air layers Budded
Sapodilla Air layers Stem Budded
approach
Cuttings
A leafy cutting is normally the terminal or subterminal part of a shoot
with leaves left. A terminal cutting comprises the tip of a shoot, while the
subterminal comprises the portion just below the tip of a branch; the upper
leaves are left on and the basal leaves removed. These cuttings are held under
conditions that prevent water loss (hermetic polyethylene chambers or a
mist bed) in some porous medium with good drainage and moisture-holding
capacity. Cuttings can be used for many tropical fruits, including guava,
cashew, acerola, jaboticaba, golden spoon, litchi and passion fruits. When
propagating hardwood or semi-hardwood cuttings, all the leaves are removed
or absent, as for Spondias.
Most stem cuttings are treated with compounds that have auxin-like
activity, such as naphthalene acetic acid (NAA) and/or indole butyric acid
(IBA), to improve rooting percentage, accelerate the rooting process or to obtain
more roots per cutting. The rooting products are applied to the basal cut end as
prepared powders or in liquid form (50% alcohol/50% water) at concentrations
of 1000 to 10,000 ppm, depending on the species, variety and type of cutting.

Tree Management 67
Tissue culture
This approach (in vitro propagation) uses micro-cuttings, which can be
meristems or portions of cells from di erent parts of the plant (Fig. 4.1b). The
process involves two phenomena: dedi erentiation from its original tissue
condition to callus, followed by redi erentiation to generate all the tissues of a
whole plant, which involves somatic embryogenesis. In many crops and under
certain tissue culture protocols, somaclonal variations can be a problem.
Somaclonal variation is thought to be due to DNA changes in the cell nucleus
caused by tissue culture stress in the presence of plant growth regulators such
as auxin and cytokinin.
This in vitro approach is used for rapid and consistent reproduction of elite
or di cult-to-propagate genotypes. Since the tissues are so small and tender,
they are very prone to rot or infection, and therefore they are fi rst surface-
sterilized then handled under sterile conditions in special growth chambers.
During the last three or four decades, several species have been propagated by
this system to ensure freedom of diseases and true-to-type plants. The cost per
plant is normally higher than asexually propagated plants, but many plants
can be generated quickly from one elite mother plant.
Until now, soft-tissue plants like pineapple, papaya, banana and plantain
have been commercially produced, while with trees some success has been
obtained with avocado, mango, Annonas and jackfruit. This propagation
method is essential if molecular biology gene transfer approaches are to be
used for tropical fruit tree improvement.
Layering
In layering a portion of a branch relatively close to the tip and still attached
to the tree is put in contact with a moist substrate and the stem below the
substrate is cinctured. New roots emerge into the medium, then the branch
portion with the new roots is separated from the mother plant, cutting below
the roots, and a new plant is obtained.
The most popular layering system for tropical fruit trees is air-layering or
marcottage. The leaves are removed from a portion of a branch located about
30–60 cm from the tip and a 3 cm ring of bark peeled o . The girdled area and
part of the stem with bark remaining are covered with a ball of moist sawdust
or peat moss. The ball is wrapped tightly with plastic sheet or aluminium
foil. Sometimes auxins can improve the rooting of these air layers. After 1–3
months, when the roots are visible and have turned from a white to cream
colour, the air layer can be harvested by cutting below the new rooted area.
The wrap is removed and the ball with roots transplanted into a pot or bag with
a substrate and held under medium shade. When this new plant shows signs
of growth, the bag or pot can be taken into full sun for some months until an
adequate size is attained for transplanting to the fi eld. Air-layered plants lack a
tap root and can be more easily blown over by strong winds, the same as plants
propagated by cuttings, but this is not a problem under normal fi eld conditions.

68 Chapter 4
Grafting or budding
Grafting is a system where a piece of stem containing two to six buds, called
a scion, is used, while in budding just a single, detached bud is joined with
the stem of a plant that provides the root system (Fig. 4.1c). The plant that
provides the root system is called the rootstock. The bud or stem piece with the
buds (scion) is of the variety that will become the tree canopy.
The most common methods of grafting are cleft, splice (whip), side and
saddle graft, while the main budding methods are ‘T’ or shield, patch, chip
and veneer. In some cases, branches of di erent trees are brought in contact to
form a union, and this is called approach grafting. For grafting or budding to
be successful, the cambium layers of both components have to be in intimate
contact to regenerate the union with all needed parts: xylem, phloem and
cambium. Many tropical fruits are, or can be, propagated by this system, and
most of them will do well with di erent grafting or budding methods. Some
species are more di cult and a specifi c type of grafting or budding has to be
used. The skill and experience of the grafter plays a very important role as to
which method is best for certain species, while in other species several methods
will produce satisfactory results.
Every grafted or budded plant has two genotypes, that of the root system
(rootstock or stock) and that of the canopy (variety or scion). The orchard
canopies should be genetically identical because the buds are taken from a
tree or trees of the same variety, so they are cloned on di erent root systems.
The ideal would be for the roots to be from a single clone, which occurs when
they are from vegetatively propagated plants or come from seeds with nucellar
embryos because of their asexual origin (apomixis). In most other fruit trees,
the seedlings used for rootstocks are of sexual origin so they vary among
themselves. Since the rootstock can signifi cantly infl uence the canopy growth
and development, any variation can show up once planted in the fi eld. The
root and canopy maintain their original genetic make-up and do not exchange
genes, except in the few cells around the graft union.
Natural propagative structures
Plants produce organs or structures that are used for its propagation (Table
4.1). Examples of these structures would include runners in strawberry
and suckers in date palms. Some tropical fruits do produce suckers. Banana
or plantain suckers have a solid base called a corm. This corm is the normal
propagation material for these species. The sucker arises from the corm of the
mother plant and can be transplanted whole or with most of the pseudostem
removed, leaving the base and the corm. This piece is buried ~10 cm deep.
Pineapple suckers arise on top of the fruit (crown sucker), at the base of the
fruit (slips) and at the base of the plant (basal sucker) (Fig. 4.1d). The preferred
propagation material is the sucker at the base of the fruit, but they are not
produced all year round by the plants. These suckers are detached from the

Tree Management 69
mother plant and left to dry for a few days so that the wound forms its scar,
and after that they can be used for a new pineapple planting.
Differences between sexually and asexually propagated
fruit trees
Trees originating from seeds tend to be vigorous since they are in a juvenile
state that does not produce fl owers and fruits. After these plants reach a
certain age and become adults, they are able to fl ower and fruit. For some
fruit trees the juvenile period can last 8–10 years. During this juvenile period,
the plant will grow vigorously and become very large unless subjected to
modern tree management practices. Additionally, trees from sexual seeds are
normally genetically di erent from their mothers and among themselves, and
potentially have di erent growth patterns and have fruit of di erent quality
characteristics, making marketing more di cult.
Asexually propagated trees are clones of their mother plants and bear the
same type of fruit. Additionally, the material used for asexual propagation is
normally obtained from a plant in the adult phase, not in the juvenile phase.
This adult material will have already fruited and its quality can be evaluated.
The trees obtained from adult plants will fl ower and fruit upon receiving the
external signal that induces fl ower development and will not have a juvenile
phase such as a plant from seed. If no external stimulus is required, they will
fl ower immediately. This earlier fl owering results in smaller trees. In the case
of grafting or budding, the existence of a graft or bud union can restrict the
internal xylem and phloem transport and additionally infl uence tree size
reduction and precocity. Dwarfi ng rootstocks are being sought for species that
normally grow very large.
NURSERY TREE MANAGEMENT AND PLANTING
Tree management starts in the nursery, since many problems in fruit
production can be avoided by just taking care of this phase of development.
Seedlings
At this stage many trees will show twisted or malformed roots or stems due
to the position of the embryo at sowing time. In some species this can be
controlled or reduced by sowing the seed in the correct position. This is
unpractical with some species because of the small seed size, and when the
seeds are polyembryonic (citrus, mango) the orientation of some of the
embryos, relative to the correct position of the seed at sowing, is not normal,

70 Chapter 4
and there will be a number of seedlings that come out with a swan-neck-
shaped stem base or root. Seedlings with this condition should be discarded
since they do not become good plants or rootstocks. Only seedlings with
straight stems and roots should be transplanted to the nursery pots or bags.
Plants in nursery containers
Plants left too long in nursery pots or bags normally have deformed root
systems. The main root will reach the bottom of the container and will not
be able to grow through so it will bend and start growing horizontally in a
circular fashion, following the lower rim form of the container. The lateral
roots, upon reaching the walls of the container, will grow around the root
ball and form a sort of peripheral basket or root mesh (see Fig. 3.5b). This
condition is also referred to as root-bound. When transplanted, the root-ball
prevents the development of a good root structure; the plants remain stunted
and are poorly anchored in the soil. Therefore only plants that are not root-
bound or have not stayed in the container too long should be selected and
planted.
Transplanting in the nursery into larger or taller containers that allow
the roots more time before they become deformed is preferable. If planting
material of the desired variety is in short supply, then the deformed plants can
be used. Remove the deformed plant from the container and cut the main root
above the point where it started to bend at the bottom. The lateral roots should
also be cut to disrupt the peripheral root basket, if present, by making several
vertical cuts on the outer part of the root-ball.
Planting
Planting in warm climates can be done almost any time of the year. If the
weather is too cool, planting can be delayed. If no irrigation is available,
planting should be done at the beginning of the rainy season. Some people
recommend adding 20–50 g triple superphosphate in the bottom of the hole,
separated by a 3–5 cm layer of soil from the root-ball.
PRUNING
Pruning fruit trees involves training the tree to the desired shape. The shape of
the trees will depend upon the species and the specifi c management objective.
Several models that are based upon developmental fate of the apices, the
confi guration of the branching points and plagiotropy (horizontal growth)
and orthotropy (vertical growth) of tree axes exist. The growth of the trunk
can be monopodial, rhythmic and indeterminate.

Tree Management 71
The models of tree growth habits provide a basis for comparing pruning
and training protocols for di erent tropical fruit trees. Pruning to shape a
tree aims to control the number, orientation, size and angle of branches
and thereby improve the trees’ structural strength to carry a fruit load,
reduce wind damage, increase light penetration, improve air circulation,
stimulate fl ower-shoot development and enhance fruit yield and quality. Too
much pruning can delay fl owering and lead to vegetative growth. Pruning
is not practised in monoaxial crops with short life cycles (banana, plantain,
pineapple and papaya), except that excess suckers are removed in bananas
and plantains; in papayas normally short lateral branches arising from the
main stem are removed. Broken, weak, drooping and diseased branches and
leaves should be removed. Pruning of many tropical trees may reduce yields
during the following season (mango, litchi), but some form of tree size control
is necessary to maintain a manageable tree size and structure. The trend is to
maintain the trees at a 3–5 m height by yearly or more frequent pruning for
ease of fl ower cycling, pruning and harvesting.
Avocados, mango and litchi have monopodial rhythmic growth (Table
4.2). In avocado, the branches are morphologically identical to the main
trunk, with fl owers borne laterally with only minor e ects on the vegetative
shoot. In the case of guava, mango and rambutan, the rhythmic fl ushing
of vegetative growth and fl owering infl uences when and how pruning is
carried out. Flowers develop on shoots that are not fl ushing, and the objective
of pruning should be to increase the number of shoots that can fl ower
simultaneously. Carambola can be extensively pruned to remove overlapping
branches and topped to maintain 2–4 m height without signifi cantly reducing
yield, and it can also be trellised.
In the past, it was said that only deciduous fruit trees had to be pruned;
nowadays most of the evergreen tropical trees are being pruned in modern
orchards. Normally there has to be a formation pruning at the beginning
of the plantation, and later production and sanitary pruning has to be
performed.
Formation pruning
This practice is of major importance in deciduous fruit trees, where it takes
several cycles to achieve the desired form. Most tropical fruit species have a
natural tendency to develop a desired form, but often they need to be helped to
achieve well-balanced and anatomically proportioned canopies. This canopy
should be not too tall, without structural faults that might cause the trees
or main branches to break easily with a full fruit load. The object is to prune
the trees to achieve the right form and avoid overcrowding of branches in the
canopy. The main pruning actions eliminate all shoots arising below the graft
union. Shoots below the graft union can lead to a loss of a grafted canopy as

72 Chapter 4
Table 4.2. Tropical seedling fruit tree growth is normally continuous and the trees develop different growth phenomenon and branching
habits. Flowering then either becomes synchronized to vegetative growth or is more responsive to the environment (Verheij, 1986).
Branching Vegetative growth and fl owering Example Growth characteristics
Single-stemmed
(monoaxial)
Continuous Concurrent Papaya, coconut Constant growth and concurrent fl oral development,
stable number of leaves thus a constant top to root
ratio. Improvement via altered growing techniques
(nutrients, water) to maintain high growth rate.
Terminal Banana, pineapple Supported by current photosynthesis. Suboptimal
growing conditions postpone fl owering.
Improvement via altered growing techniques
(nutrients, water) to maintain high growth rate.
Equidistant planting patterns frequently used in
single or double row.
Branched
(polyaxial)
Flushes Concurrent Passionfl ower Rapid, continuous shoot growth and concurrent
fl owering, rectangular planting patterns.
Separate in loci Avocado, durian, mango,
Annona spp., soursop,
jackfruit, abiu, lanson
Rhythmic growth. Major competition between
intermittent vegetative growth and fl owering.
Synchronous vegetative growth with almost
simultaneous fl owering and fruiting, feedback
control between top and roots to maintain balance.
External stimulus for fl owering and growth rhythm.
Improvement by manipulation of tree (juvenility
reduction, pruning, fruit thinning, girdling,
defoliation) before growing conditions (nutrients,
water) have favourable response.
Separate in time Atemoya, avocado,
mango, mangosteen,
litchi, carambola,
rambutan, guava, longan,
chiku
Feedback control between top and root growth.
Improvement in yield via manipulation of the
tree using pruning, fertilization and irrigation.
Improvement as for separate in loci category above.

Tree Management 73
these shoots can grow taller and more vigorously than the grafted canopy.
Another objective is to eliminate all excessive primary branches and all water
sprouts that are very vigorous and succulent shoots in a juvenile stage that
will take time to become adult branches.
The modern tendency is to have canopies close to the ground. In the case
of trees that tend to have a main stem with vigorous upright growth, they
are tipped at about 80–90 cm in order to induce lateral branching that starts
below that point and close to the ground (Fig. 4.2a). If the branching starts
higher on the main stem, as in non-pruned trees, the canopy will leave too
much empty space between the canopy and the ground. This space is wasted
and results in a higher tree, and it is more di cult to carry out spraying,
fertilization, pruning and harvesting.
Once the tree has been tipped, several lateral branches will develop.
Three to four of these lateral branches are retained to become the main
sca olds of the tree (Fig. 4.2b). The lateral branches are selected so as to
form a glass shape, with a 120 or 90° angle between the third and fourth
branches (Fig. 4.2c). The branches should not arise too close to each other
on the main stem, with 20–30 cm between them or between at least of one
of them and the other two branches. If all the main lateral branches come out
too close together on the main stem, too much of the canopy weight will be
concentrated at one point. This concentration of weight can become a problem
in later years when the trees are large, and they often break at this junction or
form a water pocket that accumulates water, which is undesirable.
If a primary branch grows too fast in relation to the others, it should be
checked by tipping or bending, in order to promote a balanced growth of all
the primary branches. In some species these primary branches have to be cut
back to about 60 cm, in order to induce them to form secondary branches (Fig.
4.2d), and this is followed by further pinching (Fig. 4.2e) to form a canopy
closer to the ground. A more open growth angle of the primary branches is
achieved by pulling them with strings attached to the ground or with strings
that have a weight hanging at the other end or by putting pieces of twigs
or bamboo between the branches in order to open their growth angles.
Sometimes the inner part of used tyres is put between the branches so that the
insertion angle to the stem is wider.
Formation pruning can start in the nursery or at planting time if the
plants are large or already show unwanted growth proportions, or after they
have achieved a certain size in the fi eld. During the fi rst years of growth in the
orchard, every 3 or 4 months the plants should be inspected and any water
sprout or sprout coming from below the graft union removed. If this is done
frequently, it can be done with the hands and no tools are needed. Longer
intervals will require pruning shears. The shoots should be cut at their base, to
prevent re-sprouting of more than one shoot.
74 Chapter 4
Production pruning
There is a growing tendency to prune adult evergreen fruit trees periodically to
manage their size. Tree size of less than 3–4 m is needed in order to facilitate
all management operations being carried out from the ground, such as
spraying and harvesting. The canopy should also not be overcrowded with
branches, which limits good light penetration and ventilation. The aim of
production pruning is to improve tree health, plant growth and fl ower bud
formation and so improve fruit yields and often fruit quality (Fig. 4.3a).
At planting
(a)
(b) (c)
(d) (e)
Three primary branches
Second pinching
Six to nine secondary branches
Aerial view
<120º
<120º
Development
90–110
cm
70–90
cm
Cut the top
Fig. 4.2. Formation pruning, which can start in the nursery or soon after planting,
develops a central pole cut to 70–90 cm from 100 cm, retaining only three evenly
spaced lateral branches that form the basic framework of the tree and develop from
the lateral buds, ensuring that these lateral branches arise at different positions
on the pole. Additional pinching of the apical buds induces additional secondary
branches; pinching of the apical buds may be done a number of times.
Tree Management 75
Maintaining the trees at less than 3–4 m can be achieved by topping trees
either with large machine pruners or by hand, using di erent cutting devices
such as machetes, chain saws or pruning shears. This pruning becomes
a periodic or yearly operation and is often carried out immediately after
fruit harvesting in order to allow the trees plenty of time to regenerate their
productive structures. This pruning has resulted in signifi cant increases in
yields and fruit quality, and in disease reduction.
Another technique used, in species like mango, is a peripheral pruning
after harvest, eliminating the outer 30–40 cm of the canopy in order to
stimulate a uniform re-growth so that all new shoots have the same age, in
order to induce uniform fl owering (Fig. 4.3b). This includes eliminating all
fruit stalks left from the former harvest since they have been shown to have an
inhibitory e ect on production.
(a)
(b)
Fig. 4.3. Production pruning begins after the formation pruning and the induction
of subbranches. The most common practice is to remove branches inside the
canopy, especially those that are diseased and crossing other branches; this will also
maintain tree height. Peripheral pruning is also practised to maintain canopy size
and tree height.

76 Chapter 4
Guavas are forced to produce year round, and normally the actively
growing shoots are tipped once they become too large. This tipping forces re-
growth and new fl owers to be induced, to keep production high and constant.
In guava cycling, as soon as the harvest is fi nished, a peripheral pruning of
the canopy is performed, in order to force new shoots that will bear fl owers.
Cherimoya and atemoya are also tipped to remove last season’s growth and
force new shoots to arise below this cut. The new shoots lead to adequate and
more uniform fl owering since new fl owers arise from these new shoots. If the
climate permits, this pruning can be done at shorter than yearly intervals, as
soon as the previous growth and leaves start to mature. This shortened period
can lead to fruit harvests every 7 to 9 months. In orchards where the trees in
a row are closely spaced and are starting to touch and shade each other, they
can be pruned as a hedge, by pruning the trees on both sides and leaving a
fl at surface where the hedge is a bit wider at the bottom than at the top. This
is achieved by pruning at a 20° angle. The top of the hedge would also be
trimmed horizontally at this time.
In banana and plantain all but one of the suckers taller than 1 m are
eliminated, leaving one replacement sucker or son that will be the next to
fl ower; the smaller suckers (grandchildren) are not cut. This practice is done
every 6–8 weeks.
Sanitary and maintenance pruning
Sanitary pruning is done to eliminate any growth that has been damaged by
diseases or by insects. It also includes branches that are broken or growing
in the wrong direction or interfering with neighbouring branches. Branches
growing towards the ground and interfering with fertilization or weeding
practices should be also eliminated. Any structure left from the former harvest
should also be eliminated. The person doing this pruning needs training in
order to make the correct decision as to what growth to eliminate. This could
include old, diseased or broken leaves in the case of papaya, plantain or
banana.
Structural regeneration pruning
This is performed to induce the regeneration of the productive structures and,
in the case of large plants, to reduce their size to facilitate fi eld operations and
avoid overcrowding. In yellow passion fruit, the plants are pruned after about
3–4 years, when they start to decline, and the decision has to be made between
replanting the whole fi eld or rejuvenating the old vines. If rejuvenated,
pruning is performed so as to leave the main stem and the primary branches,
which are cut back too about a metre, and all secondary branches are cut to

Tree Management 77
leave a short cane. This pruning will force new vigorous growth. Co ee plants
are cut back to 30–40 cm and they will start to regenerate their canopies, and
in this way they will be rejuvenated and return to a more manageable size and
be more productive. Regeneration pruning can also be done in old and large
trees of several species where size has reached unmanageable proportions and
there is a need to reduce it.
Pruning to change a variety
A grower can change the variety of an orchard that is mature but still healthy
and vigorous. The mature trees are pruned to leave the bases of the three or
four sca old branches (Fig. 4.4). Scions or bud-wood can be grafted on to
the stubs of the sca old branches directly or on to the shoots coming from
them. This operation reduces the time for the orchard to come back into full
production, as compared to starting with new plants from the nursery.
When large sca old branches are cut back and left without shade from
the canopy, care has to be taken to avoid sunburn. Normally the whole trunk
and the remaining portions of the sca old branches are protected with
whitewash prepared at the farm or with white latex paint. The wounds have
to be disinfected and protected; in some cases aluminum foil is used to cover
them. An alternative to this could be to cut one or two of the branches and
graft them, while the others are left intact and will provide protection and keep
the normal sap fl ow within the plant; the next year the remaining part of the
plant will be cut back and grafted (Razeto, 1993).
Pruning cuts and tools
Pruning cuts, if smaller than 1 cm in diameter, are normally not treated, but
larger cuts are treated with a paste containing copper, such as copper sulfate
or other fungicide, and sometimes insecticide, to avoid the entrance of some
pathogens or insects. Under very wet situations and if certain diseases are
present, almost any cut has to be disinfected; sometimes it is best is to wait for
a dry spell to prune.
Pruning tools include pruning shears, pruning saws, chainsaws and
even machetes. Machetes are not recommended, but they are used to prune
trees that have a soft wood. Tools should be disinfected before pruning the
next tree, in order to avoid transmission of diseases from one tree to another.
In some cases, such as with fi re blight, tools should be disinfected between
each cut (Harris, 1983). Disinfection is of the utmost importance with some
tree species or when disease is present, to protect the rest of the grove from
becoming infected. In banana plantations, the machetes used for eliminating
suckers are disinfected after each cut is made, to avoid the spread of ‘Moko’,

78 Chapter 4
the bacterial wilt disease. Disinfection products include an iodine-containing
odophor such as the commercial product ‘Vanodine’, sodium hypochlorite
(bleach) at a 10% diluted solution or denatured methyl alcohol (shellac
thinner). Bleach can be very corrosive on equipment, so it should be used
with care.
Fig. 4.4. To regenerate an old orchard and change the variety, growers cut back the
old tree to the main lateral branches (stag-horning) and graft the new variety scions
on to the old branches.

Tree Management 79
IRRIGATION
In some parts of the tropics with abundant rainfall, such as the Atlantic
coast of Costa Rica, which receives ~4000 mm/year, irrigation is not
necessary, even for bananas; drainage is the main concern, to remove the
excess rainwater. In tropical areas with high rainfall, most have a dry season
of a couple of months, when irrigation is needed. Installation of irrigation
will depend on an economic analysis of the situation as to whether artifi cial
irrigation will improve fruit size, production and quality. The analysis should
take into consideration the species, trees’ growth phase, temperature, wind
and relative humidity during the dry months. If the dry season occurs during
the cool season, when little wind occurs or high relative humidity is present,
irrigation needs are less than if the dry season is hot and windy and the
relative humidity is low. A problem arises in abnormal years with a longer
than usual dry period, when productivity can fall signifi cantly. Two or three
dry months under the tropical heat can have detrimental e ects on yields of
some species not adapted to long dry spells and it can be wise to irrigate during
the dry season.
Many fruit crops that originated in the monsoon areas need a dry period
to restrict tree growth, mature the foliage and induce fl owering. The mango
is an example of a tree that evolved in a monsoon environment and fl owers
better if subjected to cool weather and dry soils. In many places, mango is
grown with only rainfall, though yield may be reduced. Some fruit trees are
subjected to a water stress even when irrigation is available, with irrigation
resulting in heavier and more uniform fl owering. A number of tropical species,
such as many Spondias, have evolved mechanisms to withstand the dry season
by shedding their leaves to minimize water loss.
Tropical fruit trees should be irrigated or receive adequate rainfall in order
to yield their maximum. Most modern orchards install an irrigation system
even if the rainfall is adequate to keep the plants growing; irrigation under
these circumstances will provide extra water in case of mild stress or when the
rainfall pattern changes.
Irrigation systems
The irrigation system installed will depend on many factors: amount and cost
of water, availability of money, steepness of the land, and the technical level of
the farmer. Several systems are used in tropical fruit orchards.
Surface irrigation
Surface irrigation includes furrow and basin irrigation, with furrow
irrigation being the most popular. The land needs to be fairly level or to have
been levelled during preparation (Avilan et al., 1989). Land levelling can

80 Chapter 4
be a costly operation, but once it is done, furrow and fl ood irrigation will be
cheap though very ine cient in water usage. Furrows are more suited to fl at
or almost fl at land where the slope is 0.5–1.0%. The furrows can be made to
follow the contour, with the same slope of 0.1–1.0%. Higher slopes can lead to
erosion, which will destroy the irrigation ditches and the furrows will become
excessively deep.
In furrow irrigation, water is conducted along the tree rows and around
the trees. For young plants, one furrow is often su cient, later increased to
two furrows, one at each side of the trees, and this could be increased to four
furrows in older orchards. Furrows can also be dug around the trunk or have a
side furrow that directs water under the canopy. Furrows should be no longer
than 120–180 m in loamy soils and 60–80 m in very permeable soils. If a
furrow is excessively long, the time for the water to reach the end will allow
for excessive infi ltration at the start beyond the root zone and waste water and
fertilizers. Short furrows have the opposite e ect of insu cient penetration
and faster onset of water stress.
In the basin system, around each canopy drip line a 25–30 cm mound of
soil is prepared to retain the water and allow fl ooding of the canopy area till
the soil becomes saturated. This system has 35–40% water-use e ciency and
is used where water is abundant and cheap or free.
Furrow and basin irrigation are less e cient in water usage because of
the excessive infi ltration at the start of the furrow and the loss of water in
transporting to the fi eld. Care is taken to ensure no fl ooding or excess irrigation
water comes in contact with the base of the trunk, as this can lead to serious
trunk diseases and even tree death.
Low-pressure irrigation
Drip and micro-sprinkler irrigation are low-volume, low-pressure systems (Fig.
4.5a, b). These systems can be used on steep and uneven land, and require no
land levelling. Pressure compensation built into emitters and sprinkler heads
means that all points receive similar amounts of water. Water is applied only
where needed to the plant roots, and these systems are the most e cient for
reducing water wastage. The distribution of the water in pipes and hoses
results in very little evaporative loss, saving 30–40% of the water in relation to
conventional surface systems.
Drip irrigation consists of having hoses running along the tree rows (Fig.
4.5a), and these hoses will have several drippers, normally three or four under
the canopy drip line, with one or two hoses on each side of the trees along
the rows. In sandy soils, lateral movement of water is poor, so more drippers
will have to be used or the frequency of irrigation increased. Sometimes
micro-sprinklers are a better alternative in sandy soils. Normally with micro-
sprinklers (Fig. 4.5b), there will be one per tree to keep the soil under the
canopy moist. Some large banana companies use sprinklers that hang from
cables. Watering is more frequent and a lower volume applied each time, so

Tree Management 81
that the plants do not become water-stressed, resulting in improved yields
(Baldini, 1992). The systems represent a higher initial investment, and skilled
labour is needed to install and maintain, with the benefi ts of lower water usage
and greater e ciency of application. In many parts of the world, these systems
lend themselves to ‘fertigation’ or ‘ferti-irrigation’, a combination of irrigation
and fertilization. A calibrated mixer injects a fi xed amount of a concentrated
liquid fertilizer stock solution into the main irrigation supply line. The amount
injected is determined by knowing the amount of water to be applied, fl ow rate
and the amount of concentrated fertilizer to meet the plant’s needs.
Sprinkler irrigation
Sprinkler irrigation is still quite popular for fruit trees. Permanent and semi-
permanent or portable sprinkler systems are used. Sprinkler irrigation can
be over or under the tree canopy (Fig. 4.5b). The above-canopy system
washes pesticides o the leaves and can contribute to disease spread because
of the humidity and splashing the droplet causes. In the case of banana and
plantain, under-canopy irrigation is used. Mini-sprinklers that cover a circle
with a diameter of about 12–14 m are installed every 10–11 m in a square
arrangement. Under-canopy irrigation avoids the disadvantages of the big
sprinklers used in the past, which covered almost 1 ha. In very few situations,
(a)
(b)
(c)
Fig. 4.5. Irrigation of orchards has moved away from furrow and fl ood irrigation to
the use of drip tubes (a) and micro-sprinklers (b). To determine water needs a simple
system is the use of tensiometers at different depths (c). Electrical tensiometers are
also available.

82 Chapter 4
especially with small trees like guavas that are pruned, travelling sprinkler
systems can be used successfully. Sprinkler irrigation does not require level
land, uses little labour and improves soil structure; water volumes can be
regulated; it wastes less water than surface irrigation, but it requires more
pump power, and it is more expensive to install than furrow or basin irrigation.
Sprinkler systems sometimes interfere with farm machinery operations.
Sub-irrigation
Sub-irrigation can be used in very permeable soils like the coralline soils of
Florida. Water is pumped into the drainage system and this makes the water
table rise, so that the root zone becomes saturated.
Irrigation control
An excess of water or drought a ects plant functions and thus tree yields.
Drought will reduce water availability and consequently nutrient uptake; the
stomata will close and less carbon dioxide is available for photosynthesis. With
fewer sugars produced by photosynthesis, translocation of carbohydrates
will be reduced, as well as hormonal transport from the roots to the canopy.
Excess water will create a shortage of oxygen in the soil, leading to anaerobic
conditions, which are toxic and reduce root activity and growth, often leading
to root death. Root death results in reduced water absorption and hormone
production by the roots and more pathogenic activity, which can cause root
rot and fi nally tree death.
Irrigation has to meet the plant’s needs, which depend on the species, the
tree size and age, foliar density and the climatic conditions. Any irrigation
plan has to take into account the water retention of the soil, the rate of water
penetration, the distribution and depth of the root system and the amount
of water the crop uses. The amount of water to apply will depend on soil
structure and texture, and depth of the root system. Irrigation must take into
account the depth that water should reach in the soil profi le, and this is based
upon root distribution and where the majority of roots are active. During tree
growth, root distribution shifts from the roots being concentrated near the
surface to becoming deeper in distribution. If irrigation is too shallow, the root
system will also remain shallow, forcing more frequent irrigation. If irrigation
is too abundant, water will pass below the main root zone and take with it
many nutrients needed for plant growth.
After heavy rain or irrigation, the soil is saturated and begins to drain
under gravity, and it reaches a point called fi eld capacity (FC) about 24 h
later; that water is held by the soil against gravity. The potential (force) that
this water is held at is about 7 kPa (7 cbars, ~0.07 atmospheres) and it is
readily available for the plants. At FC, there is more water in a heavy than in
a light soil. If no further water is received by the soil, it will start to dry out,

Tree Management 83
until it reaches the so-called permanent wilting point (PWP), where most
plants are no longer able to extract water from the soil and the water potential
is too strong. At PWP, plants start to wilt, irrespective of the time of day.
Water at PWP is retained by the soil with a force of 1500 kPa (15 bars, ~15
atmospheres).
Several methods are available to determine plant needs and the amount
of irrigation that must be applied. Simple approaches involve digging a small
hole with a shovel to see if there is moisture at a certain depth or checking
whether the annual weeds in the orchard are wilting. In some cases, growers
use rain gauges (pluviometers) to determine the amount of rain and calculate
how much irrigation water to apply knowing the crop’s total water needs on
a weekly basis. Total crop water needs depend upon the surface area covered
by the crop canopy and the potential evaporation. In certain banana-growing
areas in Honduras, irrigation needs are estimated based on research data
showing that the plants need about 2000 mm of water falling on the ground,
~40 mm/week, supplied with three applications of 13 mm each time. The
amount applied is reduced by the amount of rainfall recorded in the rain
gauge in 2-day periods. For example, if it rained 6 mm they would only irrigate
to apply 7 mm.
A more accurate method is to use tensiometers (Fig. 4.5c). Tensiometers
are pieces of pipe with a porous tip where water can fl ow in and out. The
pipe is fi lled with water and at the other end there is a manometer, which will
measure the tension by which water is retained by the soil. The manometer is
calibrated in centibars (cbars) or hundredths of a bar, a bar being equivalent to
1 atmosphere. In a saturated soil, there will be practically no tension and the
manometer should indicate 0 cbars; at 10–25 cbars there is a good amount of
water available, while at >25 cbars, water is becoming more di cult for the
plant to extract and at 75–80 cbars irrigation is essential.
Tensiometers are usually installed at a number of locations spread
throughout the orchard and at di erent depths to represent the root-zone
conditions. If the tensiometer with the porous tip 30 cm deep shows high
tension, irrigation is necessary, while if the one with the tip at 60 cm shows
tension it means more water has to be applied to reach that depth. The 30
cm depth indicates when and the 60 cm tip indicates the amount of water
required. For avocados, irrigation should be carried out at 40–50 cbars
tension. In basin or furrows irrigation, 50–60 cbars would indicate the need
to irrigate, and with drip irrigation 10–15 cbars would be the threshold.
In the last few years more sophisticated remote indicators or sensors have
become available. Some sensors measure the electrical resistance of gypsum
or other blocks whose resistance varies according to the soil water content.
Other instruments measure the time an electromagnetic impulse takes to
travel through the soil (TDR – time domain refl ectometry), and that varies
according to soil moisture content, and the neutron emitter that receives the
refl ected emissions, which also vary according to soil moisture.

84 Chapter 4
A more accurate calculation of a crop’s water needs is the crop’s
consumptive water usage, which combines leaf transpiration and water
evaporated by the soil and the leaf surfaces. These losses are a ected
by temperature, relative humidity, wind speed and radiation, and their
combined value is called evapotranspiration. A type ‘A’ evaporimeter, which
is a circular tank 1.20 m in diameter and 25 cm deep fi lled with water, is
used in meteorology; daily measurements are made to establish how much
evaporation occurred and it is expressed in mm/day. This value multiplied by a
location factor and the crop coe cient (Kc) (ground area covered by the crop)
will give the daily evapotranspiration or consumptive water use and is used to
calculate how much and when to irrigate according to what is evaporating.
FERTILIZATION
Fertilizer needs and dosages
A number of mineral elements are required for plant growth and development
and are classifi ed, according to the amounts needed, into macronutrients and
micronutrients. Macronutrients are needed in higher amounts and include
nitrogen, phosphorus, potassium, sulfur, calcium and magnesium. The micro -
nutrients are needed by plants in lesser amounts and include iron, zinc,
boron, manganese, copper, molybdenum and cobalt. Both groups are equally
important; when plants do not have enough of any one of the macro- or
micronutrients, they are unable to complete their life cycle. In an orchard, a
shortage or defi ciency is overcome by the application of fertilizer.
The amount of fertilizer to apply can be established in many ways. In
some tropical regions the nearest university or agricultural extension o ce
can provide a fairly good estimate as to the amount of fertilizer to apply for
specifi c crops; published information can be a useful initial orientation as to a
specifi c crop plant’s needs, and some neighbouring farmers’ fertilizer practices
can help as a starting point. A more technical approach is to determine the
amount needed to replace that ‘exported’ by the harvest. This estimate needs
to be adjusted for the amount of nutrients lost through pruning and e ciency
of nutrient uptake and the amount unavailable to the plant roots. Another
system is to establish trials using di erent dosages of fertilizers for a given
species or variety under your climatic and soil conditions. These trials can
take a long time, and often the answer is needed sooner than the results are
obtained. The other approach is to carry out soil and plant tissue chemical
analysis.

Tree Management 85
Soil analysis
Soil samples are collected before planting from a soil depth of between 10
and 30 cm with a shovel or auger. It may also be necessary to sample the
subsoil. In fairly uniform soils, soil is sampled at various points, following a
‘W’ pattern across the fi eld. The soil samples are mixed for analysis. If the soils
appear to vary in a fi eld, each di erent area should be sampled and analysed
separately. The analysis results will provide the soil’s pH and an estimate of the
available macronutrients and, when data are available, a recommendation
as to the fertilizer needs. For nitrogen content, the results will not be as exact.
Any defi ciencies noted at this time should be corrected. It is important to
remember that the soil analysis indicates what is present but not necessarily
available for the plants; sometimes an extreme pH can block the availability
of some nutrients. So the soil’s analysis has to be complemented by the plant
analysis. The soil analysis can be repeated every 2 or 3 years to detect any
signifi cant changes.
Plant analysis
Plant or foliar analysis indicates the level of a particular element present in
the plant. The amount is compared with a standard table, which have been
developed for many species after long fi eld studies. This analysis makes it
possible to know if the level found in the tissue is normal, defi cient or excessive.
For leaf analysis, it is important to know how the standards were developed, as
the type, number and age of leaf, what type of shoot (with or without fruit)
and at what time of the year samples were taken can change the standard
values. The samples taken will need to duplicate this sampling protocol
carefully in order to have comparative results. In banana, leaf or petiole pieces
are used instead of whole leaves. The sampling has to be performed by trained
personnel and the samples analysed by a certifi ed and trusted laboratory. The
analysis results will indicate what nutrients are insu cient for the plant’s
growth or what is in excess. A decision can then be made to correct a nutrient
that is limiting growth by applying more or less of that nutrient in your
fertilizer programme (Table 4.3).
Dosages will initially follow recommendations from other growing areas
until reliable local or your own information is available. The nutrients most
commonly defi cient are nitrogen, phosphorus and potassium, and sometimes
magnesium, and these are included in a regular fertilization programme.
Roughly, in the adult stage plants need around 100 kg of nitrogen, 50 kg of
phosphorus (P
2
O
5
) and 100 kg of potassium (K
2
O) per hectare per year. These
very broad referential numbers vary signifi cantly with species, soil type and
plant growth stages. Banana, plantain and pineapple normally need much
more nitrogen and potassium than other tree crops. A common problem

86 Chapter 4
Table 4.3. Nutrient levels that are suffi cient for growth for various selected tropical fruit crops (after Reuter and Robinson, 1986; Jones
et al., 1991).
Nutrient levels adequate for growth (%)
Tissue Nitrogen Phosphorus Potassium Calcium Magnesium Sulfur
Annona spp. Leaves 2.5–3.0 0.16–0.2 1.0–1.5 0.6–1.0 0.35–0.5
Avocado Leaves 1.6–2.0 0.08–0.25 0.75–2.0 1.0–3.0 0.25–0.8 0.2–0.6
Banana Leaves 3.5–4.5 0.2–0.4 3.5–5.0 0.8–1.5 0.25–0.8 0.25–0.8
Guava Leaves 1.3–1.6 0.14–0.16 1.3–1.6 0.9–1.5 0.25–0.4
Litchi Leaves 1.3–1.5 0.15–0.2 0.8–1.2 0.56 0.21 0.1–0.16
Mango Leaves 1.0–1.5 0.08–0.25 0.4–0.9 2.0–5.0 0.2–0.5
Papaya Petioles 1.01–2.5 0.22–0.4 3.3–5.5 1.0–3.0 0.4–1.2
Passion fruit Leaves 4.75–5.25 0.25–0.35 2.0–2.5 0.5–1.5 0.25–0.35 0.2–0.4
Pineapple Leaves 1.5–1.7 < 0.1 2.2–3.0 0.8–1.2 < 0.3

Tree Management 87
is a tendency to use an excess of nitrogen on most crops, which can lead to
excessive vegetative growth and environmental contamination, besides being
a waste of fertilizer and increasing production costs.
Timing of application
Nitrogen, if applied conventionally, should be applied three or four times a
year in equal amounts, with the fi rst application before fl owering or the main
growth fl ush. The next applications will follow at 3- or 4-month intervals.
If there is no irrigation, the fi rst application should be done as soon as the
rains start, and the other two or three divided so that the last is made 2 weeks
before the rains stop. Potassium can be split into two or three equal fractions
and applied with the second and third, and eventually the fourth, nitrogen
application, if nitrogen is applied four times a year. Magnesium can also be
split into two or three applications. Phosphorus, since it is readily immobilized
in the soil, can be applied all at once for the whole year, normally with the fi rst
application of nitrogen. Nitrogen should not be applied close to harvest, as this
results in fruits that are more succulent, mature later and are not as sweet and
tasty. Nitrogen is preferably applied right after harvest
Fertilizer application
Solid application
When low-volume or sprinkler irrigation systems are not installed, con-
ventional soil fertilizer application systems are used. This application of dry
fertilizers to the soil is made by broadcasting the fertilizer on to the soil surface
over the outer half or third to the canopy drip line. There is no need to apply
fertilizer near the tree trunks, as the old roots have little nutrient-absorption
ability. Small banana growers apply dry nitrogen and potassium fertilizer
four times a year, while phosphorus is normally applied once a year; these
applications are done by applying the fertilizer in a semicircle about 60 cm
long and 30 cm wide in front of the ratoon sucker, about 30 cm from its base.
When the plants are closely spaced, fertilizer can be broadcast uniformly
in the space between rows, as it is assumed that this area is fi lled with roots.
Alternatively, a shallow semicircular furrow about 40 cm long and 5 cm deep
is excavated with the edge of a hoe or square shovel 30–40 cm from the main
stem; the fertilizer is put in the furrow and covered. Sometimes fertilizers are
applied following the irrigation furrows or in the basins, which ideally should
be wet. Fertilizers such as urea should not be left exposed but covered with a
rake or irrigated into the soil to avoid volatilization of the active components.
The fertilizer should be applied when the soil is wet and moved from the

88 Chapter 4
surface into the soil by irrigation or rainfall. When irrigation facilities are not
available, the farmer should wait for a day when he is certain that there will
be rain and fertilize just before it rains. Fertilizer applied during the dry season
will not be available to the plants until it rains, unless an irrigation system
is available.
In many orchards, a wide and 10–15 cm deep furrow is made that follows
the drip line of the canopy, and the fertilizer is placed in that furrow and
buried to avoid losses. Four to six holes about 30–40 cm deep can be made in
the vicinity of the drip line in a square or a hexagon layout, and the fertilizers,
sometimes including manure, are buried.
Dilute application
Large banana companies used to fertilize every 2–3 months with nitrogen and
potassium, using ‘cannons’ or large sprinklers that covered a circle of about
a hectare. They fi rst irrigated with water containing no fertilizer until the soil
was wet, then injected the soluble fertilizer into the water until the calculated
amount had been applied to the fi eld and ended the process with plain water.
The last phase of irrigation without fertilizer ensured that the fertilizer had
been taken into the soil and that leaves and metallic structures in the fi eld
were washed, so that fertilizer residues, especially urea, did not burn the leaves
or corrode the metals. This approach applied a large amount fertilizer a few
times a year. When sprinklers, micro-sprinklers and drippers are used, smaller
amounts of fertilizer are applied with each irrigation. The advantage of using
smaller amounts at more regular intervals is that the fertilizer is placed near
the root zone and less fertilizer is lost because of leaching. Small, regular,
almost continuous application ensures that most of the fertilizer is used by the
plants, reduces plant nutrient stress and the application rate can be readily
adjusted to the stage of plant development. Nitrogen applications near harvest
time have to be reduced or eliminated to avoid delayed fruit maturation,
excessive fruit succulence and to achieve a better-fl avoured fruit. The
reduction in nutrient stress associated with almost continuous application of
fertilizer results in higher yields and allows growers to recover the investment
made in the irrigation infrastructure, in addition to signifi cant reduction in
fertilizer and water usage.
Foliar fertilization
Although the roots are the main organs that the plants use to absorb needed
nutrients, foliar applications can be made to correct certain defi ciencies. Foliar
fertilization is normally used to apply micronutrients and in exceptional cases
to supplement for a defi ciency of a major element, especially nitrogen in the
form of urea. Because of the large plant needs for major elements and the low
concentrations tolerated by the leaves, especially urea, it is almost impossible
to fi ll the needs through the leaves. Microelements are also normally taken up
by the roots, but in situations with low micronutrient availability or the root

Tree Management 89
inability to absorb them, foliar sprays provide a very rapid response, and after
2–3 weeks improvement can be noticed. Pineapple in Hawaii is sprayed at
regular intervals with an iron solution to correct a defi ciency; even though the
soils are high in iron, most is unavailable to the plant.
Many fertilizer preparations contain microelements that are sprayed on
to the foliage from one to four times a year. Spray application frequency is
dependent on the micronutrient, the species and the defi ciency severity. Foliar
fertilizations should not be performed at fl owering time or when the fruits are
very small. Some micronutrients are applied as sulfates, such as zinc sulfate,
but since this is an acid product, it has to be mixed with lime to avoid leaf
damage. Urea, which has a high biuret content, can also cause severe burns.
Soluble forms of single essential micronutrients and mixtures of a number of
micronutrients, as their sulfates, nitrates and chlorides and complexed with
chelating compounds (EDTA), are available commercially. These products can
be tried and used if satisfactory results are obtained.
For best results, foliar fertilization by spraying young leaves will make it
easier for the product to be absorbed, since the leaf cuticle is thinner. If the
spray drop size is small, repeat applications at lower concentrations enhance
uptake. Since a liquid is applied to the foliage, the more foliage it covers and
the longer it stays as a liquid the more nutrients will be absorbed by the plant.
Application during cooler, high-moisture and calm air conditions is the best
to obtain maximum benefi ts, with early-morning sprays being recommended.
Covering both sides of the leaves, especially the underside, with the greater
number of hairs and pores and less wax, aids in foliar nutrient uptake. The
addition of a spreader sticker to the foliage fertilizer mix can also increase
uptake. Some foliage spraying can be done in combination with disease
and insecticide sprays after checking for compatibility. Phosphates are not
compatible with salts of copper, manganese, zinc and iron; copper fungicides
should not be mixed with sulfates, nitrates or chlorides. If a new product or
procedure is being used, test the mixture on some plants before spraying the
whole fi eld.
CROP PROTECTION
Fruit crops can be a ected by insects, mites and nematodes, and diseases
caused by bacteria, fungi, viruses and similar organisms. Vertebrate enemies
such as rats, squirrels, monkeys, birds and bats can also be a problem. Since
fruits are expected to be without blemishes, and more so if they are for export,
the tolerance is zero for damage. It is not possible to obtain blemish-free-
quality fruit without using pesticides. For many tropical fruit crops, there
are few approved pesticides, and the residues tolerance is low, and varies
with market.

90 Chapter 4
Insects
Insect pests are a major problem in agriculture, including tropical fruit
production. Most insects are harmful because they chew plant parts or
suck their fl uids. Some insects are borers that damage the branches or
stems. Additionally, some sucking insects can transmit viruses, viroids and
mycoplasmas that cause diseases if they come from an infected plant. There
are also benefi cial insects such as honey bees, silk worms, those involved in
pollination, predators that eat harmful insects and parasites that lay their eggs
into the insect pest; the parasite eggs hatch and the larvae consumes the pest
larvae or pupae.
Insect control using integrated pest management (IPM) requires
knowledge of the insect life cycle and habits. Questions asked include: Where
do they lay their eggs? How much time does it take to become larvae? How long
are the larvae active before becoming pupae? Where do they stay during these
stages? What are their feeding habits? What species and what part of the plant
do they attack? What are their migratory habits? Knowing this information
allows the IPM person to design a strategy for controlling them.
A major pest such as fruit fl ies requires a preventative approach for its
control, since once the fl ies lay their eggs, the fruits are di cult to market
unless subject to a kill or sterilization treatment. Frequent preharvest sprays
and traps can be used for fruit fl y control, though costly. Fortunately, fruit fl ies
do not generally lay their eggs into unripe fruit, therefore harvesting at early-
ripening stages avoids major fruit fl y injury. Fruit fl ies are a signifi cant insect
problem in fruit export, since the USA, Japan and others have very stringent
quarantine regulations and in many cases require the fruit to be treated
before entry.
Insect traps in the fi eld (Fig. 4.6a) give an indication about the insect
species present and their population size, and this is used to decide if spraying
is necessary. Some traps used have light as an attractant and have either a
pan fi lled with soapy water to reduce surface tension so that the insect sinks
into the water, or kerosene or water with a contact insecticide is used to kill
them. Other traps use a lure, which can be a pheromone or a particular
scent that will make the insect go to the trap and be killed in similar ways.
Another way is the use of attractive plastic sheets with a sticky substance in
the nursery (Fig. 4.6b) and in the fi eld (Fig. 4.6c), which will immobilize and
thus kill the insect.
Among the exclusion methods used are barriers and tall plant fences,
which will not keep insects out but will reduce the number of insects visiting
the fi eld. Anti-insect fabrics exclude insects completely but they are expensive,
although they are used to exclude aphids from papaya trees in some areas (see
Fig. 3.9b). Another exclusion method is bagging the fruit (Fig. 4.6d), which is
very e ective in preventing fruit fl y and other insects from laying eggs.

Tree Management 91
Cultural practices can be used, such as ploughing or discing to kill insects
present in the soil, weed control to remove shelter and an alternative place
to reproduce, and pruning damaged parts and burning them. In the case of
fruit fl ies, all fallen fruit should be buried at least 40–50 cm deep so that if
the larvae evolve into pupae there will be no chance for the fl ies to reach the
surface. High-pressure water sprays can also be used for control, and in other
cases, hand collection of the leaves with larvae that have hatched from eggs or
of larvae shaken from the plants.
Biological control includes the use of predators’ parasites as well as
bacterial and fungal enemies. In many cases insect damage can be solved
by releasing predators or parasites that can be acquired commercially and
released in the orchard. This approach provides a very e ective long-term
biological control. Examples include the e ective control of many scales and
aphid insects in di erent parts of the world. The bacterium Bacillus thuringensis
is used widely to control many leaf-eating larvae and the fungus Beauveria to
control a number of pests such as whitefl y and di erent beetles.
(a)
(c)
(b)
(d)
Fig. 4.6. To protect the trees and fruit, various approaches can be used to reduce
the insect pest levels by use of traps (a), which may include a lure or sticky traps
in the nursery (b) and in the orchard on the trunk (c), and protecting the fruit by
bagging (d).

92 Chapter 4
Chemical control is obtained by using insecticides. Insecticides act by
poisoning the insects that ingest the sprayed plant. The ingestion of the
insecticide can occur by direct contact, inhalation of the toxic vapours or
when systemic products are used that get into the plant sap through either the
leaves or the roots, and as the insect sucks this poisoned sap, it is killed. There
are also repellent substances that keep the insects from attacking the plants;
in some cases they are plants. For chewing insects, products that act through
ingestion or contact are used. For insects in older stems, trunks, roots and
seeds, control is obtained either by fumigation or with a contact insecticide.
For insects sucking aerial or root parts, a contact and a systemic insecticide
can be used.
Most newer insecticides are less harmful to the environment, the farmers
and the consumers. The new products are very specifi c and short-lived in the
fi eld, so that their poisoning e ect is not as long-lasting as it used to be with
the old insecticide compounds. The grower should always make sure what
the best product is for controlling an insect and if it has been approved by the
government or the importer for that species, especially in case of an export
product. For fi eld safety, the requirements as to how soon after spraying
people can re-enter a fi eld and what is the minimum time before harvest that
the insecticide can be applied should be determined. Some friendlier products
include pure soap, oils and plant extracts, which can be very e ective for
some insects.
Mites
Mites belong to a di erent group and are related to spiders. They normally
rasp the underside of the leaves and feed on the sap, damaging the plant and
its ability to photosynthesize. Mites can become more of a problem when
the plants have a shortage of water or fertilizer. Normally they are on the
underside of the leaves, making it more di cult for sprays to reach them.
Their control should start with feeding and watering the plants. Biological
control measures include the use of predatory mites. The chemical approach
includes the use of some fungicides such as sulfur and some dithiocarbamate
fungicides. Specifi c acaricides are based on pyrethroids, and in recent years
many new compounds have been developed, most of them interfering with
mitochondrial activity. For best control, it is often necessary to apply another
product 5 days later to kill the new adults that emerge from the eggs. Rotating
products avoids the build-up of resistance. During spraying it is essential to
cover the underside of the leaf where mites are found.

Tree Management 93
Diseases
Diseases are caused by bacteria, fungi, viruses and other organisms, such as
mycoplasmas and viroids.
Bacteria
These organisms are the cause of moist foul-smelling wounds, necrosis, galls
and wilting, because they can infect the vascular system and clog it. Bacteria
can be controlled with antibiotics such as streptomycin, but these products
are fairly expensive. The measures to reduce their appearance and progress
are to select propagating material that is clean and avoid excess moisture.
Disinfecting any tool before using it on another plant and destroying any
diseased material by burning are other simple techniques.
Fungi
Fungi are the most common cause of plant diseases. For a fungal attack to be
successful it will depend on the status of the plant; the presence of mechanical
breaks in the cuticle, the plant’s susceptibility to the fungal strain, the plant’s
stage of development; the fungal development stage and the environmental
conditions. Relative humidity of 90–100% is optimal for fungal development,
with dew sometimes creating favourable conditions.
All fungi need a certain combination of temperature and relative
humidity to become a problem; hence many fungal diseases can be a serious
problem in some situations and seasons but not in other conditions. Fungal
diseases can be avoided through resistance in a few cases. Well-fertilized
plantings with a good drainage system and careful irrigation to avoid excess of
water will reduce the risk of root diseases. Disinfection of pruning and other
wounds, control of host weeds and spraying with specifi c fungicides all help
in controlling diseases. Preventive applications of fungicides are often used
with some crops, such as papaya against anthracnose, where all stages of fruit
development are present versus application only at certain stages, such as
during mango fl owering.
Fungicides act via contact or systemically, with many products being
available in the market. In most cases, fungicides have to be rotated to avoid
resistance development; this is especially acute for postharvest use. It is
important to check the toxicity of the product and the waiting time before
harvest. Fungicides in general are less toxic than insecticides, and in the last
years several new products have been developed that are more e ective and
also friendlier to the environment. Several older products are still available
that are cheap and e ective. Examples include copper sulfate, copper oxide,
Bordeaux mixture (a copper derivate), sulfur and some oils.

94 Chapter 4
Virus and viroids
Viruses are very simple microscopic agents that possess either RNA or
DNA that is encapsulated in a protein coat. They are capable of replicating
themselves inside the plant using the plant cell’s own metabolism. Viroids
di er from viruses in being composed of only a single strand of ribonucleic
acid but are not protected by a protein coat. Virus diseases are normally
transmitted by contaminated propagation material, such as seeds, cuttings,
layers, budwood or entire plants, hands or tools that are contaminated or by
insect transmission. Insect transmission is probably the most important way
viral diseases are spread, and aphids are one of the most important vectors, as
well as some other insects such as thrips.
Once a plant is infected with a virus, it is not possible to cure that plant.
Sanitation is by removal of the diseased plants and using plants that serve as
host vectors for the virus and insect. Careful disinfection of hands and tools
such as grafting knives, pruning shears or saws is a must. Seed disinfection
can also be e ective with some plants of the Solanaceae family. If an economic
analysis can justify its use, an anti-aphid screen or fabric can be used, as is
done in some papaya fi elds.
Viruses can kill a plant or reduce production and quality of fruit. Some
cause di erent types of leaf spots such as mosaics, while others can cause
leaf yellowing, stunting and curled leaves. Some species will show no obvious
visible symptoms but are still infected. Clean material can be obtained by
meristem culture combined with thermotherapy, micro-grafting, ovule and
nucellar embryo culture. Some viral diseases are not seed-transmitted and
the seeds act as fi lters. Care has to be taken with the plant material taken to
the farm and to keep it as clean as possible. At country level, strict quarantine
is important to avoid the importation of new viruses carried unknowingly
by people.
Nematodes
Nematodes are non-vertebrate animals that can be plant parasites. Most of
them attack the roots, causing galls, wounds, tumours and lesions. Their
feeding reduces plant vigour and yields. Several genera of nematodes –
Meloidogyne (knot nematode), Ditylenchus, Tylenchus and Radopholus – cause
plant diseases, and the last two are parasites of citrus roots.
Nematodes are more common in sandy soils and their control, till
now, has been by the use of fumigants. Fumigants are very poisonous and
the results obtained from their use have been mixed. Once you start on a
fumigation programme you have to continue. Post-plant nematicides are also
available. Other approaches are rotations that involve trap crops, organic
matter addition and soil solarization, which can be used with short-cycle crops
such as pineapple and banana.

Tree Management 95
Vertebrate enemies
Birds, bats, squirrels, rats, monkeys, pigs and other animals, including
elephants, can become a real problem in orchards. These animals cause
damage to the tree trunk and eat the fruit. Birds can devastate a whole crop,
with several methods having been tried and discussed in Chapter 3.
For rodents, metallic plates around the trunks can be used to prevent them
from climbing to the tops or to prevent damage to the bark of the base of the
tree. Poisoned baits or para n blocks containing an attractant and a poison
that are fairly weatherproof are also a good solution. The use of repellents or
trunk protectors can sometimes be useful.
Other less important miscellaneous problems include algae, mosses and
parasite plants. Algae can be controlled with copper.
Integrated pest management (IPM)
This approach involves the use of many of the above procedures and
practices in combination to obtain the best control with the least
environmental impact. A careful analysis of the crop and pests’ development
cycles need to be established to develop a strategy to combat the problem
at its most susceptible phase. Computer systems can assist by analysing
the environmental conditions and predicting the development of certain
problems. IPM potentially can save money, protect the environment and
allow the orchardist to market a product with fewer pesticide residues. For
tropical fruit the market demands products that are of the highest quality,
and without blemishes or insect and disease damage, making it di cult to
avoid the use of some chemicals (Penman et al., 2003).
GIRDLING AND SCORING
Girdling consists of taking o a ring of bark, 4 mm to 3 cm wide, from the
main trunk (Fig. 4.7a), sca old branches or smaller branches to stop the
movement of sugars in the phloem, especially from the canopy to the roots.
This procedure is usually done with two knives or blades that are joined or
welded together to cut through the bark and phloem, which are removed once
the cuts have gone around the whole stem. Sometimes spiral girdling is used,
and semi-girdles of two Cs in opposite positions but at di erent heights of the
branch are used to avoid a complete blocking of the fl ow.
Scoring is a mini-girdle by which the bark of the trunk or branch is cut in
a circumscribing form with a saw blade or a knife. This wound is very narrow
and will take about 3 weeks to heal, while a normal girdle will take about
3–4 months. Scoring is made by orchardists to manage fruit tree size and to
improve yield. However, scoring has variable e ects on vegetative growth,

96 Chapter 4
possibly due to imprecision that is inherent in cutting a tree with a saw or
knife blade.
Girdling and scoring are used in some fruit trees for di erent purposes.
In the case of layers or air layers (marcotts or gootees), a 3 cm-wide ring of
bark is removed at the point just below the zone where new roots are expected
to arise; the interruption of the phloem fl ow stimulates adventitious root
formation above the girdle. Sometimes the branch from which grafting or
budding material is to be obtained is girdled below these buds 2 or 3 weeks
before material is to be removed. The objective is to induce bud swelling, which
results in higher grafting and budding success rates.
Another objective of girdling is to stimulate fl owering. In very hot areas
with no cool or dry period to induce mango fl owering, a 3-cm girdle is done on
a primary branch to induce fl owering. The problem is that 5% of the branches
often die. The girdling technique is also used on avocados, macadamias and
litchis, in order to improve fl owering, fruit set or fruit size. For better fruit
set, girdling or scoring should be done at petal fall, while for larger fruit size
(a)
(d) (e)
(b) (c)
Fig. 4.7. Girdling is used to aid in inducing fl owering (a). To protect young trees
that are susceptible to full sunlight, simple structures such as palm fronds are used
(b), to plastic shade structures (c) and wrapping pineapple fruit with paper to reduce
sunburn (d). Some crops may need to be protected from rainfall (e) during fl owering
and fruit development, with rain shelters built over the crop in the fi eld.

Tree Management 97
girdling is done at the end of the initial small fruit drop. Girdling has been used
to improve avocado fruit size, especially ‘Hass’ (McNeil, 2001). A longer-term
objective is the elimination or modifi cation of biennial bearing in some tropical
and subtropical fruit trees. Litchi girdling is done before fl ush initiation,
around September in the northern hemisphere, to induce more fl owering
and increase fruit yield. Only one of the three or four main branches should
be girdled, in order to avoid the danger of killing the tree. Girdling should be
preceded by a heavy irrigation and fertilization for better results. Normally if
there was a poor crop the previous year, girdling will work, while if there was
a heavy crop, no girdling should be done. It is recommended that girdling or
scoring be done for no more than 3 years in a row. Girdling is not a standard
practice and, in many cases, growers will use it when they have marginal
yields and will forget about this practice when good yields are being obtained.
DEFOLIATION
Most tropical fruit trees are evergreen and usually do not shed their leaves.
An exception is some Spondias species that lose their leaves during the dry
season as a drought-protection mechanism. The trees begin fl owering while
completely bare. Some other trees, such as avocados in the subtropics, might
have a heavy loss of leaves just before fl owering time. Artifi cial defoliation is
practised in di cult-to-graft trees, where the portion of the branch from which
bud-wood is going to be taken is defoliated 2 or 3 weeks prior to collecting the
scions. This defoliation stimulates the lateral buds to start swelling and become
more active, and ensures better budding or grafting success.
Cherimoya and atemoya, being more subtropical, are sometimes classifi ed
as semi-deciduous. The lateral buds of these species are covered by the leaf
petioles (see Fig. 6.1) and are not at the leaf axils, as in most plants. Thus the
leaves have to fall or be removed for the bud to burst. Defoliation is done to
induce uniform sprouting of these buds just before the new growth is about to
start and natural defoliation has not been complete. This is achieved by hand-
stripping or with the application of urea or other defoliants, at the time of
pruning, to induce a new cycle of fl owering and fruiting.
PLANT GROWTH REGULATORS AND OTHER STIMULANTS
Plant regulators have been studied for many years and some practical uses
have been developed. Among the most common uses of plant regulators is the
improvement of rooting of cuttings or air layers with auxin-like compounds
such as indole butyric acid (IBA) or naphthalene acetic acid (NAA). These
auxins improve rooting percentage, accelerate adventitious root emergence
and increase the number of roots a layer or cutting will form.

98 Chapter 4
Some trials have been made to enhance fruit set in cherimoyas and
atemoyas. Auxins will reduce fruit drop while gibberellins will improve fruit
set and growth rates. In some trials seedless fruits of atemoya have been
obtained, but repeated sprays have to be done during the fi rst 2 months and
fruit size is smaller and fl avour is poorer. Similar results have been obtained
with cherimoya and sweetsop. None of these practices are commercially used.
In guava, trials have been done to reduce the number of seeds with auxins
and gibberellic acid; the latter has reduced the number of seeds and enhanced
soluble solids but is not used commercially.
In commercial pineapple production, fl ower induction is carried out to
obtain more uniform fl owering. The plants are sprayed, once they achieve the
right size, with ethylene gas dissolved in water or ethephon (2-chloro-ethyl
phosphonic acid), an ethylene-releasing compound. Also, acetylene generated
from calcium carbide reacting with water can induce fl owering; this product
can be applied dissolved in water as a spray or by putting a little amount of
it in the cup formed by the leaves in the centre of the plant. Since ethylene is
the ripening hormone that plants and fruits produce naturally, many fruits
are treated with ethylene gas or ethephon to hasten and to obtain uniform
ripening. This is done commercially with bananas. For citrus, it helps achieve
better rind colour.
Some growth retardants, such as pachlobutrazol and uniconazole,
which act as gibberellin antagonists, are used commercially to prevent
mango shoots from re-sprouting too soon. This treatment allows the shoot
to achieve a minimum age, so that when it does sprout it is a fl ower panicle
instead of a vegetative shoot. These products have not been approved by the
US government but are used outside of the USA.
Potassium nitrate or ammonium nitrate is used to induce fl owering in
mango. This foliar application treatment is more successful if the shoots have
achieved certain age and are mature. This treatment is more successful in the
tropics than the subtropics. In the case of longan, potassium chlorate can be
applied to the soil to induce fl owering. Dinitro-orto-cresol (DNOC) has been
used to burn mango infl orescences in order to force a second fl owering a few
weeks later, when climate or market conditions are more suited.
POLLINATION
Some fruit species have self-sterility problems and will not produce good yields
in single-variety planting. The species can be completely or partially self-sterile
or the plants can have di erent sexes or because of dichogamy, when there is
no coincidence of the maturation time of the female and male organs of the
fl owers. Often more than one genotype has to be present in the fi eld or artifi cial
pollination has to be done. In other cases, a certain number of male plants
have to be inter-planted among female plants. For avocados that have A and

Tree Management 99
B fl owering habits, there has to be a combination of both types in the orchard
to get proper pollination, fruit set and yields. Macadamia or carambola require
two or three varieties in the orchard to achieve higher yields, since most
varieties are partially self-incompatible and intercrossing results in higher
yields. In carambola, sometimes a branch of the rootstock is left on the tree
to ensure better fruit set. Date palms are dioecious and wind pollinated, and
one male plant is planted for every 10–14 female plants, distributed uniformly
throughout the orchard. Male date infl orescences can be shaken in front of
female infl orescences to achieve better fruit set, and sometimes pure pollen is
obtained and applied to the female fl owers with dust blowers.
The dichogamy in cherimoya, atemoya, soursop and other Annonas makes
it necessary to hand-pollinate either by collecting pollen of fl owers that opened
the day before in glass jars or by collecting pollen from fl owers in the fi eld in
the male stage to apply it to fl owers in the female stage. This can be done with
a No. 4 fi ne paint brush made with camel hair or with a hand mist blower (see
Fig. 6.4) using pollen dissolved in Lycopodium spores to be able to pollinate
more fl owers. In soursop, which has moist pollen and large fl owers, this can be
done with the fi ngers.
Yellow passion fruit is self-sterile and carpenter bees (Xylocopa) have
to be present to get proper fruit set. When bees are absent, hand-pollination
has to be carried out using cloth gloves to collect the pollen from fl owers in
the contiguous plant row to ensure that it comes from a di erent genotype,
since plants come from seed. This is done in the afternoon since fl owers open
at noon.
The presence of bees, carpenter bees or common fl ies is a great help in
achieving better yields. For bee pollinatation, four to six bee hives per hectare
should be in the fi eld at fl owering. In some places, mango trees in fl ower are
sprayed with a solution of manure to attract as many domestic fl ies as possible
to the orchard to improve pollination.
TEMPORARY SHADE, SHELTER AND BAGGING
Some tropical fruit species that originated in forests may benefi t from being
under partial shade during their fi rst years in the fi eld. This is the case for
mangosteen, durian, jackfruit, acahachairu and other species. Semi-shade is
provided by planting certain bushy plants, such as pigeon peas, castor beans,
bananas or small fast-growing trees, around the planting hole or making a
tent-like structure with palm leaves (Fig. 4.7b), branches with leaves or shade
cloth (Fig. 4.7c). This shade will avoid sunburn damage and provide the plants
with less-stressful growing conditions. Some fruits, such as pineapple, are also
susceptible to sunburn and are protected by tying the leaves together over the

100 Chapter 4
fruit, placing straw around the fruit and the use of plastic or paper wrappings
(Fig. 4.7d).
Rain shelters are sometimes constructed to protect a fruit crop from
incessant light rain showers (Fig 4.7e). This can be seen when grapes are
grown in the tropics and mango in Okinawa, to protect the fruit from disease
and for pollination, respectively.
To harvest high-quality, blemish-free fruit, bagging when the fruits
are young is often practised (Fig. 4.7d). Often the fruits are sprayed with a
fungicide, thinned and the remaining selected fruits bagged. The thinning
allows a grower to ensure that each remaining fruit has su cient leaves on
the stem for fruit growth and development. Though there is a cost associated
with this bagging operation, the harvested fruits are blemish free, without
disease and insect damage, and more than 90% are marketable. For fruits
that are not bagged, 50–70% of the harvested fruit maybe marketable and a
smaller percentage will be of the highest grade. Another advantage of bagging
is that it evens out labour needs by moving some of labour requirements from
harvesting to thinning and bagging and thereby o ers more continuous work
for good workers whom you wish to retain.
FURTHER READING
Chandler, W.H. (1958) Evergreen Orchards. Lea and Febiger, Philadelphia, Pennsyl-
vania.
Coronel, R.E. (1983) Promising Fruits of the Philippines. College of Agriculture, University
of the Philippines at Los Baños, Laguna, Philippines.
Morton, J.F. (1987) Fruits of Warm Climates. J. Morton, Miami, Florida.
Popenoe, W. (1974) Manual of Tropical and Subtropical Fruits. Facsimile of the original
1920 edition, Hafner Press, New York.
Samson, J.A. (1986) Tropical Fruits, 2nd edition. Longman, New York.

© CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1 101
(R.E. Paull and O. Duarte)
5
POSTHARVEST TECHNOLOGY
INTRODUCTION
Postharvest handling refers to all the steps that take place at harvest and
from harvest through to when the consumer receives the fruit (Fig. 5.1). The
goal of postharvest handling is to maintain quality. Quality is defi ned as the
absence of defects or degree of excellence and includes appearance, colour,
shape, injuries, fl avour, taste, aroma, nutritional value and being safe for the
consumer (Abbott, 1999; Shewfelt, 1999). Fruit qualities when consumed are
decided in large measure before harvest and depend upon variety grown, crop
management (fertilization, irrigation, etc.), environment (climate, soil) and
other preharvest factors. The objective of postharvest handling is to maintain
quality by preventing mechanical injury, water loss and disease development,
limiting unwanted physiological changes and preventing chemical and
microbial contamination (Cook, 1999).
Farmers, packers, shippers, wholesalers, retailers and consumers fre-
quently have di erent perspectives with regard to quality and often place
di erent emphasis on the di erent components of quality. In research, quality
is related to some intrinsic character(s) (appearance, colour, acids, sugars, etc.)
and how these characters change during handling. The research data give us
information as to how a product should be handled postharvest. The data need
to be complemented with simulated shipping studies and the evaluation of the
product from initial commercial shipments.
POSTHARVEST LOSSES
During postharvest handling, losses frequently occur, referred to as shrink in
the commercial and retail trade. These losses mean a complete loss of all the
resources that went into production. Estimates of tropical fruit postharvest
losses in both developed and developing countries vary widely from 10 to
80%. The losses given in published reports highlight the total postharvest

102 Chapter 5
losses of products but do not consider the loss of quality or nutritional value
and the downgrading that may reduce the price received. Losses may be due
to mechanical injury, physiological damage or pathogens. Once a tree comes
into production, the most expensive cost is the cost of labour to harvest and
to prepare the fruit for market; that in developed countries ranges from ~30
to 80% of the total operating costs for the year. The reduction of losses in a
systematic way requires a knowledge of postharvest physiology, its applied
technical aspect, handling and the appreciation of its biological limitation,
represented as storage potential (Paull et al., 1997).
Harvested by hand – maturity
– grade
Ø
Ø
Ø
Ø
Ø
Ø
Ø
Ø
Ø
Ø
Ø
Ø
Ø
Placed into shoulder bag
Poured into field bin or lug box
Transported to packing house
Water- or hand-unloaded
Culling
Disinfestation treatment
Sprayed with wax and fungicide
Culling
Weight/colour sorting
Hand packed into cartons
Cooled to 10–12
o
C
Loaded into shipping container
Air or sea transport to market
Fig. 5.1. Generalized fruit-handling scheme for tropical fruit.

Postharvest Technology 103
GENERAL CHARACTERISTICS OF TROPICAL FRUIT
Like all fresh fruit and vegetables, tropical fruits have four general
characteristics that need to be understood and considered during postharvest
handling. These characteristics are the reason why postharvest technology is
needed. The characteristics are:
1. All fresh fruits after harvest are still living and respiring.
2. All fresh fruits have a high water content (~90%), and after harvest it is no
longer being replaced from the plant yet they continue to lose water.
3. All fresh fruits are subject to pathogen attack before and after harvest.
4. All fresh fruits are made up of diverse morphological structures, with the
edible tissues varying between di erent fruits, and hence they have di erent
composition and physiology.
The edible fruit tissues in tropical fruits are derived from many tissues, such as
the fl oral receptacle in strawberry and cashew, the fused clustered fl owers in
pineapple, the entire pericarp in tomato, the exocarp and mesocarp in apple
and peach, the mesocarp only in papaya, the endocarp in citrus, the aril tissue
arising from the funicle in litchi and durian, and the seed coat in rambutan
(Fig. 1.1). This diversity needs to be understood as it infl uences how a fruit will
respond to postharvest handling treatments.
The objective of postharvest handling is to minimize adverse changes in
these characteristics, especially respiration, dehydration and pathogen attack.
Losses postharvest are tied directly to these adverse changes, often associated
with poor storage environment and mechanical injury. Mechanical injury,
besides causing unsightly scarring, also increases water loss and susceptibility
to pathogens. The component of the storage environment over which we have
the greatest control postharvest is temperature. Proper storage temperature
and avoiding mechanical injury are the two most important variables in
postharvest handling.
POSTHARVEST PHYSIOLOGY
Tropical fruit physiology does not di er from the basic knowledge gained from
studies of temperate and subtropical fruits. Di erences occur between fruits in
the major substrates involved in ripening, the rate of ripening and senescence,
and in some cases variation in the order in which various components of
ripening occur. The aspects of tropical fruit physiology that make these fruits
unique are:
• their chilling sensitivity;
• the generally more rapid ripening of climacteric tropical fruits when
compared to temperate fruits; and

104 Chapter 5
• the frequent need in postharvest handling to expose them to high
temperatures or other stresses during insect disinfestation prior to export.
Respiration is the conversion of sugars such as glucose into carbon dioxide,
water, chemical energy and heat. The chemical energy (ATP, NADH) is used
to synthesize the molecules needed to keep the cells alive and to grow. The heat
represents the inability of the cell to capture fully all the potential energy in
the sugar. The simple overall equation for glucose respiration highlights the
relationship between storage environment and respiration, which is directly
related to postharvest storage life.
Glucose + Oxygen → Carbon dioxide + Water + Chemical energy + Heat
The respiration rate is decided by the amount of the substrate, in
this equation glucose but it can be starch or fats, and oxygen. The rate of
conversion, indicated by the arrow, is determined by temperature. Lower
storage temperatures lead to a slower rate of respiration and a greater storage
life; the higher the temperature the higher the rate of respiration and the
shorter the storage life. Storage life includes all the time spent in the various
marketing steps from harvest to the consumer.
The initial use of cold to preserve or extend the shelf-life of fresh
commodities in many cultures is lost in antiquity. Examples of the use of cold
for storage of fresh produce range across the use of clamps, cellars, basements,
caves and ice houses. Industries developed around the harvesting of ice in the
winter for use in the summer. The limitations of cold for tropical plants were
well recognized by the 18th century; for example, the Palace of Versailles’
greenhouse was designed to keep tropical plants alive during winter. An
understanding of the range of suitable temperatures for fruits and vegetables
followed the development of reliable calibrated thermometers in the 1700s by
the Dutch instrument maker Gabriel Fahrenheit and the Swedish astronomer
Anders Celsius. The choice and acceptance of common fi xed temperature
points (freezing and boiling points of pure water) led to standardization of
temperature scales.
Climacteric and non-climacteric
Like temperate fruits, tropical fruits can be divided into climacteric and non-
climacteric (Table 5.1). This division is based on the respiratory pattern after
harvest. In climacteric fruits such as banana and papaya, there is generally
a dramatic and rapid change in respiration, ethylene and other quality
characteristics during ripening. In commercial handling, ethylene can lead
to earlier ripening of climacteric fruit but not of non-climacteric fruit. Non-
climacteric fruit such as pineapple and citrus should be already ripe and ready
to eat when harvested since they show little change in respiration and quality
characteristics after harvest.

Postharvest Technology 105
Respiration and ethylene
Tropical fruits vary widely in their respiration rate and ethylene production
(Table 5.2). The rate and production depend upon the stage of ripeness as well
as variety, preharvest environment and culture. Respiration rates also vary
with type and maturity of the fruit and the presence or absence of the plant
growth regulator ethylene.
Respiration varies from <35 mg/kg/h for pineapples to nearly 300 mg/
kg/h for ripening avocados and ripe bananas (Table 5.2). Green bananas have
a much lower respiration rate of about 50 mg/kg/h. Knowledge of the rate of
respiration of di erent fruits is essential in determining the potential heat loads
in refrigerated cold rooms and containers. The ethylene production rate is
also essential information, as its presence can lead to premature ripening and
senescence of fresh commodities. In mixed storage, where fruits and vegetables
are held in the same room or shipping container, the ethylene released from
ripening fruit can lead to rapid yellowing of leafy vegetables.
Respiration rate and storage life are related (Fig. 5.2). Fruits with high
respiration rates have shorter postharvest lives, hence the need to reduce
respiration and thereby increase postharvest life. Temperature management is
the major method of controlling respiration rate, although it is limited in most
tropical fruits by their chilling sensitivity, which leads to injury.
Table 5.1. Classifi cation of selected tropical fl eshy fruits according to their respiratory
pattern.
Climacteric Non-climacteric
Avocado (Persea americana Mill.) Carambola (Averrhoa carambola L.)
Banana/plantain (Musa spp.) Litchi (Litchi chinensis Sonn.)
Breadfruit (Artocarpus altilis Parkins Fosb.) Mangosteen (Garcinia mangostana L.)
Cherimoya (Annona cherimoya Mill.) Mountain apple (Syzygium malaccense
(L.) Merril & Perry)
Durian (Durio zibethinus J. Murr.) Pineapple (Ananas comosus (L.)
Merrill)
Guava (Psidium guajava L.) Rambutan (Nephelium lappaceum L.)
Mango (Mangifera indica L.) Rose apple (Syzygium jambos (L.)
Alston)
Papaya (Carica papaya L.) Star apple (Chrysophyllum cainito L.)
Passion fruit (Passiflora edulis Sims) Surinam cherry (Eugenia uniflora L.)
Sapote (Casimiroa edulis Llave.)
Soursop (Annona muricata L.)
Chiku (Achras sapota L.)

106 Chapter 5
Chilling injury
The ability to use temperature to extend the postharvest life of tropical fruits
is limited by chilling injury. For example, temperate fruits such as apples and
pears can be stored at 0°C for months, while the maximum storage life for
papaya is about 21 days at 10°C (Fig. 5.3). Papaya, when held at less than
10°C, has reduced storage life due to chilling injury (Chen and Paull, 1986).
The symptoms of chilling injury are similar for most commodities and include
pitting, skin darkening, failure to ripen completely and increased susceptibility
to decay. Carambola and the subtropical fruits longan and litchi are crops that
are somewhat resistant to chilling injury, requiring a considerable time (>14
days) at 1°C before injury occurs.
In any discussion of chilling injury, two aspects must be considered:
the storage temperature and the duration or exposure at that temperature.
Although there is not an exact, fruit-specifi c, reciprocal relationship between
temperature and time, it takes a longer time at a higher temperature to develop
injury than at a lower temperature. As storage temperature is lowered from
30°C, the duration of storage life increases and the limitation is fruit ripening
and senescence. Storage life reaches a maximum at 15°C for Brazilian
Table 5.2. Respiration and ethylene production rate of selected tropical fruits at
20°C.
Class
Respiration Ethylene
Range
mg/kg/h Commodity
Range
μl/kg/h Commodity
Very low <35 Pineapple, carambola
Low 35–70 Banana (green), litchi,
papaya, jackfruit,
passion fruit,
mangosteen
0.1–1.0 Pineapple, carambola
Moderate 70–150 Mango, rambutan,
chiku, guava,
durian, lanzone
1.0–10.0 Banana, guava,
mango, plantain,
mangosteen, litchi,
breadfruit, sugar
apple, durian,
rambutan
High 150–300 Avocado, banana
(ripe), sugar apple,
atemoya
10–100 Avocado, papaya,
atemoya, chiku
Very high >300 Soursop >100 Cherimoya, passion
fruit, sapote,
soursop
Vital heat: kJ/tonne/h = mg CO
2
/kg/h × 11. BTU/tonne/h = mg CO
2
/kg/h × 10.4
Postharvest Technology 107
banana, 10–12°C for papaya, 8°C for rambutan and 5°C for carambola (Fig.
5.3). Lowering the temperature further leads to a shorter storage life, but the
limitation changes from being fruit ripening to the development of chilling
injury, as ripening is completely inhibited. Recommendations for optimum
storage of most tropical fruits are just inside the chilling range (8–12°C),
as this allows ripening to be controlled, and, if removed before the chilling
stress threshold is exceeded, the fruit still has a number of days of useful
marketing life as it ripens. Unfortunately, similar data are not available
or are fragmentary for many other tropical fruits. The actual relationship
between storage temperature and duration can vary with cultivar, preharvest
conditions, the stage of ripeness and postharvest treatments.
Moisture loss
Frequently, injury induced by water loss is confused with chilling injury.
Water loss by tropical fruit postharvest is dependent upon the commodity,
cultivar, preharvest conditions, vapour pressure defi cit, wounds, postharvest
heat treatments and the presence of coatings or wraps. Tropical fruits can
be grouped as to water moisture loss rate, from low (coconut) to medium
Fig. 5.2. Relationship between respiration rate and postharvest life of tropical fruits
held at their optimum storage conditions (Paull, 1994).
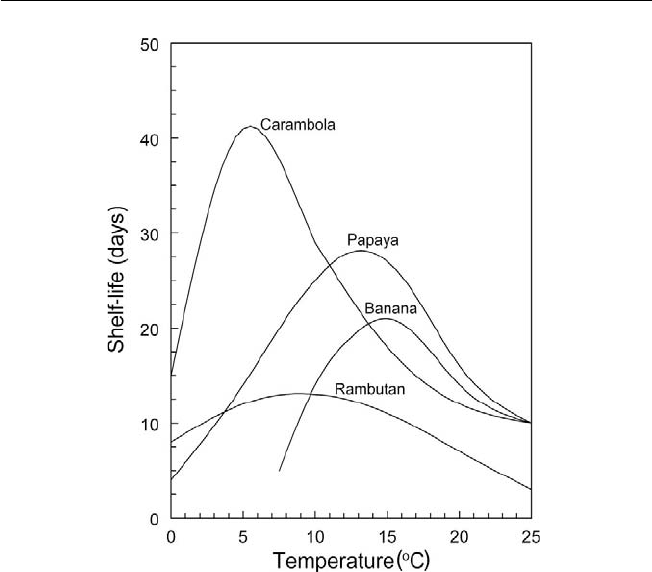
108 Chapter 5
(avocado, banana, pummelo) to high (guava, litchi, mango, papaya, pineapple,
rambutan). On a per unit area basis, the stem scar is frequently the site of
highest water loss, although most water is lost through lenticels, stomata and
skin cuticle. Tropical fruits have water loss rates between about 0.1 and 0.3%/
day/mbar WVPD (water vapour pressure defi cit) (Table 5.3). Fruit having
lost 6–8% of their fully turgid initial weight begin to show signs of mass loss.
Usually the initial sign is skin wrinkling, although skin discoloration is the fi rst
symptom in some fruits. Loss of moisture, besides a ecting overall appearance,
is also an economic loss if fruit is sold by weight.
Nutritional attributes
Composition is a signifi cant quality attribute, and storage temperature
can infl uence vitamins and other nutrients in many fruits and vegetables.
Ninety per cent of the vitamin C in the US diet is derived from fresh fruits
and vegetables. Loss of vitamin C is generally more rapid at higher storage
Fig. 5.3. The shelf-life at various storage temperatures for carambola, rambutan,
Brazilian banana and papaya fruit (O’Hare, 1993, 1995; Paull, 1994).
Postharvest Technology 109
temperatures and slower in acid fruit (pineapple) than more neutral fruit
(avocado, papaya). A 40% loss of vitamin C occurs in tangerines at a higher
storage temperature (7–13°C) for more than 8 weeks, while a negligible loss is
found in lemon at 13°C, although it is signifi cant at 24°C, and no signifi cant
loss in grapefruit held at 8°C and 12°C, with an increase in concentration
following 2-month storage at both temperatures. Vitamin C loss can be
signifi cant during fruit ripening, and the impact of temperature is not
regarded as nutritionally signifi cant.
Flavour and aroma attributes
Flavour is determined largely by the sugar to acid ratios. Changes in these two
components can vary independently and so alter fl avour. Storage temperature
can infl uence the rate and direction of change. Both soluble solids (mainly
sugars) and acidity decline more in grapefruit held at 8°C than at 12°C, while
guavas show no dramatic change during storage at temperatures between 10
and 15°C. The stage of maturity at harvest has a signifi cant e ect on changes
during storage.
Improper storage temperature can lead to signifi cant alteration in the
volatiles produced during ripening. Jonathan apples stored at ~10°C lose the
volatiles butyl, isopentyl and hexyl acetates and alcohols. The rate of loss
is reduced up to 6000-fold when stored at 0°C (Wills and McGlasson, 1970,
1971). Soursop aroma develops during ripening and is lost if the fruit is stored
at 10°C for 2 days. O -fl avours due to ethanol and acetaldehyde in citrus
fruits are frequently associated with chilling injury or long-term storage of
tangerines and grapefruit (Cohen et al., 1990).
Table 5.3. Comparison of water loss from different tropical fruits.
Tropical fruit
Water loss
% loss/day % loss/day/mbar WVPD
Avocado 0.5–1.0
Banana 0.4 0.3
Guava 0.3
Mango 0.1
Papaya Sunset 0.08
Plantain 0.5
Rambutan 0.32
Chiku 0.3

110 Chapter 5
STORAGE POTENTIAL
Storage potential is normally defi ned as the postharvest life of a commodity
held at its optimum storage temperature. This potential is dependent upon
cultivar, preharvest environment and culture, maturity at harvest and storage
conditions. Postharvest life is terminated because of physiological, mechanical
and pathological stress, with associated symptoms such as excessive water
loss, bruising, skin scald, failure to ripen and decay.
The commercial postharvest storage potential for most fresh fruits and
vegetables are given in numerous publications. The values given for storage
life (Table 5.4) should be regarded as the maximum, as they are based upon
laboratory studies using appearance criteria and may not allow for loss of
the other quality criteria, such as texture, nutritional value and fl avour. The
range in storage life for each fruit indicates the variability in the information
available, di erent criteria probably being used to evaluate the end of storage
life, and di erences associated with stationary or transport storage. Often
simulated laboratory studies used do not allow for the vagaries of commercial
handling and storage. An important aspect of this variation is associated with
the retailing phase, where proper temperature maintenance is frequently lost
for various reasons.
Storage temperature
Low temperature has been used to extend the shelf-life of fruits and vegetables
since antiquity (Qi, 1982; Paull, 1993, 1999). The additional benefi t of low-
temperature storage is in protecting non-appearance quality attributes,
such as texture, nutrition, aroma and fl avour. Time of day when harvest
is performed can infl uence storage life. In addition, delays in cooling after
harvest can reduce fruit storage life and quality. In commercial handling, shelf-
life of commodities may vary greatly from laboratory studies. The distribution
chain rarely has the facilities to store each commodity under ideal conditions
and requires handlers to make compromises as to the choice of temperature
and relative humidity (RH). These choices can lead to physiological stress and
loss of storage life and quality. This limitation, especially late in the handling
chain during retailing, requires all participants in the distribution chain to
increase their understanding of the need to improve management of handling,
temperature and RH, to limit losses in quality.
If cooling is delayed after harvest, storage life can be signifi cantly reduced.
Simple shading, if there is a delay, can limit loss of quality. An unshaded
commodity directly exposed to the sun can rapidly warm to 10°C higher than
the ambient air. A 2-h delay after harvest can mean a loss of marketable
strawberries of about 93–80%, and 6 h leads to an increased loss of ascorbic
acid, soluble solids, fructose, glucose and sucrose, and titratable acidity and

Postharvest Technology 111
fi rmness. Most tropical fruits do not show as rapid change in quality, but delays
in cooling should be kept to a minimum. Apples that have a long postharvest
life and lower rate of respiration should be cooled within 3 days of harvest,
to prevent loss of apple fi rmness and acidity during storage for 7.5 months at
~3°C. For most climacteric tropical commodities, the delay in cooling allows
continued ripening and overall loss of storage potential. This is a problem,
especially for commodities such as papaya and atemoya, which have a short
shelf-life (10–14 days). In general, the higher the metabolic rate (respiration)
the shorter the overall shelf-life, the greater the impact of delayed cooling on
preserving quality (Fig. 5.2).
Fresh fruits and vegetables probably receive the greatest temperature abuse
at the retail level (LeBlanc et al., 1996). Mean temperatures of display cases
used for fruits and vegetable are ca. 8°C. The majority of those commodities
that should have been stored at <4°C are above the recommended temperature
range. The same percentage is found for commodities that should have been
held >12°C.
Table 5.4. Recommended storage temperature and storage potential as postharvest
life of selected tropical fruits. Data extracted from commercial publications.
Fruit
Optimum storage
temperature (
o
C)
Relative humidity
(%)
Postharvest life
at optimum
temperature (days)
Acerola 0 85–90 50–58
Atemoya 13 85–90 28–42
Avocado Mexican 5 85–90 14–28
West Indian 10 85–90 14–28
Banana 14 90–95 7–28
Breadfruit 13 90–95 14–40
Carambola 1 90–95 21–28
Cherimoya 13 85–90 14–28
Durian 4 85–90 42–56
Guava 10 90–95 14–21
Jackfruit 13 90–95 14–45
Langsat 11 90–95 10–15
Litchi 1 90–95 21–35
Longan 2 90–95 21–35
Mango 10–12 85–90 14–25
Mangosteen 13 85–90 14–25
Papaya 8–12 85–90 7–21
Passion fruit 12 85–90 14–21
Pineapple 10 90–95 14–36
Rambutan 12 90–95 7–21
Sweetsop 7 85–90 28

112 Chapter 5
Storage relative humidity
Relative humidity is the percentage that air is saturated with water vapour
at a particular temperature and pressure. At low temperatures such as 0°C,
the amount of water vapour required to saturate the air is fi vefold less than
at 25°C (Ga rey, 1978). This di erence in amount of water vapour at lower
temperatures means that condensation is a greater problem on fruit when
small changes in temperature occur. Relative humidity is used as a handy
measure of water vapour pressure (WVP) in air relative to standard pressure.
Psychrometric charts give a graphical representation of the relationship
between temperature, RH and WVP in moist air. The water vapour pressure
defi cit (WVPD) is the di erence between actual vapour pressure and the
saturated vapour pressure and determines the rate of evaporation from a fresh
commodity at the same temperature (Paull, 1999).
Relative humidity in storage rooms is dependent upon the surface area
of the refrigeration evaporator coil and the temperature di erence between
the coil and the air, along with air exchange rates, temperature distribution
in the room, commodity and packing material used. Practical di culties are
encountered in maintaining RH in large storage rooms within a narrow range
at high relative humidity. To illustrate the di culty, to maintain 95% RH at
0°C, the mean temperature di erential between the air and the evaporator
must be ~0.5°C. A measurable and controllable temperature di erence
of ~1°C is available at 90% RH. These small di erences test the limits of
sensitivity needed to measure temperatures to this degree of accuracy. At high
RH, a small fl uctuation in temperature (<0.5°C) can result in condensation
on cool surfaces. Poor air distribution could mean that air at 0°C, 95%
RH from the coil would be 70% RH in an area at 5°C. Fibreboard and wood
absorb water and may decrease RH in a room. A fi breboard box held at 50%
RH has a moisture content of 7% (dry mass basis); at 90% RH, the moisture
content would be 16%. High RH will not prevent moisture loss if the product
temperature is not near the air temperature. Newer refrigeration controls,
more rugged humidity detectors and humidifi cation technologies have
increased the ability to vary both temperature and RH and reduce energy
consumption.
The nature of the commodity evaporative surface is determined by
commodity type and cultivar, and both have a major infl uence on the rate
of evaporation. Surface area to volume ratios are a signifi cant commodity
factor infl uencing evaporation (Ben-Yehoshua, 1987). The ratio varies
widely for di erent commodities: individual edible leaves have 50–100 cm
2
/
cm
3
, strawberries 2–5 cm
2
/cm
3
and bananas 0.5–1.5 cm
2
/cm
3
. In order to
compare fresh commodities, it is necessary to incorporate these factors into
loss units such as %/day/mPa WVPD (Table 5.3). This enables the time needed
to reach a permissible water loss as a percentage of the original mass at which
a commodity becomes unmarketable or has to be sold for a lower price to be
calculated.

Postharvest Technology 113
Recommendations on RH have been made for most commodities
(Table 5.4), with variation in the recommendations from di erent sources.
These di erences in recommendations may refl ect general conclusions for
a particular commodity group or specifi c observations. There is fairly good
agreement as to the degree of water (or mass) loss from initial fi eld condition
before a commodity shows wilting symptoms (Robinson et al., 1975; Sastry et
al., 1978). The wilting symptoms most often reported are less gloss, wrinkling
or fl accidness. Maximum permissible losses can vary from ~10% for onions,
~7% for papayas and other tropical fruit to ~3% for lettuce. The corresponding
loss rates are 0.02% initial mass/day/mbar WVPD: 3.6, 0.5 and 7.5,
respectively (Table 5.3). This loss can be modifi ed by postharvest handling
practices such as packaging, waxing and wax removal during washing. This
loss criterion occurs at about double the loss required for the fi rst visible
symptoms to appear and integrates the overall loss rate, which normally
declines with storage. Mass loss is linear and related to WVPD; hence loss can
be reduced by lowering the WVPD via reducing air temperature, increasing
humidity or creating a barrier to water loss. High air fl ow may be necessary
during cooling; once cool, RH is crucial in determining rate of moisture loss.
Moisture loss from pre-climacteric avocado, mango, banana, plantains
and pear fruit hastens ripening (Fig. 5.4). There is a linear negative
relationship between water loss and green life of avocado, banana and mango
(Fig. 5.4). For example, the green life of bananas is about 22 days at 20°C with
95% RH and ~16 days at 13% RH. There is also about a 50% reduction in the
postharvest life of custard apple at low RH and 65% reduction for plantains.
There are numerous studies relating reduced chilling injury symptom
development in sensitive tropical commodities to high RH. Lemon chilling
injury symptoms in the peel are reduced at high RH, though RH had no impact
on fl esh chilling injury symptoms.
Mechanical injury
Mechanical injury causes unsightly blemishes and favours decay by breaking
the cuticle, which also favours water loss. Three types of mechanical injury
are recognized as occurring preharvest, at harvest and during handling; they
are impact, compression and abrasion. Often personnel handling fruit do
not recognize that they are causing injury as the symptoms do not become
obvious until the fruit ripens. Examples of this would be abrasion injury to
green bananas, which turn black as the fruit ripens, and in papaya, which
appears as sunken green areas. Impact and compression injury is sometimes
not visible on the skin but is recognized as water-soaked areas in the fl esh. The
water-soaked areas are often only on one side of the fruit and are caused by the
kinetic energy of the impact being dissipated in the fruit cells, which rupture.
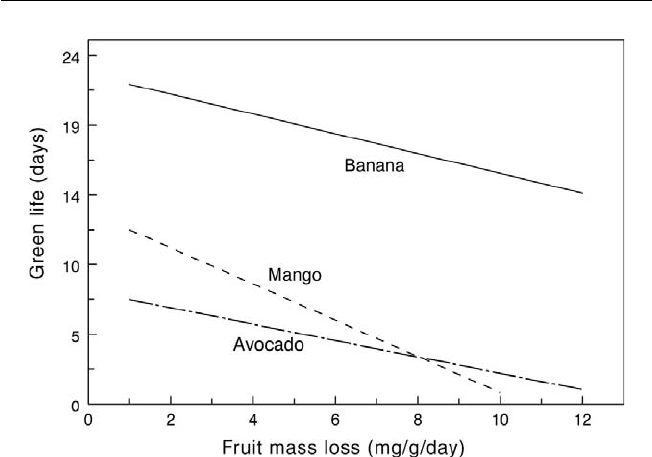
114 Chapter 5
Careful handling is the most e ective method of limiting injury. This
involves careful harvesting practices, placing fruit in buckets or boxes,
ensuring that the bags and buckets are clean and free of dirt and grit that can
abrade the skin.
Controlled and modifi ed atmosphere storage
During respiration, the other substrate besides sugars is oxygen, while the
major product is carbon dioxide. Controlled and modifi ed atmosphere storage
methods normally reduce the oxygen levels to slow respiration and increase
carbon dioxide levels to slow conversion of substrates. Controlled atmosphere
(CA) storage is a more controlled, active regulation of oxygen and carbon
dioxide levels, while modifi ed atmosphere (MA) storage is more passive and
relies on gas exchange. Both CA and MA methods are supplements to good
temperature management and do not replace temperature management
(Kader, 1993; Yahia, 1998).
Generally oxygen levels are reduced to 3–4% (Table 5.5); below 3%,
frequently the fruit may become anaerobic and o -fl avours will develop and
alcohol production occurs. Carbon dioxide is increased to 4–10%, though
greater care is needed as o -odours and fl avours frequently develop. The
potential benefi ts of MA and CA for tropical crops depend on the type of
crop, pre- and postharvest handling methods and length of shipping period.
Frequently, exported tropical fruits have a number of additional postharvest
Fig. 5.4. Relationship between water loss and green-life of tropical fruits.

Postharvest Technology 115
constraints such as chilling sensitivity, short postharvest life, and disease and
insect infestations.
It is essential to assure a su ciently long postharvest life for tropical
fruits to be able to be distributed in distant markets. The minimum times
required for sea freight from eastern Australia to South-east Asia, Japan and
North America, and Europe are 3, 4 and 6 weeks, respectively. Minimum
shipping periods from Mexico to Europe and Japan are about 18 and 21 days,
respectively. In addition to better postharvest handling systems (i.e. optimum
harvesting time, control of insects and diseases, and the use of proper
postharvest temperature management), MA and CA can be of major benefi t to
preserve quality and to prolong postharvest life.
For example, MA has been used for more than 30 years during banana
transport from Central America to the rest of the world. Advances in MA/CA
technology during transport have been implemented, and the technology is
much more promising for tropical fruits. Atmospheres for transport have been
developed that are passive (MA), semi-active or active (CA). The semi-active
systems are those in which a selected atmosphere is created in the container
immediately after loading, after which the container is closed and no further
control of oxygen and carbon dioxide levels is carried out. Atmospheres are
created by an oxygen sensor telling a valve to open or close to allow more air to
enter the container to increase the oxygen level, or nitrogen to reduce oxygen
levels. Carbon dioxide can also be monitored and a valve controls the amount
of air entering to prevent the carbon dioxide from getting higher than the
desired level.
The major question is whether the additional costs of applying the
MA or CA treatments are recovered in the increased storage life and quality
Table 5.5. Conclusions and recommendations on the use of MA/CA for some
tropical fruit crops.
Fruit Intended use
Atmosphere
Degree of benefi ts%O
2
%CO
2
Avocado Transport, MAP 2–5 3–10 Good
Banana Transport, MAP, storage 2.5 2.5 Excellent
Cherimoya Transport, storage 2–5 3–10 Good
Durian Transport 3–5 10–20 Fair
Lanzon Transport 5 0 Fair
Litchi Packaging, transport 5 20 Good
Mango Transport 3–5 5–10 Good
Mangosteen Transport 2 10 Fair
Papaya Transport 2–5 5–8 Fair
Pineapple Transport 2–5 5–10 Not determined
Sapodilla Not determined 5 10 Fair
Sweetsop Not determined 5 10 Fair

116 Chapter 5
maintenance. Shipping containers with atmosphere-monitoring capabilities
are in service, providing greater opportunities for CA storage of tropical
commodities when the cost can be justifi ed.
POSTHARVEST HANDLING
Most fresh fruits and vegetables are harvested by hand, sometimes with
mechanical aids. Hand-harvesting is accurate, causes minimum damage,
allows fl exible use of labour and requires minimal capital investment in
equipment. When fruit is hand-harvested, the supervision of fi eld labour is
greater, to ensure that the correct fruit maturity is harvested and diseased
and contaminated fruits are not collected (Thompson, 1996). The trend in
tropical fruit production is to maintain tree height to less than 3 m so that all
fi eld operation, including harvesting, can be done from the ground. When
mechanical harvesting aids are used in the fi eld, such as ladders and moving
harvest platforms, harvest rate is slower and has a higher potential for worker
injury and hence liability. Mechanical harvesting is used for fruit that is
destined for processing as it is more rapid and requires less management,
though it causes more mechanical injury.
During collection in the fi eld and transport to the packing area, fruit
should be protected from mechanical injury and high temperatures due to
direct exposure to the sun. Upon arrival at the packing area, the fruits are
normally washed, cleaned and graded, and unsuitable fruit culled; sometimes
fruits are coated with waxes or other barrier compounds to reduce water loss
and improve appearance, and the fruit is treated for disease control, often in
the coating materials, before being packed into shipping cartons. The washing
is done to remove dust and other contaminants. Potable water should be
used, with chlorine or another sanitizer added to reduce cross-contamination
of fruit. Chlorine is the most common sanitizer, available in a number of
formulations, and requires 100–150 ppm of chlorine ion at pH 6–7.5 and
a contact time of 1–2 min. Fruit should be rinsed with potable water upon
removal from the chlorine solution.
National, international and commercial grade standards are used to cull
fruit that does not meet the standards. The standard used will depend upon the
market requirement. The grade standards include a description of maturity,
skin colour, fi rmness, shape and size, and freedom from decay, defects,
mechanical injury and physiological disorders. The standards also include
percentage of o -grade fruit allowed in a batch of fruit. Skin colour charts are
published for many fruits, such as banana, pineapple and papaya.
Packing into cartons or other containers (clamshell) is done to aid in
marketing. The package needs to meet the following requirements: protect the
fruit from injury, allow temperature management, protect the fruit from water

Postharvest Technology 117
loss and facilitate special treatments. Most tropical fruits are hand-packed
either by weight or by a fi xed number per container.
Postharvest cooling is carried out to reduce the rate of respiration and
ethylene e ects. Cooling also reduces water loss from the fruit, rot organism
growth and the development of mechanical injury symptoms. The most
common method used is room cooling, where fruits are placed in a refrigerated
room that is above the chilling injury threshold for the fruit. Room cooling
is slow and, if the cartons are stacked on a pallet, can take days to reach the
room temperature setting. Forced air cooling draws refrigerated cold air
through the cartons themselves and thereby increases the rate of cooling.
Another less frequently used method for tropical fruit is hydro-cooling, where
cold water is used as the coolant instead of air and is faster.
POSTHARVEST INSECT DISINFESTATION
Postharvest treatments are required to disinfest economically important host
fruit, vegetables, dried fruits and vegetables, nuts, fl owers and ornamentals
of insect pests before they are moved through marketing channels to areas
where the pests do not occur (Paull and Armstrong, 1994). Examples of these
pests are various tephritid fruit fl ies and mango seed weevil. Alternatively, the
importing country has a ‘zero tolerance’ for all live insects, whether or not they
are economically important. While there are many insects and arthropods of
quarantine importance, fruit fl ies represents a major group of destructive pests
that attack a wide range of fruit, including most tropical fruit. Disinfestation
treatment technologies and strategies include pest-free zones, non-host status,
fumigation, irradiation, insecticides, heat and cold treatments, controlled
or modifi ed atmospheres, and combinations of these treatment methods.
Inspection by regulatory personnel is required to ensure quarantine treatment
procedures and regulations, including handling requirements to preclude
reinfestation after treatment, are followed.
Many of these disinfestation treatments cause damage to the commodity
that limits postharvest life and reduces quality (Paull and Chen, 2000).
Research e orts are directed to the use of physical disinfestation treatments,
such as heat, cold and irradiation. Such physical disinfestation treatments
are non-polluting and they leave no toxic residues. No single quarantine
treatment or system can be expected to work equally against all insects
or for all host fruits, and the response to any treatment can vary greatly
between commodities.
POSTHARVEST DISEASE
During growth and development fruits are continually being attacked by
pathogens. However, disease is only seen when the fruit is injured and the

118 Chapter 5
right environmental conditions exist (Snowdon, 1990). Some infections, such
as anthracnose, are called latent infections, as the initial infection occurs with
little pathogen growth while the fruit is green and only becomes visible as the
fruit ripens and the fruit’s natural resistance mechanisms are breaking down.
The major factors that determine whether a pathogen attack will be successful
in germinating and colonizing the fruit are the cuticle, which acts as a
morphological barrier, the presence of stomata and lenticels as a pathway for
entry, and the broken peduncle. Other factors postharvest are the physiological
state of the fruit such as having been subject to chilling temperatures, the
presence of inhibitors that frequently decline during ripening and the storage
temperature. As with respiration, lower temperature reduces pathogen growth
and limits disease development.
Control methods include preharvest and postharvest disease control using
fungicides. The loss of postharvest fungicides has led to renewed interest and
the use of alternative methods to control disease postharvest. The approaches
have included heat treatments such as the 49°C for 20 min hot water dip
for papaya and the use of natural biostatic compounds and chemicals such
as sodium bicarbonate. These approaches are often not as e ective as some
fungicides and need to be used in a systems approach that includes more
e ective sanitation.
FOOD SAFETY
Since fresh fruits and vegetables are raw agricultural products grown in fi elds,
they can be expected to be in contact with a wide range of microorganisms
(Brackett, 1999; Zagory, 1999). Many are plant pathogens and other
microorganisms, but human pathogens at low levels can also be expected.
Recent food safety outbreaks have raised concerns about human pathogen
survival and proliferation on fresh produce. The increase in outbreaks could
refl ect better reporting, more complex handling systems, multiple sources,
greater opportunities for contamination and wide distribution of potentially
contaminated products. The US Centers for Disease Control reported that,
from 1983 to 1992, only 3% of traceable outbreaks of food-borne diseases
were associated with source and supply. Most were associated with postharvest
handling (77%) and the home (20%). These incidents occurred primarily
during the warmer summer months. Poor sanitation and improper hygiene
were the major source.
The management of temperature and relative humidity (RH) used during
shipping and storage is a critical component in maintaining a safe food supply.
Temperature and relative humidity are two major criteria used to defi ne
critical limits in monitoring programmes associated with the hazard analysis
and critical control point (HACCP) system. HACCP is a preventive quality-
assurance system and is required so that safety programmes can be properly

Postharvest Technology 119
implemented. The HACCP system involves an evaluation of every step in the
handling chain by the di erent individuals handling a commodity from harvest
to the consumer. The critical points where contamination could occur are
identifi ed and procedures developed to ensure that safety is maintained. The
procedures are called standard operating procedures (SOP), and responsibility
is assigned to someone in authority to ensure that these SOPs are carried out.
Such safety programmes involve a number of principles, including developing
a plan to carry out inspections, good record-keeping and steps to ensure safety
throughout handling, shipping and storage, and monitoring to ensure that
procedures are being implemented, and, most importantly, assignment of
responsibility.
In the USA, major retailers and food service chains are putting restrictions
on and demanding compliance by suppliers to avoid food microbial
contamination. These require self- and third-party audits, as the buyers set
the terms. The objective is to verify use of good agricultural practices (GAP)
and good manufacturing practices (GMP). Voluntary guidelines have been
issued by the US Food and Drug Administration (FDA). The document Guide
to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables can
be found on the FDA web site (www.foodsafety.gov/~dms/prodguid.html).
Third-party audits are performed by private companies, and some of their
web sites contain information as to how audits are carried out and the
postharvest practices that are evaluated. In Europe, GlobalGAP (http://www.
globalgap.org/cms/front_content.php?client=1&changelang=1&parent=
&subid=&idcat=9) has been developed by large retailers and includes social
and environmental aspects. Most of the guidelines are common sense, and
are possibly already being done but not being monitored and recorded. A
comprehensive food safety programme based upon risk assessment includes
employee training, audits, food safety record-keeping and a senior manager in
charge of the programme.
The goal of all food safety programmes is the prevention of contamination.
The harvest considerations to prevent contamination are only picking dry
fruit, leaving fruit that has bird droppings on them, use of clean bags or
buckets for collection, training workers on the importance of hand-washing
and cooling the product quickly. Further steps to reduce contamination are
excluding animals from the orchards and packing facilities and the use of
potable and chlorinated water to prevent cross-contamination. Clean bins and
containers should be used to transport fruit.
FRUIT RIPENING
Ethylene gas, which is a natural plant growth regulator, is used postharvest
to ripen fruit. Fruit ripening is carried out with ethylene (~1000 ppm) under
controlled temperature and relative humidity conditions. The objective of

120 Chapter 5
ripening with ethylene is to place fruit on the retail shelf at the desired stage
of ripeness for the consumer. Banana is most commonly ripened in this way
at terminal markets or by wholesalers. Another fruit that is sometimes ripened
with ethylene is avocado. Pineapple, a non-climacteric fruit, is treated with an
ethylene-generating compound about 6 days before harvest or postharvest,
to enhance the degreening of the skin; the treatment has little e ect on
other aspects of fruit quality. Papaya can be induced to ripen faster with
ethylene treatment, but it is not practised commercially as it leaves little time
for retailing.
In the absence of ethylene gas, acetylene can be used at a slightly higher
dose. Calcium carbide, which in the presence of water releases acetylene, is
widely used in developing countries. Fruit can also be ripened by adding an
already-ripening fruit, such as passion fruit or avocado, which generates
higher amounts of ethylene. Crushed leaves of some species also generate a lot
of ethylene and these are used for fruit ripening.
ORGANIC TROPICAL FRUIT
Organic standards place additional requirements on those in the postharvest
chain handling organic fruit. The requirements relate specifi cally to what
chemicals can come into contact with the organic commodity. There are
limitations on the use of chlorine in the wash water to prevent cross-
contamination and the types of chemicals that can be used as cleaners and
sanitizers. Synthetic fungicides and many wax coating materials are not
allowed and alternative natural products are used. Care is also needed during
shipping and storage to ensure that organic products are clearly marked and
no direct contact occurs with conventional products.
LOGISTICS
During the movement of fresh products to market (Fig. 5.1), wholesalers
and retailers frequently do not have enough facilities set to the optimum
conditions for each commodity. Inventory management and marketing largely
determines how a product will be handled (Prussia, 2000). These limitations
are especially true for specialty commodities, such as minor tropical fruits,
handled in small quantities.
A major logistical issue is to maintain quality while integrating improve-
ment in technology into the supply chain (Prussia and Shewfelt, 1993;
Thompson, 1996; Hewett, 2003). The integration needs to manage the
available resources at all steps in the chain (Epperson and Estes, 1999). The
most important resource is the human capital, who can jeopardize safety and
quality and who manage the chain so as to reduce losses (shrink). This requires

Postharvest Technology 121
an understanding that the value of a commodity increases as the product
moves through the chain to the consumer and that money and information
should move freely back from the consumer to the producer. Between the
extremes of farmers’ markets and street stalls in developing countries and
almost vertical-integration producers/shipper/supermarkets, information and
money fl ow must occur. Frequent disruption of this communication fl ow can
occur when there are numerous suppliers and buyers separated by wholesale
and spot markets.
In addition to information fl ow, the reduction in seasonality of many
commodities by global sourcing and new production technologies has
heightened the need to manage inventories. No longer is a fi rst-in-fi rst-
out credo su cient. Supermarkets strive to set quality standards, delivery
schedules and minimize inventory at both the distribution warehouse and
the individual outlets. This is achieved, in part, by forward contracts, so that
inventory and quality can be managed, storage costs minimized and in-store
losses reduced.
The management’s practices used at all the steps from harvest to the
consumer (Fig. 5.4) play a crucial role in maintaining quality and safety of
tropical fruit. The requirements to maintain quality and safety are the same
in developed and developing markets. In most cases, application of current
knowledge will bring the handling system to a high level of quality and
safety assurance. A major limitation for small-scale operations is often lack
of resources, infrastructure, marketing systems and the availability and
understanding of the tropical fruits’ handling requirements. These limitations
can be overcome by consolidation, using marketing companies, associations
and cooperatives.
In all logistic chains, and particularly for tropical fruit handling,
managers play a central role. This role is equally as important in developing
markets, where labour costs maybe much lower than in developed markets.
E ective supervisor and worker training, and retraining, as to their role in
maintaining fruit quality and safety, is crucial. The role and the responsibilities
of supervisors and workers must be stressed to maintain quality and safety,
and worker productivity and development of problem-solving skills. Frequent
fruit-handling logistics problems are seen when supervisors do not have
problem-solving skills or the authority for implementation of changes. Often
supervisors must ask permission from the owner or a few owner-trusted
persons to make changes in the postharvest handling of a specifi c shipment,
thus reducing worker productivity.
CONCLUSIONS
The quality of fruit sold to consumers and the measure of postharvest success
depends upon fi ve factors. The fi ve factors are the initial fruit quality at

122 Chapter 5
harvest, which is set by how it is grown; the maturity of the harvested fruit;
careful handling to avoid injury; proper storage environment (temperature,
relative humidity, atmosphere); and sanitation to prevent disease and human
pathogen contamination. Prevention of mechanical injury, temperature
maintenance and sanitation are crucial.
FURTHER READING
Arpaia, M.L. (1993) Preharvest factors infl uence the postharvest fruit quality of tropical
and subtropical fruit. HortScience 29, 982–985.
Kader, A.A. (2002) Postharvest Technology of Horticultural Crops, 3rd edition. University
of California Agriculture & Natural Resources, Oakland, California.
Kays, S.J. and Paull R.E. (2004) Postharvest Biology. Exon Press, Athens, Georgia.
Wills, R., McGlasson, B., Graham, D. and Joyce, D. (2007) Postharvest: an Introduction to
the Physiology and Handling of Fruit, Vegetables and Ornamentals. CABI Publishing,
Wallingford, UK.

© CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1 123
(R.E. Paull and O. Duarte)
6
ANNONAS: CHERIMOYA, ATEMOYA
AND SWEETSOP
Three Annona species will be covered in this chapter. One species is thought
to have originated in Central America (sweetsop or sugar apple, Annona
squamosa), while cherimoya (Annona cherimola) origins are the highland
plateaus of the Andes. The third is not a species but a cross between sweetsop
and cherimoya and called atemoya (A. squamosa × A. cherimola), the ‘ate’
from the Brazilian word for sweetsop and ‘moya’ from cherimoya. All three
are found cultivated or naturalized throughout the tropics. Cherimoya is
better adapted to cooler, drier and subtropical climates, and sweetsop in moist,
tropical climates and in drier, subtropical climates.
BOTANY
Family
Annonaceae, in the Order Magnoliales, commonly referred to as the custard
apple family, consists of about 75 genera, widely distributed. The family is in
the basal group of the dicots. Some are grown as ornamentals, while only four
genera produce edible fruit. This chapter will focus on the three Annona species
widely grown for fruit.
Important genera and species
The genus Annona is the most important, being the second largest genera in
the family, and among its 100 or more species, seven species and one hybrid
are grown commercially. All are native to the American tropics. Leaves are
alternate, simple and entire, and fl owers may be solitary or in clusters, with
two series of three thick and fl eshy petals. The other closely related genus with
some commercial fruit is Rollinia.

124 Chapter 6
The important commercial species are:
A. cherimola Mill. Cherimoya (English); chirimoya (Spanish);
cherimolier (French); anona (Mexican); noina
ostrelia (Thai); chirimoya (German)
A. squamosa L. Sugar apple, sweetsop (English); anon, riñón
(Spanish); noina (Thai); nona seri kaya (Malay);
custard apple (Indian)
A. squamosa × A. cherimola Atemoya, custard apple (English); atemoya
(Spanish)
Annona diversifolia Sa . Ilama (English, Spanish)
Annona glabra L. Pond apple (English); mamon (Philippine)
Annona montana Magfady Mountain soursop (English); guanábana
cimarrona (Spanish)
Annona muricata L. Soursop (English); guanabana, atio
(Philippines); guanábana, catoche (Spanish);
durian belanda, nona sri kaya (Malay); nangka
belanda (Indonesian); thurian-khaak (Thai);
sitaphal (Indian); fruta de conde, graviola
(Brazilian); zapote agrio, catoche (Mexican);
corossol épineux (French)
Annona reticulata L. Custard apple, bullock’s heart (English); anon,
anona, corazón (Spanish)
Rollinia orthopetala R.DC. Biriba (Brazilian).
Area of origin and distribution
Cherimoya (A. cherimola Mill) is thought to have originated in the highlands of
Ecuador and Peru. The antiquity of the fruit is attested to by ancient artifacts
shaped in the form of the fruit in Peru. Distribution through Central America
and Mexico probably occurred at an early date, as it has become naturalized in
the cool highland areas. Distribution continued from Mexico to the Caribbean
islands and then to the African coast and the Mediterranean. Introduction to
Africa and the Far East is attributed to early Spanish navigators.
The cherimoya is considered the best of the Annonas and is cultivated in
subtropical regions and in the tropical highlands. In most areas, it is grown
as a garden tree or as part of a subsistence farming system. Commercial
production occurs in Spain, Bolivia, Chile, Peru and New Zealand (George
and Paull, 2008). Experience has shown that the California coastal regions
are more conducive to cherimoya production, having higher relative humidity
(RH) (70–80%) in spring and summer than in the interior valleys, where the
RH can drop to less than 40% during the hotter part of the day in summer.
The tree grows to about 7.3 m; it is vigorous when young but tends to
decline in growth with age. New buds cannot sprout until the leaves have
been shed, as the leaf bases grow over the axillary buds, as in sweetsop and
Annonas: Cherimoya, Atemoya and Sweetsop 125
atemoya (Fig. 6.1). Leaves are 10–25.4 cm long, light green and arranged
alternately. Leaves tend to fall before spring, and many people call it a semi-
deciduous plant.
The sweetsop (A. squamosa L.), also called sugar apple, probably originated
in the Caribbean region and is the most widely distributed and also the most
commonly grown Annona species in the tropical regions of the Americas,
Africa, Asia and the Pacifi c. It is less tolerant of cold temperatures than
the cherimoya but more tolerant than the soursop, as it is found thriving
in subtropical areas, such as the coastal areas of south Florida. The fruit
is frequently found in village markets but has not shown any potential for
large commercial cultivation, due to the small fruit size, frequent cracking at
maturity and poor shelf-life. It is intensively cultivated in Taiwan, where about
5000 ha are planted, producing around 50,000 tonnes; the peak harvest is
between July and March
The tree is normally smaller than the cherimoya, attaining heights of
3.0–4.6 m, with slender branches. The leaves are oblong–lanceolate, narrower
than those of the cherimoya and 10–15 cm long (Fig. 6.1). All leaves are shed
before new shoots appear. Flowers are axillary, in clusters of two to four on
leafy shoots (Fig. 6.1). Fruit set is better than in the cherimoya.
The fruit is more nearly heart-shaped, 5–10 cm in diameter. It is yellowish-
green in colour, but a purple-fruited variant is also known. The exterior parts
of adjacent carpels are not completely fused, and these rounded protuberances
3 mm
Fig. 6.1. Leaves, axillary buds and fl ower of sweetsop. The fi gure shows the buried
nature of axillary buds, which can be vegetative or a mixed fl ower and vegetative
bud. The leaf must abscise before the bud can develop, the same as in atemoya and
cherimoya.
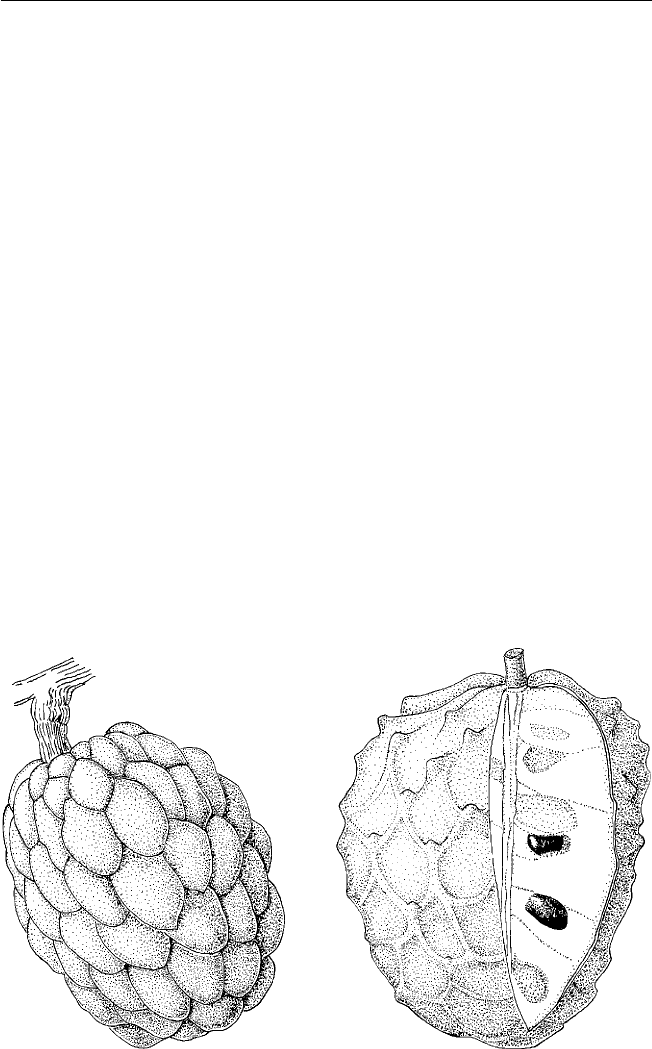
126 Chapter 6
frequently separate, exposing the white fl esh upon ripening (Fig. 6.2). The
fruit matures during the summer although production can extend for several
months.
Atemoya is a hybrid between A. squamosa and A. cherimola; P.J. Wester
of Florida produced the fi rst hybrids in 1908 and called it the ‘atemoya’,
using the Brazilian name ‘ate’ for sweetsop and ‘moya’ from cherimoya. In
1927, hybrids were also developed in Poona, India. Numerous cultivars have
been selected in Israel (Gazit and Eistenstein, 1985). There is considerable
variation among seedlings. Leaves are glabrous and larger than those of the
parents, with fl owering and fruiting seasons resembling those of A. squamosa.
Seedlings also di er widely in the external and internal structure of the fruit.
Favourable characteristics inherited from the cherimoya parent include
many seedless carpels and carpels that adhere together instead of breaking
apart into individual tubercles, as in the sweetsop (Fig. 6.2). The atemoya is
more tropical in its requirements than cherimoya. While young trees require
some protection from frost, older trees have shown tolerance in Australia.
The atemoya is currently grown commercially in Israel, Australia, California,
Florida and Hawaii and in other countries for domestic consumption.
A. glabra L. (pond apple) has greater potential as a rootstock than for
fruit. It grows wild in southern Florida around lakes and rivers and is widely
distributed in the lowlands of tropical America. It is a vigorous grower,
reaching heights of 12 m and grows well in swampy areas. The fl ower is
fragrant, creamy-yellow and reddish inside. The fruit is about the size of the
sweetsop and heart-shaped; its yellow pulp is described as insipid.
Fig. 6.2. Fruit of sweetsop and atemoya.

Annonas: Cherimoya, Atemoya and Sweetsop 127
ECOLOGY
Soil
All species are capable of growing in a wide range of soil types, from sandy
soil to clay loams. Nevertheless, they di er in their soil preferences. Higher
yields occur on well-drained sandy to sandy loam soils, except in the case of
A. glabra, which can succeed in shallow ponds. Drainage is essential to avoid
root-rot diseases; hence the interest in A. glabra as a rootstock, related to its
tolerance of wet soils. Cherimoyas and atemoyas prefer sandy or sandy loam
soils with uniform moisture but with a good drainage to avoid root rots, and
a dry period at the end of winter at the time of leaf fall will enhance fl owering
uniformity. Sweetsop is capable of growing in a wide range of soil types, but its
shallow root system does not allow for fl ooded or waterlogged soils. It will do
best if good drainage exists and if soil pH is around 5.8–6.6.
Rainfall
In cherimoya and atemoya, rainfall and high humidity during the peak
fl owering season greatly enhance fruit production by preventing desiccation
of stigmas, prolonging their receptive period and increasing fruit set and early
fruit growth. Similar conditions are also ideal for other Annonas. The sweetsop
is probably the most drought-tolerant species and it grows and produces poorly
where rains are frequent. For example, sweetsop does much better in northern
Malaysia, where dry periods occur, than in the southern part, which has year-
round high moisture. For cherimoya and atemoya, a dry period at the coldest
time of the year enhances leaf fall and fl owering at the end of spring and early
summer. This dry period is not required for sweetsop.
Temperature
Temperature is the limiting factor, with frost killing young trees, especially
sweetsop, but older trees showing some tolerance. Cherimoya (7–18°C
mean minimum) is more tolerant to low temperatures (Fig. 6.3), followed
by atemoya (10–20°C mean minimum). Cherimoya can initiate vegetative
growth at ~7°C, while atemoya starts at ~10°C. Except for cherimoya, the
annonas do not require chilling periods and do well under lowland conditions
(George and Nissen, 1987b). Cherimoya is susceptible to high temperatures,
with a growing temperature range of 21–30°C (George and Nissen, 1986b).
Both cherimoya and atemoya are semi-deciduous, and growth declines at
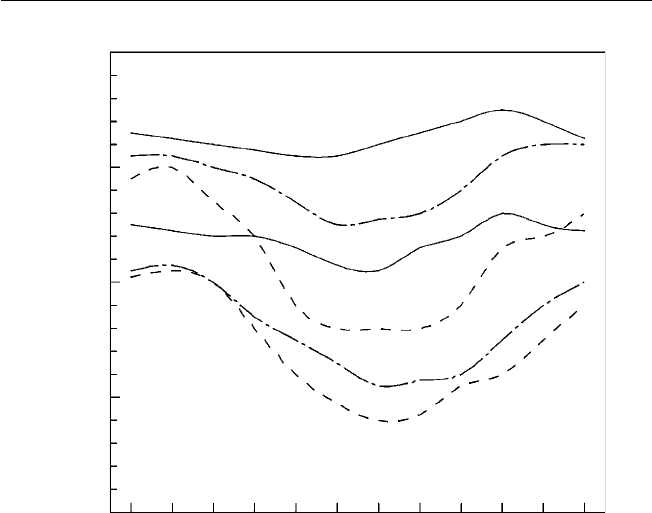
128 Chapter 6
the end of winter and the beginning of spring, during which many leaves
fall, followed by fl owering by the end of spring. In areas with year-round good
growing temperatures and no dry period, atemoyas can be forced to complete
their fl owering and fruit cycle in 8 months. An 8-month fruit cycle allows for
three harvests in 2 years. To achieve three cycles in 2 years requires careful
management of irrigation, fertilization, pruning and defoliation.
Poor pollination is a frequent problem with all three species and
occurs under high temperature (30°C) and low humidity (30% RH).
Lower temperature (25°C) and high humidity (80% RH) greatly improves
pollination. Hand-pollination is recommended for cherimoya and atemoya to
achieve more uniform fruit shape (Fig. 6.4). Sweetsop exposed to >30°C and
relative humidity <60% leads to poor pollination, even if hand-pollinated.
At 25°C and a relative humidity >80% sweetsop pollination is greatly
improved, with too high RH and rain negatively a ecting anther dehiscence.
High temperatures will induce strong vegetative growth in atemoya and
cherimoya. A temperature of 22–28°C is ideal for fruit set in atemoya, while
cherimoya needs slightly lower temperatures for fruit set.
Australia (max.)
Thailand (max.)
Thailand (min.)
Spain (max.)
Spain (min.)
40
30
20
Temperature (ºC)
10
0
JFMA ASONDMJJ
Month
Australia (min.)
Fig. 6.3. Seasonal changes in mean monthly minimum temperature for areas suited
to sweetsop (Bangkok, Thailand), atemoya (Mareeba, Australia) and cherimoya
(Malaga, Spain) in southern-hemisphere equivalents (after Marler et al., 1994).

Annonas: Cherimoya, Atemoya and Sweetsop 129
Light and photoperiod
Light penetration to the base of vigorous atemoya trees with a dense canopy
in close spacing can be 2% of full sunlight, and there is very little fruit set.
Pruning practices and spacing need to be adjusted for this shading e ect on
growth. No photoperiod responses have been reported. All three species need
some sort of pruning to obtain better light penetration into the canopy.
Wind
The soft wood of the trees makes them susceptible to wind damage and limb
breakage. Tree shaking may also be partially responsible for the penetration of
collar-rot organisms. The fruit skin is easily damaged by rubbing and exposure
to drying winds, leaving black scars. Annona leaf stomata are very sensitive
to changes in RH and rapidly close in response to declining RH (Marler et al.,
1994). Productivity can be improved by windbreaks and under-tree sprinkling
to raise the RH above 60%.
Fig. 6.4. Hand-pollination of atemoya using a hand-blower to disperse the pollen
collected the day before on to the open fl ower (courtesy of R. Manshardt).
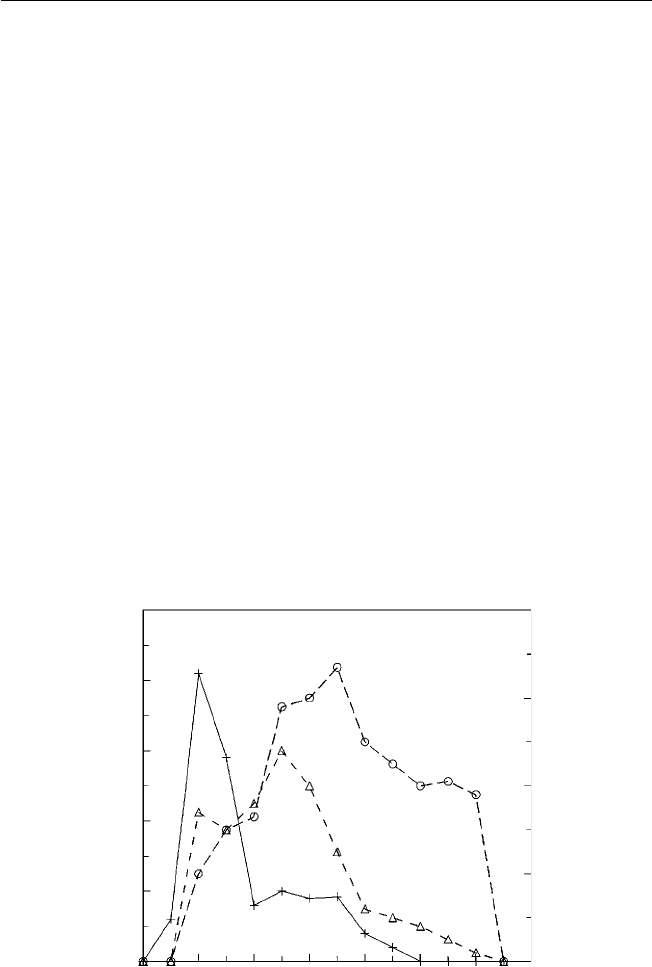
130 Chapter 6
GENERAL CHARACTERISTICS
Flowers
The fl owers of Annona species are hermaphroditic and are produced singly
or in small clusters in a compound infl orescence on an enlarged receptacle.
Flowers arise on the current season’s growth and occasionally on old wood.
Cherimoya and atemoya fl owers are fragrant and are solitary or in groups of
two or three on short, hairy stalks. The fl owers are multi-staminate and multi-
pistilate. New fl owers continue to appear towards the apex of the shoot as
fl owers produced earlier at the basal portions mature (Fig. 6.5). Defoliation of
cherimoya or atemoya manually or by using ethephon spray promotes lateral
branch growth and induces additional fl ower formation, since it exposes the
buds covered by the petiole base.
Annona species generally require 27–35 days for fl ower-bud development
from initiation to anthesis. Di erences in fl oral behaviour in the various
areas may be attributed to both genetic variability and climatic di erences
(Kshirsaga et al., 1976). Flowering can extend from 3 to 6 months or even
longer, with heavy peaks. In sweetsop, two major fl owering periods occur after
periods of vegetative fl ushes, with the second peak coinciding with the onset of
the monsoon in India (Kumar et al., 1977). Atemoyas in Australia produce the
25
20
15
10
Mean weekly shoot increment (cm)
5
0
02468
Weeks from the start of bud break
10 12 14
0
20
Flowers set
Flowers
Shoot
Total number of flowers/tree/wk and % set/wk
40
60
80
Fig. 6.5. Atemoya: newly emerging vegetative-fl ush shoot increment, total number
of fl owers produced per week and percentage fl owers set per week. Peak fruit set
occurs after completion of vegetative fl ush (after George and Nissen, 1988).

Annonas: Cherimoya, Atemoya and Sweetsop 131
fi rst fl owers during spring and have a second fl owering during the summer, the
latter being the more productive (Sanewski, 1991).
Pollination and fruit set
Natural pollination
The fl owers exhibit both dichogamy and a protogynous nature. This poses
a serious problem in obtaining high yields. The fl owering seasons of A.
squamosa and A. cherimola coincide. When sweetsop pollen is shed at about 2
a.m., cherimoya fl owers are receptive, opening around 7–9 a.m. and, when
cherimoya pollen is shed at 3–6 p.m., sweetsop fl owers are receptive. This
fl ower synchrony, together with complementary functional sexes, favours
cross-pollination, leading to natural hybridization. This is attested to by the
frequent appearance of hybrid seedlings under the trees of sweetsop and
cherimoya when grown in close proximity. Sweetsop is a more e ective pollen
source than cherimoya in cross-combination studies.
The atemoya female parts are receptive between 4 p.m. and 8 a.m.
and appear moist and sticky (Thakur and Singh, 1964). Atemoya pollen
is discharged in the afternoon of the same day from 3 to 6 p.m., if the RH is
above 80% and the temperature >22°C. At lower temperatures, pollen is
released on the afternoon of the second day. The pollen sacs turn a greyish
colour as pollen is discharged. Pollen grains from fl owers that appear early in
a fl owering season have thick walls, are high in starch, germinate poorly and
give poor fruit set. Pollen of later fl owers shows a high proportion of individual
pollen grains without starch grains, which germinate well. Upon opening,
fl owers are receptive for about 24 h. It has also been shown that spraying the
fl owers with water or putting a drop into the fl ower can signifi cantly increase
fruit set, probably because of the increased humidity slowing the drying of the
stigma and pollen grains. Night-time irrigation with micro-sprinklers or late
afternoon rains also increases fruit set.
Nitidulid beetles (Carpophilus and Uroporus spp.) are the important
pollinators of Annona fl owers, with wind- and self-pollination being low
(1.5%). Fruit set of ‘African Pride’ atemoya increases linearly with increasing
numbers of nitidulid beetles per fl ower. Three or more beetles per fl ower
increased fruit set to nearly 25%. Studies also show that these beetles breed
rapidly in rotting fruit media and that their populations can be increased by
maintaining a rotting-fruit attractant.
Hand-pollination is frequently practised to ensure pollination and good
fruit shape. Pollen must be collected in the evening from fully open fl owers,
when the sacs have turned from white to cream. The fl owers are held in a
paper bag, not a closed container, and should discharge that afternoon. The
fl owers are shaken over a shallow tray or paper to collect the pollen, which is
transferred to a small container and held in the refrigerator for use the next

132 Chapter 6
morning. Twenty to 30 fl owers can give enough pollen to pollinate 50–60
fl owers. Pollination is done daily, using a small (No. 4) organic hair brush,
so that pollen adheres better to it, or a pu er (Fig. 6.4). Pollen diluted with
Lycopodium spores (1:1 dilution) allows pollination of more fl owers. About
150 fl owers can be pollinated in 1 h, with a success rate of 80–100% being
achieved.
Hand-pollination is less successful when carried out on very humid,
overcast days and on young vigorous trees. In Honduras, hand-pollinatation
of ‘Gefner’ atemoya was better when done between 4.00 and 6.00 p.m.
than between 6.30 and 8.30 a.m. when the day temperatures were ~30°C
and relative humidity was low (Duarte et al., 1997). A fruit set of ~47% was
obtained against ~12% for morning pollination. This result was probably
obtained because of the lower night temperatures and higher humidity and the
use of pollen collected in the fi eld and applied immediately. When the Peruvian
‘Cumbe’ cherimoya was grown in Honduras, better fruit set was obtained
using pollen from a di erent variety (‘Bronceada’ from Chile) and afternoon
pollination rather than pollination in the morning (Duarte and Escobar, 1994).
Growth regulators for fruit setting
Hand-pollination in commercial orchards is tedious, time-consuming and
a costly practice. Attempts have been made to use growth regulators, with
considerable variation in the results obtained. Auxin-induced (indole acetic
acid (IAA), naphthalene acetic acid (NAA)) fruit grow very slowly with
less fruit drop, while gibberellic acid (GA
3
) promotes fruit set and growth;
however, it does not aid in early fruit retention (Duarte et al., 1974a; Yang,
1988). Application of the two substances separately at appropriate times has
produced seedless fruit of 200–300 g (Saavedra, 1979). Gibberellin (1000
ppm) is more e ective than hand-pollination and produced seedless fruit in
atemoya cultivars ‘African Pride’ and ‘Gefner’. Repeated spraying is necessary
to prevent fruit abscission during the fi rst 2 months. Seedless fruit are smaller,
with less fl avour and less fruit splitting than occurs in the seedy fruit that
result from pollination (Yang, 1988). Similar results have been obtained
for cherimoya and sweetsop. GA spraying is not recommended as a general
management practice for atemoya, because of variable results, although it
could be used in areas with poor natural pollination.
Fruit
Fruits of cherimoya, atemoya and sweetsop are compound fruits, generally
conical or roundish or heart-shaped. Cherimoyas can weigh up to 1000 g,
with an average of about 150–500 g, while atemoyas and sweetsops are
smaller. The skin can be smooth with fi ngerprint-like markings or can be
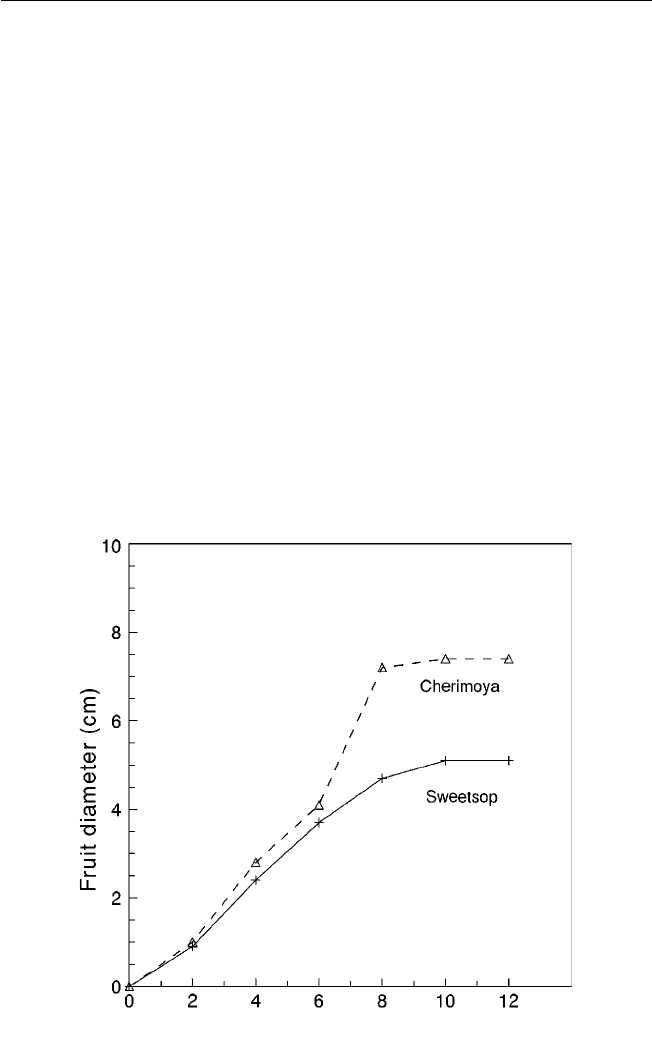
Annonas: Cherimoya, Atemoya and Sweetsop 133
covered with conical or rounded protuberances. The mature fruit is soft and
can easily be opened to show its white, juicy fl esh, of pleasing aroma and
subacid fl avour, containing numerous – sometimes few – brown or black, hard
and glossy, bean-like seeds. Atemoya has smaller seeds, while sweetsop has the
largest seed number.
Fruit growth shows the typical sigmoidal curve, with maturation
occurring in 16–24 weeks, depending upon species and growing conditions
(Fig. 6.6). Low humidity (<60% RH) and temperature (<13°C) near fruit
maturity can increase the severity of fruit skin russeting, as well as delaying
fruit maturation. High temperature can cause premature fruit ripening and
fermentation of the fruit.
CULTIVAR DEVELOPMENT
Genetics, cytogenetics and breeding
The chromosome numbers of A. cherimola, A. reticulata, A. squamosa and
atemoya are 2n = 14. A. glabra has been shown to be a tetraploid (2n = 28),
Fig. 6.6. Increase in fruit diameter from anthesis for sweetsop and cherimoyas (after
Thakur and Singh, 1965).
Weeks from anthesis

134 Chapter 6
and the occurrence of high chiasma frequency and the absence of multivalents
during meiosis suggest that it is of amphidiploid origin.
Cross-pollination between species is conducted primarily to determine
compatibility for increasing fruit set. Atemoya is the only hybrid that has
gained importance and it has inherited the glabrate leaf character of A.
squamosa and a leaf size almost as large as that of A. cherimola. Flowering and
fruiting seasons are similar to those of sweetsop. Skin, pulp and seed characters
of both parents are inherited in varying degrees by each plant. A desirable
hybrid would be between the cherimoya and soursop that combines the larger
fruit size and acidity of the soursop and the cherimoya’s sweetness, fl avour and
texture. Attempts to cross the soursop with cherimoya, ilama, bullock’s heart
or sweetsop have not been successful and may refl ect a considerable genetic
distance of soursop from the other species (Samuel et al., 1991).
Problems in breeding
The long reproductive cycles, higher levels of heterozygosity and the costs
associated with evaluating large populations of crosses limits breeding
programmes. Existing commercial cultivars show considerable variation in
growth, fruit set, fruit size and quality. No single variety has all the desirable
characteristics. The length of the juvenile period varies, with earliest
production occurring in 2 years and full production in 5–6 years. This
juvenile period is extremely variable with scions on seedling rootstocks. The
seedling rootstocks are derived from extremely heterogeneous, open-pollinated
seeds; hence it is di cult to fi x specifi c characters in a short period. Breeding
programmes have focused on selections from seedling populations. Early
maturity, better fruit appearance and, in the subtropics, greater cold tolerance
are the most frequent objectives.
Cultivar development
Except for cherimoya and atemoya, very few named clonal cultivars have been
developed among the Annonas (Table 6.1). Most of the plantings have been of
seedlings. In California, some old cultivars of cherimoya include ‘McPherson’,
‘Deliciosa’ and ‘Bays’. Considerable work has been done in Peru on the
development of cultivars, but they are not widely known outside Peru; one of
them, ‘Cumbe’, is considered exceptional, because of size, surface smoothness,
fl avour and very few seeds. Chile, Spain and New Zealand grow the cherimoya,
as it is more tolerant of cold temperatures, with more successful self-pollination
than the atemoya. New Zealand’s principal cultivars are ‘Reretai’, ‘Burton’s
Wonder’ and ‘Burton’s Favourite’. Chilean cultivars ‘Bronceada’ and ‘Concha
Lisa’ have also performed well in Australia, the former having been reported to
Annonas: Cherimoya, Atemoya and Sweetsop 135
possess a postharvest cold-storage life of 3 weeks. Pascual et al. (1993) report
seven cherimoya cultivars in Spain, of which ‘Fino de Jete’ and ‘Campa’ are
the most extensively cultivated, due to their superior yield and quality. Isozyme
studies indicated that these two cultivars showed identical banding patterns
for 15 enzymes, indicating that they were most probably the same cultivar. A
cluster analysis of isozyme patterns showed that Spanish cherimoya cultivars
were distinctly di erent from cultivars in California (Pascual et al., 1993)
and atemoya (Ellstrand and Lee, 1987). Spain has focused its programme on
germplasm collection and in situ evaluation. Similar programmes are taking
place in Mexico and Chile. In Spain polymorphic molecular markers are being
developed for cherimoya, most of which can be transferred to other Annonas.
Six atemoya cultivars have been described in Australia (Sanewski, 1991).
‘Pink’s Mammoth’ is reported to have been introduced from British Guiana
to Australia. It takes 6–7 years to begin producing commercial-size yields of
fruit, which are large, weighing from 800 g to as much as 2 kg, and it is a less
precocious bearer than ‘African Pride’. ‘African Pride’ was probably introduced
into Australia from South Africa, although its origin may have been Israel.
Table 6.1. Selected cultivars of cherimoya and atemoya.
Cherimoya Atemoya
Name Origin Name Origin
Andrews Australia African Pride Southern Africa or
Israel
Bays USA – California Bradley USA – Florida
Booth USA – California Gefner Israel
Bronceada Chile Island Gem Australia
Burton’s Wonder New Zealand Kabri Israel
Campa Spain Malalai Israel
Concha Lisa Chile Nielsen Australia
Cristalino Spain Page USA – Florida
Cumbe Peru Pink’s Mammoth Australia
E-8 Ecuador
Fino de Jete Spain
Kempsey Australia
Libby USA – California
Lisa USA – California
Mossman Australia
Negrito Spain
Reretai New Zealand
White USA – California

136 Chapter 6
It produces good yields of small to medium-sized fruit. ‘Bullock’s Heart’ (an
atemoya) is similar to ‘Pink’s Mammoth’. ‘Island Gem’ is an early-maturing,
small-fruited, heavy yielder. Two new cultivars developed in Australia are
‘Maroochy Gold’ and ‘KJ Pinks’, which have superseded the old, above-
mentioned cultivars (George and Paull, 2008). In Florida they developed the
cultivars ‘Bradley’ and ‘Page’. The former produces small-sized fruit with
relatively smooth and thin skin. The latter produces medium-sized, well-
shaped fruit with prominent skin segments. ‘Gefner’, an Israeli cultivar, is the
main cultivar grown in Florida and Hawaii; it produces small to medium-sized
fruit resembling ‘Page’.
In order to develop cultivars adapted to cooler environments, Australia
has concentrated on self-progenies and interspecifi c crosses of A. cherimola
with A. reticulata and A. diversifolia. Progenies of A. cherimoya–A. reticulata
crosses are late-maturing, showing fl owering and fruiting characteristics of
A. reticulata, which fl owers in the autumn and has mature fruit in late spring.
Selections possessing most of the fruit qualities of commercial cultivars have
been established in various areas for evaluation.
India and Taiwan have produced a few named cultivars of A. squamosa
that are propagated vegetatively. The main Taiwan cultivars are ‘Ruan-zhi’,
‘Cu-lin’, ‘Da-mu’, ‘Tainung no. 1’ and ‘Xi-lin’. Indian cultivars include ‘Arka
Sahan’, ‘Barbadose Seedling’, ‘Washington’, ‘Red Sitaphal’, ‘Balanagar’,
‘Mammoth’, ‘Purandhar’ and ‘Arka’. Thailand has developed ‘Fai Kaew’, ‘Fai
Krung’, ‘Nang Kaew’, ‘Nang Sir Krung’ and ‘Nang Thong’. In Florida they
selected ‘Lessard’, ‘Kampong Mauve’ and ‘Red Sugar’, and they also plant
‘Cuban Seedless’, a cultivar developed in Cuba that is seedless with medium-
sized fruit; there is another Cuban cultivar that is low in fi bre content. Seedling
populations have been established in Taiwan to select superior lines with
better yield, quality, early maturity and higher edible fl esh ratio and better
postharvest behaviour (Chen and Paull, 2008).
CULTURAL PRACTICES
Propagation
The Annonas, except for the cherimoya and atemoya, are usually propagated
by seed (George and Nissen, 1987b). There is a rapid loss of seed viability (6
months), and seeds should be planted as soon as possible after removal from
the fruit. Cherimoya seeds can take up to 30 days to germinate, and GA
treatment (10,000 ppm) can signifi cantly increase germination and enhance
seedling growth (Duarte et al., 1974b). Seedlings require at least 3–4 years to
bear fruit (Sanewski, 1991). In some seed lots, germination occurs quickly,
while in other lots it can take several months; therefore ideally it is better to
use fresh seeds in all Annonas.

Annonas: Cherimoya, Atemoya and Sweetsop 137
Clonal propagation by cuttings, layering, inarching, grafting and budding
have been tried (Table 6.2). Inconsistent results have been obtained with
cherimoya when 1-year-old cuttings are treated with rooting hormones, and
cuttings from mature trees have been di cult to root (Duarte et al., 1974b).
Atemoya, as a hybrid, has to be propagated asexually by cuttings from selected
trees or grafting or budding. There are cultivar di erences in the rooting ability
of atemoya, with ‘African Pride’ having a higher rooting response (60–80%)
than ‘Pink’s Mammoth’ and cherimoya (<20%). Atemoya tip cuttings are
superior to stem cuttings, with rooting percentages between 50 and 60%,
as compared with about 25% for stem cuttings (Sanewski, 1991). Time of
cutting removal is crucial for success, cuttings taken at the end of the cool
season showing the greater success. Roots should occur in 8–12 weeks and
the cuttings are ready to pot in 16–20 weeks. Air-layering can be used with
some cultivars, although cherimoya is not propagated easily. A modifi cation
where the new shoot is clamped and only the shoot tip is exposed is successful.
Inarching of A. squamosa, A. cherimola, A. glabra and atemoya to A. reticulata
rootstock has been successful, with only A. glabra giving less than 70% success.
Although inarching has given good results, it is time-consuming and costly for
large-scale propagation (George and Nissen, 1987b).
Cherimoyas are normally grafted or budded. Grafting is superior to budding
in percentage takes and subsequent growth, with side-whip graft and cleft-
graft techniques giving the best results (Duarte et al., 1974b). The branches
should be defoliated 1–2 weeks before scion wood is cut to induce bud swelling.
T-budding and chip-budding methods are successful. There are considerable
graft incompatibilities among Annona (Table 6.3) and Rollinia species and types.
Cherimoya has been found to be a vigorous rootstock for ‘Pink’s Mammoth’
(atemoya), although atemoya is not compatible with A. glabra, A. montana,
Table 6.2. Propagation methods and success for Annona spp. (after George and
Nissen, 1987b). Root-cutting success rate is unknown.
Method Species
Atemoya Sweetsop Cherimoya
Seedling
Genetically Variable Uniform Variable
Use Not recommended Good Rootstock
Tip and stem cutting Some cvs only Some cvs only Not successful
Micropropagation Possibly high Unknown Unknown
Layering Unknown High if modifi ed
techniques used
Unknown
Air-layering <5%
<8.3%
<5%
Budding >70% >70% >70%
Grafting >70% >70% >70%
138 Chapter 6
Table 6.3. Rootstock and scion compatibility of different Annonas (after Sanewski, 1991).
Scion
Rootstock Atemoya Gefner
Pink’s
Mammoth Page/Bradley A. cherimola A. glabra A. muricata A. squamosa
Atemoya C C
–– C
Gefner C
Pink’s Mammoth C
Page/Bradley C
A. cherimola
CC CC
–
C
A. glabra
N–CC–
A. muricata
N–CCN
A. palustris
––––N
A. reticulata
NPC C P – C
A. squamosa
CC––C
C, compatible; P, partial; N, not compatible; –, unknown.

Annonas: Cherimoya, Atemoya and Sweetsop 139
A. muricata and A. reticulata as rootstocks (Sanewski, 1991). This is complicated
by cultivar di erences in compatibility with common rootstocks. Atemoya
cultivars ‘Bradley’ and ‘Page’ are compatible with custard-apple rootstocks, but
‘Gefner’ shows partial incompatibility with the same rootstock (Table 6.3).
Sweetsop is usually seed-propagated. The seeds should be sown within
a week after extraction from the fruit. Inarching can be done to A. reticulata,
with 70% success (George and Nissen, 1987b). The same success is obtained
for grafting and budding. Rooting of tips has been successful in some cases, as
well as air layers, but they are more costly.
Rootstocks
There are several possible combinations and incompatibilities among Annona
and Rollinia species. Cherimoya rootstocks normally include seedlings of
cherimoya and, in some cases, soursop and sweetsop. For atemoya, cherimoya
and atemoya are normally used as rootstocks and also A. squamosa, but it is
susceptible to bacterial wilt caused by Pseudomonas solanacearum. Sweetsop
is normally not grafted or budded, but it could be done with seedlings of
sweetsop, cherimoya or A. glabra (Table 6.3). A. muricata or Annona palustris
are not good rootstocks for sweetsop.
Field preparation
A soil sample should be taken 4–6 months before planting to determine lime
requirements and soil nutrient levels. Soil phosphorus can also be adjusted
at this time or in the planting hole. Minimal tillage can be achieved with a
2-m-wide band cultivated where the trees are to be planted. Drainage should
be installed at this time to avoid fl ooding, with either contour or subsurface
drains. Windbreaks should be established prior to transplanting. Napier or
Bu alo or Bana grass (Pennisetum purpureum Schumacher) can be used when
shelter is required in the fi rst 12 months.
Transplanting and spacing
Transplanting should be done at the beginning of the wet season if there are
seasonal dry periods and no irrigation facilities. In the subtropics, planting
should not occur if there is a risk of frost. Plants should have attained a height
of 30–50 cm at transplanting time, with the union of grafted or budded plants
placed 15 cm or so above the ground. Trees should be irrigated as soon as
possible after transplanting, with wind and sun guards sometimes required.

140 Chapter 6
Transplanting distance depends upon the species of Annona. The
periodically pruned small sweetsop can be spaced at 3.7 m × 4.6 m. In dry
areas with less luxuriant growth, closer within-row spacing than 3.7 m can be
considered. Closer spacing would increase humidity and benefi t the longevity
of stigma receptivity. For machine operations, wider spacing of 5.0–5.5 m ×
6.0–6.5 m can be used.
Cherimoya can be planted at 5 m × 6 m within and between rows, with
appropriate pruning practices. Row spacing of 5–10 m and between rows
7–12 m are recommended for atemoya in Australia, depending on cultivar,
rootstock and pruning to an open goblet. Narrower spacing is used for
‘African Pride’ on A. squamosa rootstock, and the widest spacing is for ‘Pink’s
Mammoth’ on A. cherimola. In Florida, narrow plant spacing of 4–6 m and
row spacing of 6–7 m are used (Campbell, 1985). A triangular layout is
recommended whatever planting distance is selected, with the rows running
north–south to avoid shading. In Chile, narrow spacing (4 m × 2 m) has been
tried for cherimoyas, and there are some commercial plantings that use 5 m ×
1.5 m (Gardiazabal and Rosenberg, 1993). Some experimental trellis plantings
also exist.
Irrigation practices
The Annonas are grown in many areas without irrigation when rainfall is
well distributed. Except for pond apple (A. glabra), most Annonas can stand
periods of drought and prefer rather dry conditions. There must be adequate
soil moisture to encourage vegetative growth, since fl owering occurs on new
growth. Bearing atemoya trees may need up to 1440 l/tree every 4 weeks
during the low-growth phase and from 500 to 750 l/tree every 3–5 days
during fl owering, fruit set and fruit growth (Sanewski, 1991). Reducing
irrigation in late winter to force atemoya and cherimoya trees into dormancy
for 1–2 months in spring is recommended in Australia and California,
respectively. The amount and frequency of irrigation must be determined by
experience in any particular location and soil type. Water stress should be
prevented during fl owering, fruit set and fruit development, as fruits are more
sensitive than leaves to water stress (Fig. 6.7).
High soil moisture to increase humidity during the fl owering season
may prolong stigma receptivity and fruit set and growth. The use of low-
rise sprinklers under the tree canopy during fl owering increases the canopy
humidity. The stomata of Annona respond to RH, not water stress, and will
continue to lose water if the humidity is greater than 80%, making it crucial
to maintain soil moisture content (Marler et al., 1994).
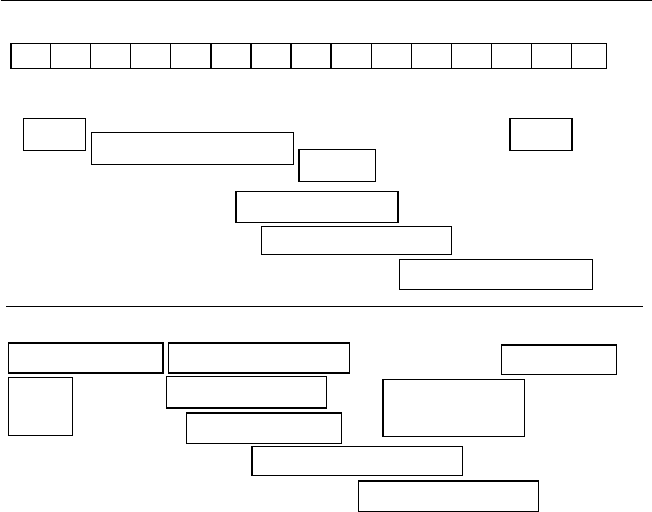
Annonas: Cherimoya, Atemoya and Sweetsop 141
Pruning
Training of trees should begin in the nursery, and pruning should continue
after transplanting. It is desirable to train the tree to a single trunk up to a
height of about 90 cm and then it should be headed back to produce lateral
branches. The lateral branches should be spaced 15–25 cm above each other
and be allowed to grow in di erent directions to develop a good sca old. After
about 2 m, they can be left to natural growth. Pruning is carried out when
the trees are dormant and, in heavy trees, involves removal of lower limbs
touching the ground and branches in the centre that may be rubbing against
each other. The objective is to allow sunlight access to the centre of the tree
(George and Nissen, 1986a).
All lateral buds can have up to two vegetative buds and three fl ower buds.
The lateral buds of atemoya, cherimoya and sweetsop are normally ‘buried’
(subpetiolar) in the base of the swollen leaf petiole (Fig. 6.1). Leaf shed must
occur prior to the elongation of ‘buried’ buds (George and Nissen, 1987a).
Removal of leaves mechanically by stripping or chemically with urea or
Flowering and fruiting
Flowering and fruiting
Harvest
Jan. Feb. Mar. Apr. May Jun. Jul. Aug. Sep. Oct. Nov. Dec. Jan. Feb. Mar.
Harvest
Pruning
Leaf drop Leaf dropLeaf growth
Flowering
Fruit set
Fruit growth
Harvest
Flower
initiation
Flower
initiation
Pruning
Sweetsop
Atemoya
Pruning
Summer pruning
Winter pruning
Fig. 6.7. Growth and management practices for sweetsop grown in Taiwan (Anon.,
1995) and atemoya (Sanewski, 1991). In Taiwan, normal sweetsop pruning occurs
in January and February, and there are extended periods of pruning of selected trees
in summer. The atemoya cycle has been converted to northern-hemisphere seasons
for comparison purposes.

142 Chapter 6
ethephon releases these buds. The buds in ‘Pink’s Mammoth’ and ‘African
Pride’ are more suppressed and may require leaf stripping in the summer.
Adventitious buds can arise at any point on a trunk.
Atemoya produces the fi rst fl owers of a season and the most fl owers on
1-year-old wood. The second and third fl ushes of fl owers occur on new growth
and produce most of the crop (Fig. 6.5). The fl owers of the fi rst fl ush may be
removed, as they show poor fruit set and have greater problems with fruit
splitting upon ripening. In south-east Queensland, each year the 1-year-old
fruiting wood is shortened to about 10–15 cm, with four to six buds (Sanewski,
1991). These pruned laterals are retained after producing fruit and allowed to
become part of the framework, thus extending the canopy by 30–50 cm each
year. However, dense shading within the canopy is a major problem. The open-
goblet system is used to produce an open, spreading tree that will produce fruit
early and avoid dense shading. The strategy is to produce one or two fruits on
each shortened, 1-year-old lateral. These laterals are removed completely after
2–3 years and replaced by new growth closer to the leader. The ideal seasonal
growth of laterals producing fruit is about 60 cm long and about 10 mm in
diameter at the base. Annual lateral growth more than 100 cm is considered
excessive for mature fruiting trees. This system allows the development of
fruiting laterals along all the main limbs, with a moderately open centre,
although trees under this system rapidly grow tall. Pruning is normally
carried out in spring before large amounts of bud growth have occurred, with
a moderate summer pruning. Defoliation during the cool season to allow early
development of the buried buds and more uniform fl owering is sometimes
carried out with an ethephon–urea mixture (Sanewski, 1991). Rejuvenation
by heavy pruning is occasionally needed.
Summer pruning of cherimoya was tried in Chile by removing the shoot
tips, leaving six or ten buds, after artifi cial pollination had been performed. In
some cases, girdling or scoring were used in combination. The best treatment
was to cut the shoots at ten buds combined with girdling the trunk during
fl owering time, with an increase of 22% in fruit yield and 25% in fruit weight
(Razeto and Diaz de Valdes, 2001).
Sweetsop is pruned so that 1-year-old branches are cut back to 10 cm.
The pruning left 120–150 branches per tree, with root pruning not being
recommended. Flower initiation begins at the basal end of the growing branch
(Lo, 1987). In Taiwan, normal pruning occurs in January/February, with
fruit harvest from July to September (Fig. 6.7). A summer pruning with fruit
thinning (June–October) of selected trees can lead to harvesting fruit from
October to the next March (Yang, 1987). Highest winter fruiting occurs when
summer growth is pruned, compared with pruning non-fruiting shoots or
pruning in late May.

Annonas: Cherimoya, Atemoya and Sweetsop 143
Fertilization
The Annonas have an indeterminate growth habit (axillary fl owering), and
applying nitrogen (N) in somewhat excessive amounts does not interfere
greatly with fl oral initiation, as is the case with plants having determinate
growth habit. However, excessive tree vigour is usually associated with reduced
fl owering and yields in many tree crops and the atemoyas are no exception
(George et al., 1989).
In Australia, continued research and fi eld observations on atemoya
nutrition have led to greater refi nements in terms of quantity of fertilizer
and times of incremental applications during the annual growth and fruiting
cycles (Table 6.4). The use of foliar nutrient analysis has become a useful
management tool in determining atemoya fertilizer programmes (Sanewski,
1991). Sampling for foliar analysis consists of obtaining the most recently
matured leaf – the fourth or fi fth leaf below the growing point. Leaves are
selected from non-bearing shoots without a leaf fl ush during late summer
or early autumn (Table 6.5). After 10 years of age, the annual amounts of
N, phosphorus (P) and potassium (K) remain the same, as tree size is kept
relatively constant by annual pruning and by competition from adjacent trees.
The annual requirements of N and K are split into four increments (Table 6.4).
In the cool subtropical areas, greatest vegetative growth takes place during the
warmer months, from spring to autumn. Reduction in N during the winter
minimizes new vegetative growth in young trees that are vulnerable to cold
temperatures. This adjustment is not necessary in the warm tropics. P is given
once per year during the early-autumn application.
Table 6.4. A guide to annual application of NPK for ‘Pink’s Mammoth’ atemoya
trees of different ages using straight fertilizers (g/tree/year) and percentage
distribution of application of annual amounts (Sanewski, 1991).
Tree age (years)
Urea
(g/tree/year)
Superphosphate
(g/tree/year)
Potassium chloride
(g/tree/year)
2 400 500 360
4 860 550 930
6 1300 780 1170
8 1600 880 1500
10 1750 880 1650
Fertilizer
Early
spring
Early
summer
Early
autumn
Late
autumn
Urea 20% 30% 40% 10%
Superphosphate 100%
Potassium chloride 10% 30% 40% 20%

144 Chapter 6
The primary sink for K in the atemoya is the fruit, rather than the leaves,
and thus there is a high requirement, with defi ciency likely. About 60% of
the K requirement is applied during the fruit-development period. Atemoyas
also have a fairly high requirement for magnesium (Mg) and calcium (Ca).
Heavy vegetative growth during the fruit-development period competes for
nutrients such as boron (B) and Ca, resulting in boron-defi cient fruit. Boron-
defi cient fruit develop hard, brown, lumpy tissues around the central core.
Defi ciency in B and, to some degree, Ca is considered a causal factor for these
lumps (Cresswell and Sanewski, 1991). Applying B at a slight excess can be
phytotoxic, especially in sandy soils. A desirable practice is to use organic
fertilizers, with inorganic fertilizer as a supplement to maintain a balance and
to control cropping (Sanewski, 1991).
For sweetsop, a periodic application of NPK is best, split into three parts
applied every 4 months. The early-spring application would include all the P
for the year and 20% of the N and K requirements. In summer, the other 70%
of N and 40% of K are applied, and the rest of K is left for autumn application.
A physiological disorder called black speck is caused by Ca defi ciency, which
is corrected by spraying with a foliar Ca solution. This physiological disorder
causes small, dark spots in the shoulder and waist of the fruit, but it can invade
the whole skin in extreme cases.
Pest management
Diseases
A number of diseases have been reported in the literature (Table 6.6).
Anthracnose, caused by Colletotrichum gloeosporioides (Glomerella cingulata),
Table 6.5. Tentative leaf nutrient standards for atemoya (Pink’s Mammoth) in
Queensland, Australia, presented as a guide (Sanewski, 1991).
Nutrient Acceptable range
Nitrogen 2.5–3.0%
Phosphorus 0.16–0.2%
Potassium 1.0–1.5%
Calcium 0.6–1.0%
Magnesium 0.35–0.5%
Sodium < 0.02%
Chloride < 0.3%
Manganese 30–90 ppm
Copper 10–20 ppm
Zinc 15–30 ppm
Iron 50–70 ppm
Boron 15–40 ppm
Annonas: Cherimoya, Atemoya and Sweetsop 145
is the most serious in areas of high rainfall and atmospheric humidity and
during the wet season in dry areas (Alvarez-Garcia, 1949; Dhingra et al.,
1980). This disease causes twig dieback, defoliation and dropping of fl owers
and fruit. On mature fruit, the infection causes black lesions.
Black canker (Phomopsis anonacearum) and diplodia rot (Botryodiplodia
theobromae) occur mostly on neglected trees and cause similar symptoms of
purplish to black lesions, resulting in mummifi ed fruit. Marginal leaf scorch is
also caused by these two fungi and causes twig dieback. Diplodia rot has darker
internal discoloration and deeper, more extensive, corky rot in fruit. A fruit
and leaf spot is caused by a soil-borne fungus, Cylindrocladium colhounii, which
can cause almost total loss of fruit during years of persistent heavy rains.
Symptoms begin with small, dark spots, primarily on the shoulders of the fruit,
which spread along the sides, enlarge, become dry and crack. Infection is skin
deep, but fruit becomes unmarketable. The control measures recommended
are good orchard maintenance with heavy mulching and lower-branch
pruning to prevent splashing of soil during heavy rainfall (Sanewski, 1991).
Bacterial wilt of atemoya is caused by P. solanacearum and is characterized
by rapid wilting and death of young trees and slow decline of old trees. There
is a general decline of vigour and defoliation on a ected limbs. Vascular
discoloration of woody tissues occurs in the roots and up to the trunk at
ground level. It has caused up to 70% tree death in 12 years in orchards using
A. squamosa rootstocks in Queensland.
In sweetsop, Phytophthora blight (purple blotch) is caused by Phytophthora
citrophthora and Phytophthora nicotianae, which infest fruit and leaves, mainly
Table 6.6. Major diseases of Annonas.
Common name Organism Parts affected, symptoms
Region or
country
Anthracnose
Colletotrichum
gloeosporioides
(Glomerella)
Flowers, fruit, leaves,
dieback, seedling
damping off
Universal
Armillaria root
rot
Armillaria
leuteobubalina
Roots, base of trees,
decline
Australia
Bacterial wilt
Pseudomonas
solanacearum
Tree wilt Australia
Black canker
(diplodia rot)
Botryodiplodia
theobromae
Leaf scorch, twig dieback Universal
Black canker
Phomopsis
Leaf scorch, twig dieback Australia
Purple blotch
Phytophthora palmivora
Spots on immature fruit,
fruit drop, twig dieback
Australia
Rust fungus
Phakopsora cherimoliae
Leaves Florida
Fruit rot
Glioclacium roseum
Fruit India

146 Chapter 6
during the wet season; the fruits get mummifi ed and stay on the tree or drop,
and the infected leaves show water-soaked spots that turn brown-black.
Another sweetsop fruit disease is attributed to Gliocladium roseum, which
a ects 20–90% of the fruit in India. Symptoms consist of water-soaked
spots, which turn soft and brown. There is also a bacterial wilt caused by P.
solanacearum that produces a rapid wilting and death of young trees and slow
decline of old trees. Vascular discoloration occurs of woody tissues of the roots
up to the trunk at ground level.
INSECT PESTS
Some insect pests occur in numerous growing areas (Table 6.7). One of the
most serious insects in Trinidad is the Cerconota moth, which lays its eggs on
young fruit. The emerging larvae tunnel into the pulp, causing blackened,
necrotic areas. It is common to fi nd every fruit larger than 7.5 cm infested.
Table 6.7. Major insect pests of Annonas.
Common name Organism Parts affected Country/region
Bephrata wasp
(soursop wasp)
Bephrata maculicollis
Fruit Mexico, Americas,
Trinidad,
Surinam
Wasp
Bephratelloides
paraguayensis
Fruit Americas,
Barbados
Cerconota moth
(soursop moth)
Cerconota annonella
Fruit Americans,
Trinidad,
Surinam
Thecla moth
Thecla ortygnus
Flowers, young
fruit
Americas,
Caribbean
Banana spotting bug
Amblyphelta
lutescens
Young fruit Queensland
Mealy bug
Dysmicoccus spp.
Stem, leaves Universal
Citrus mealy bug
Planococcus citri
Fruit Queensland
Southern stink bug
Nezara viridula
Fruit Caribbean
Caribbean fruit fl y
Anastrepha suspensa
Fruit Caribbean, Mexico
Mediterranean fruit
fl y
Ceratitis capitata
Fruit Peru, Spain
Queensland fruit fl y
Bactrocera tryoni
Fruit Australia
Potato leaf hopper
Empoasca fabae
Leaves Caribbean
Red spider mite
Several genera and
species
Leaves, fl owers American tropics
Scale insects
Saissetia coffeae
Leaves, stem Universal
Coconut scale
insect
Aspidiotus destructor,
other genera and
species
Leaves, stem Caribbean

Annonas: Cherimoya, Atemoya and Sweetsop 147
Bagging the fruit is sometimes done. This moth has been reported in the
American tropics as far south as Brazil and is a major limiting factor in
Surinam (Paull, 2008).
The bephrata wasp (Bephrata maculicollis) is widely distributed throughout
the Caribbean, Mexico, Central America and northern South America. This
wasp is considered to be the most important pest in Florida (Campbell, 1985).
The larvae infest the seeds and cause damage to the pulp, as they bore through
the fl esh to emerge when the fruit matures. The Thecla moth is widespread
through parts of the Caribbean and in the American tropics, but it is not
considered to be as serious as the Cerconota moth and Bephrata wasp. Primary
damage is to the fl owers. The larvae feed on fl ower parts, such as the perianth,
stamens and stigmas, with the fl owers failing to set fruit. Bagging the fruit can
be very e ective and an economic estimate has to be done.
The banana-spotting bug (Amblyphelta lutescens) and the fruit-spotting
bug (Amblyphelta nitida) are considered to be serious atemoya pests. Amblypelta
nitida is rare in northern Queensland, with both being pests in southern
Queensland. The bugs cause small, black, 2–10 mm spots on the shoulders of
young fruit and penetrate about 1 cm into the fruit. The damage resembles the
symptoms of diplodia rot (black canker).
Mature green annonaceous fruits have been shown to be rarely infested
by the Mediterranean fruit fl y (Ceratitis capitata) and Oriental fruit fl y (Dacus
dorsalis), but they are found, on occasion, in tree-ripened fruit. In Peru C.
capitata is one of the most important problems in cherimoya as well as some
Anastrepha species (Franciosi, 1992). Also, in Spain ‘Fino de Jete’, the major
cultivar grown, is very susceptible to the C. capitata fruit fl y. In Australia, the
Queensland fruit fl y (Bactrocera tryoni) infests ripening atemoya fruit. ‘African
Pride’ appears to be more susceptible than ‘Pink’s Mammoth’. Use of bait
sprays and fi eld sanitation are recommended measures to minimize fruit-fl y
infestation (Smith, 1991). Fruit bagging also provides protection.
Mealy bugs and various species of scale insects are found universally and
usually become a serious pest on neglected trees. The former is reported to be
a major pest on marketable fruit in some areas of Australia. Red spider mites
can become a serious problem in dry areas or during dry seasons. Scale insects
are normally a minor problem.
Nematodes can be a problem in some places like Chile, where
Helicotylenchus and Pratylenchus cause signifi cant reduction in plant growth
in certain areas (Gardiazabal and Rosenberg, 1993).
Weed management
Problem weeds, especially grasses and twining weeds, should be controlled by
cultivation and herbicides before planting. Young trees should be protected
from weed competition by hand-weeding, mulching or contact herbicides. The

148 Chapter 6
shallow root systems limit the use of cultivation under the tree. A translocated
herbicide may be needed for perennial weeds and is applied as a spot spray.
HARVESTING AND POSTHARVEST HANDLING
Harvesting season, yield and harvesting
The harvesting season is quite similar in most areas, di ering only in range
(Table 6.8). Sweetsop is usually harvested during the summer months,
from July to November, and can be extended to March (Fig. 6.7). Atemoya is
harvested in autumn in the southern hemisphere and winter in Australia (Fig.
6.7) and from October to January in Poona, India.
A major problem in Annona cultivation is obtaining commercial yields. In
order to increase yield, hand-pollination (Fig. 6.4) has become an important
aspect of cultivation practices in some areas, especially for atemoya. Use of
pollen stored under poor conditions, naturally defective pollen and pollinating
during the hottest period of the day result in very poor fruit set.
Rootstocks have been shown to greatly infl uence yield (Sanewski, 1991).
Trees of the same age and cultivar on cherimoya rootstock yield almost double
that of trees grafted on sweetsop. Cultivar di erences in yield have also been
noted (Fig. 6.8). Estimated yield per tree of 6-year-old ‘African Pride’ on
cherimoya rootstock is about 90 kg, while that of ‘Pink’s Mammoth’ of the
same age and on the same rootstock is about 30 kg. In other cases yields of
80–150 kg/tree
are recorded for cherimoya and sweetsop, equivalent to 15–25
t/ha.
Fruit is harvested when fully mature and fi rm. The skin colour changes
as the fruit approaches maturity. Skin of immature cherimoya and sweetsop
is greyish-green but turns to yellow-green at maturity. In the latter fruit,
Table 6.8. Peak harvesting seasons for some Annona species.
Countries Cherimoya Atemoya Sweetsop
Australia Mar.–Sept.
Argentina Feb.–July
California Mar.–Apr.
Caribbean June–Sept.
Chile Aug.–Dec.
Florida July–Oct. July–Sept. July–Sept.
Hawaii Aug.–Nov.
India (Poona) Nov.–Feb. Oct.–Jan. Aug.–Nov.
Portugal (Madeira) Nov.–Feb.
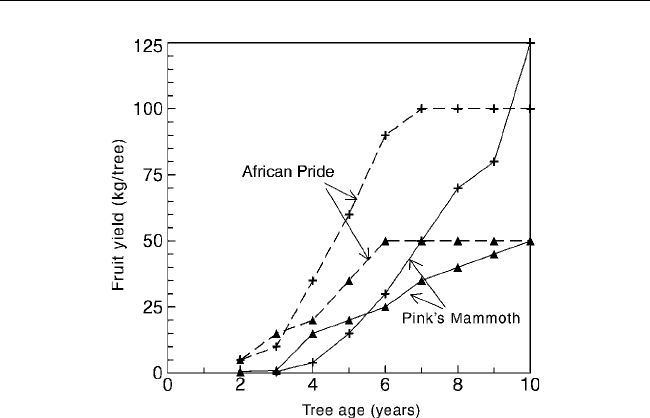
Annonas: Cherimoya, Atemoya and Sweetsop 149
adjacent carpels towards the blossom end commonly separate, exposing the
white pulp (‘creaming’). ‘Creaming’ between segments of the atemoya fruit is
considered to be an indication of maturity. Fruits are harvested when 40% of
the surface shows ‘creaming’ (Sanewski, 1991). Determining harvest time by
dating fl oral anthesis is impractical, as fl owering occurs over many months. If
a rigid hand-pollination protocol is used, with removal of naturally pollinated
fruit, days from anthesis can be used.
Fruit is hand-harvested and put into lug boxes or baskets. Sometimes
the fruit is harvested using pruning shears, in order to leave a portion of the
peduncle attached to limit stem end rots. Fruit should be harvested preferably
early in the morning, when temperatures are still cool. Atemoyas are
harvested every 3–7 days, with experienced pickers harvesting from 150 to
180 kg fruit/h.
Postharvest handling
Harvested fruit should be handled with care to prevent bruising of the skin.
This is especially important for fruits that are marketed for fresh consumption,
such as cherimoya, sweetsop and atemoya. Fruit of some cherimoya cultivars
can be kept for 7–10 days at 17°C. Normal ripening occurs at temperatures
between 15 and 30°C, and storage temperatures below 15°C cause chilling
injuries and a failure to develop full fl avour, although some Chilean varieties
Fig. 6.8. Approximate yields of two atemoya cultivars, ‘Pink’s Mammoth’ and
‘African Pride’, on cherimoya () and sweetsop (S) rootstocks with tree age (after
Sanewski, 1991).

150 Chapter 6
such as ‘Concha Lisa’ tolerate 7–8°C and ‘Bronceada’ 10–11°C. Fruit
harvested early in the season will have fewer sugars but stores better than
fruit harvested late in the season. Cherimoyas can be coated with di erent
wax formulations to reduce water loss, delay fi rmness and enhance fruit
appearance (Gardiazabal and Rosenberg, 1993).
Storage conditions recommended for sweetsop are 15°C, with an RH of
85–90%. Atemoya fruit can be stored up to 2 weeks at 15–16°C or 1 week
at 10–12°C; fruits stored at 0–5°C turn black. At lower temperatures, skin
discoloration occurs rapidly. Precooling of fruit is essential to help extend
shelf-life. Some trials have been made to store under controlled atmosphere.
Except for the atemoya in Australia, there are no reports of established
grade standards. Australian standards specify mature atemoya fruit should
be 75 mm in diameter, fi rm, with ‘creaming’ between segments on the skin.
Containers are 0.5 bushel (8–10 kg) in size, made of wood, fi breboard or
polystyrene (450 mm × 215 mm × 180 mm), well ventilated and marked with
‘custard apple’ and the number of fruit. The presence of soft fruit and even one
fruit-fl y-damaged fruit can lead to rejection of the consignment.
Compositional changes during fruit ripening
All Annonas are climacteric fruit. Cherimoya has two respiration peaks during
ripening instead of the single peak of other climacteric fruits. The fruits also
seem to release more ethylene than most other fruit and have a higher rate
of metabolism, which shortens its postharvest life. ‘African Pride’ atemoya
respiration reaches a peak about 3 days after harvest, and the eating stage is
reached in another 2 days. Total time from harvest to eating ripeness is about
5 days at 20°C.
UTILIZATION
Cherimoya is a fair to good source of vitamins, while sweetsop is a good source
of P, thiamine and ascorbic acid (Table 6.9). Atemoya is also a good source of
ascorbic acid.
These Annonas are usually consumed as dessert fruit. Once peeled, the
pulp tends to oxidize fairly soon and acquires a brownish colour. Several pies,
custards and fi ne desserts, which can include combinations with whipped
cream and meringues, are made with cherimoya and atemoya. The peeled
pulp can be eaten mixed with orange juice, as they do in Chile. A delicious ice
cream can also be made with these fruits or they can be included in yogurt.
The perishable nature of the fruit and often short supply limits availability to
local markets or air shipment to more-distant markets.

Annonas: Cherimoya, Atemoya and Sweetsop 151
FURTHER READING
Anon. (1995) Production and Marketing of Sweetsop. Special Publication No. l, Taidong
Agricultural Improvement Station, Taiwan (in Chinese).
George, A.P. and Nissen, R.J. (1987) Propagation of Annona species: a review. Scientia
Horticulturae 33, 75–85.
George, A.P., Nissen, R.J. and Brown, B.T. (1987) The custard apple. Queensland
Agricultural Journal 113, 287–297.
George, A. and Paull, R. (2008) Annona squamosa × Annona cherimola, atemoya. In:
Janick, J. and Paull, R. (eds) The Encyclopedia of Fruit and Nuts. CAB International,
Wallingford, UK, pp. 54–62.
Marler, J.E., George, A.P., Nissen, R.J. and Andersen, P.J. (1994) Miscellaneous
tropical fruits – annonas. In: Scha er. B.C. and Andersen, P.C. (eds) Handbook of
Environmental Physiology of Fruit Crops, Vol. II. Subtropical and Tropical Crops. CRC
Press, Boca Raton, Florida, pp. 200–206.
Merodio, C. and De La Plaza, J.L. (1997) Cherimoya. In: Mitra, S.K. (ed.) Postharvest
Physiology and Storage of Tropical and Subtropical Fruits. CAB International,
Wallingford, UK, pp. 265–290.
Paull, R.E. and Chen, N.J. (2004a) Cherimoya. In: USDA Handbook #66. (http://www.
ba.ars.usda.gov/hb66/051cherimoya.pdf, accessed 7 Feb 2010).
Table 6.9. Proximate fruit composition of cherimoya, soursop and atemoya in a
100 g edible portion (after Wenkam, 1990).
Nutrients Cherimoya Atemoya Sweetsop
Water (g) 68.71 78.7 75.97
Energy (kJ) 460 310 360
Protein (g) 1.54 1.4 1.89
Lipid (fat) (g) 0.13 0.6 0.57
Carbohydrate (g) 28.95 15.8 20.82
Fibre (g) – 2.5 1.41
Ash (g) 0.67 0.4 0.75
Minerals
Calcium (mg) 9 17 17
Iron (mg) – 0.3 0.3
Magnesium (mg) – 32 22
Phosphorus (mg) 24 – 54
Potassium (mg) – 250 142
Sodium (mg) – 4 2
Vitamins
Ascorbic acid (mg) 12.20 43 35.9
Thiamine (mg) 0.11 0.05 0.10
Ribofl avin (mg) 0.11 0.08 0.06
Niacin (mg) 1.00 0.8 0.89
Vitamin A 0 – 0
Seed/skin % 35.00 28.0 45.00

152 Chapter 6
Paull, R.E. and Chen, N.J. (2004b) Atemoya. In: USDA Handbook #66. (http://www.
ba.ars.usda.gov/hb66/051cherimoya.pdf, accessed 7 Feb 2010).
Pinto, A.C. de Q., Cordeiro, M.C.R., de Andrada, S.R.M., Ferraira, F.R., Filgueiras, H.A.
de C., Alves, R.E. and Kinpara, D.I. (2005) Fruits for the future 5 – Annona species.
Monograph. (http://www.icuc-iwmi.org/fi les/Publications/Annona_Monograph.
pdf, accessed 7 Feb 2010).
Sanewski, G.M. (ed.) (1991) Custard Apples – Cultivation and Crop Protection. Information
Series QI90031, Queensland Department of Primary Industry, Brisbane,
Australia.

© CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1 153
(R.E. Paull and O. Duarte)
7
AVOCADO
The avocado (Persea americana) is the most common English name but it is
also known as alligator pear and butter pear. Spanish-speaking people call it
aguacate, cura or cuprandra, while in Chile, Peru and Educador, it is called by
its Inca name, palta. In French, it is known as avocat and avocatier. The name
‘avocado’ refers to the fruit and is derived from the Aztec Nahuatl language
word, ahuacatl, meaning ‘testicle’. This name is in reference to the shape of
the fruit, which was regarded by the Aztecs as the fertility fruit. The species is
now cultivated worldwide in tropical and subtropical climates.
BOTANY
Family
The laurel family (Lauraceae) is composed of about 55 genera, with more than
2000 species. This basal family in the dicots is in the Order Laurales, which
was sometimes included in the Magnoliales. They are mostly evergreen
trees and shrubs, occasionally aromatic, and native mostly to tropical and
subtropical regions. There are about 150 species of tropical evergreen trees,
many of which are cultivated as ornamentals for their laurel-like leaves.
Important genera and species
The genus Persea Mill. is the best known for the fruit called avocado or
aguacate, P. americana Mill. (Persea gratissima C.F. Gaertn.). The fruits of
most Persea species are small and worthless. Only P. americana and Persea
schiedeana Ness. bear large fruit, the latter species being watery and fi brous
but pleasant in fl avour and eaten by people in its native habitat in Mexico and
Central America. Other species of commercial or ornamental value include
Cinnamomum camphora (L.) Nees and Eberm., camphor tree; Cinnamomum
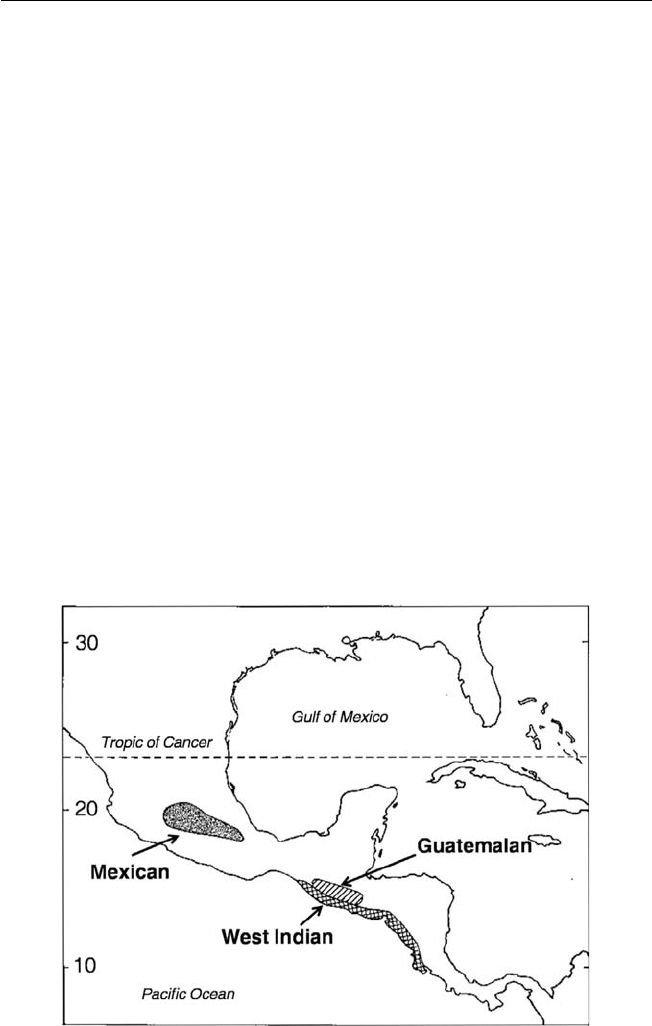
154 Chapter 7
zeylanicum Breyn., cinnamon tree; Laurus nobilis L., bay or sweet bay; and
Umbellularia californica Nutt., California bay tree.
Origin and distribution
There is general agreement that the centre of origin of the avocado is in the
eastern and central highlands of Mexico and in the adjacent highland areas
of Guatemala to the Pacifi c coast. Early European travellers during the 16th
century found avocado in cultivation and distributed throughout Central
America and northern South America. This is evidenced by the native
names given to avocado in many languages and by archaeological fi ndings.
Carbon dating indicates that Mexican avocados were used as food as early as
8000–7000 years ago (Williams, 1976). Separate human selection began
4000–2800 by MesoAmerican Indians, which led to three well-demarcated
ecotypes, known as Guatemalan, Mexican and West Indian (Coastal
Guatemala) (Fig 7.1). These three separate domestications are supported by
ethnobotanical and genetic marker studies (Popenoe, 1920; Ashworth and
Clegg, 2003). The races were separate until after European contact in the 16th
century (Chen et al., 2009).
Early accounts indicate that it was not cultivated in the Caribbean
islands during the pre-Columbian period and was introduced to Jamaica by
Fig. 7.1. Possible evolutionary centres of the three cultivated avocado races
(redrawn from Scora and Bergh, 1992).

Avocado 155
the Spaniards in about 1650. Distribution to the African and Asian tropics
occurred during the 1700s and 1800s. The fi rst recorded importation into
Florida was in 1833, into California in 1848 and into Hawaii during the early
19th century. By 1855, avocado trees were common in gardens of Oahu and
were distributed to the other islands of the Hawaiian chain (Yee, 1978). It is
now widely distributed throughout the tropics and subtropics, but the use of
the fruit di ers in di erent areas. Although the avocado has been available
in most South-east Asian countries, it has not been cultivated widely, due to
a preference for many other fruits. The main producers are Mexico, the USA,
Indonesia, Dominican Republic, Colombia, Chile, Peru and Brazil, with Mexico,
Chile, Israel, Spain and South Africa being the main exporters and Peru trying
to join this group.
The West Indian race is adapted to the humid tropics and is very chilling-
sensitive, with some salinity tolerance. The fruits of the West Indian race
are small with medium-thin, leathery skin; low oil content; loose seed and
maturing 160–240 days after fl owering (Table 7.1). The closely related
Guatemalan race is postulated to have originated at somewhat higher
elevations in Guatemala and adjacent areas, based on abundance of wild
populations (Fig. 7.1). Leaves of cultivated types of this race are not anise-
scented; Popenoe (1920) reported anise-scented wild forms. This race has
some cold tolerance and the fruit has a thick, tough skin that remains green
until maturity. The Mexican race is predominantly found in the higher
Table 7.1. Comparison of selected characteristics of three horticultural races of
avocado (Bergh, 1975; Bergh and Ellstrand, 1986).
Trait West Indian Guatemalan Mexican
Climate Tropical Subtropical Semi-tropical
Cold tolerance Least Intermediate Most
Salt tolerance Most Intermediate Least
Leaf anise Absent Absent Present
Young leaf colour Pale yellow Green with red tinge Green
Mature leaf colour Pale green Dark green Dark green
Bloom to fruit
maturity
5–9 months 10–16 months 6–9 months
Size Variable (to large) Variable (intermediate) Variable (to
small)
Colour of fruit Green or reddish Green Often dark
Skin thickness Medium Thick Very thin
Skin surface Shiny Rough Waxy bloom
Seed size Variable Small Large
Seed cavity Variable Tight Loose
Oil content Low High Highest
Pulp fi bre Less common Less common Common
Pulp fl avour Sweeter, milder Rich Anise-like, rich

156 Chapter 7
elevations of Mexico (Fig. 7.1). Leaves of the Mexican race are anise-scented
and 60% of the essential oil is the monoterpene estragol (Scora and Bergh,
1992). These races are recognized as subspecies P. americana var. americana
(West Indian), var. drymifolia (Mexican) and var. guatemalenis (Guatemalan)
and are based upon taxonomic, biochemical, isozyme and molecular data
(Bergh and Ellstrand, 1986; Furnier et al., 1990; Ashworth and Clegg, 2003;
Chen et al., 2009). Ben-Ya’acov et al. (1995) have suggested adding a new
race, which they call var. costaricensis. Due to the outbreeding nature of these
taxa and human selection and cultivation, there are many interracial hybrids
and some of the principal commercial cultivars are of hybrid origin.
ECOLOGY
Ecological requirements of avocado should be viewed in terms of the areas
of geographical origin. It is generally considered to be a subtropical plant,
except for the tropical West Indian race. Regardless of origin, the avocado has
shown adaptability to a wide range of ecological conditions, from the tropics
to approximate latitudes of 30° N and S. This wide distribution may be due
to the broad genetic di erences of the three horticultural races. In some high
Andean regions, Mexican avocados grow well under these harsh conditions.
Soil
Avocado is grown in a wide variety of soil types. Deep soils of volcanic origin,
sandy loam soils, calcareous soils and other soil types have supported good
growth. Soil pH may range from 5 to around 7. Since avocado is highly
susceptible to root rots, good drainage is crucial and a high water table
undesirable. Trees show dieback in parts of fi elds when the water table is less
than 1 m.
Avocado has little tolerance for saline conditions. Kadman and Ben-
Ya’acov (1970a) produced various degrees of leaf burn by irrigating an
avocado orchard with water containing 150–170 mg chlorine/l. It has also
been shown that West Indian seedlings are more tolerant to salt than Mexican
seedlings, with Guatemalan having an intermediate tolerance (Table 7.1).
Climate
Rainfall
Most cultivars are sensitive to water stress and to excess moisture caused
by poor drainage. In Hawaii, avocado trees have grown well under annual
rainfall of 3125 mm, largely due to the excellent drainage provided by the

Avocado 157
‘a’ a’ soil (crushed lava rock). Generally, a moderate rainfall range between
1250 mm and 1750 mm per annum with good distribution is desirable.
Almost all avocado-growing areas have wet and dry periods, necessitating
some form of supplemental irrigation.
Avocado infl orescences are not damaged by moderate amounts of rain
for short periods, although relatively dry conditions are preferred during
fl owering. Avocado roots are shallow, and prolonged dry conditions during
the critical periods of fl owering and fruit set can cause fl ower and young-fruit
drop. Among the three races, West Indian cultivars are more adapted to high-
summer rains, while the Mexican races possess greater tolerance to water
stress and lower humidity.
Temperature
Humans have attempted to broaden the range of the three races for economic
reasons by cultivation in areas with seasonally adverse conditions. The West
Indian race is best adapted to a humid, warm climate and monsoon rains, with
optimum temperatures around 25–28°C (Table 7.1). Higher temperatures
depress photosynthesis, thus lowering yields. This race is susceptible to frost
and has a foliage tolerance to a minimum temperature of 1.5°C. Mature trees
of Mexican cultivars have been observed to tolerate temperatures as low as
−4 to −5°C without damage to the foliage and wood, although fl owers are
damaged. The Guatemalan race is adapted to a cool tropical climate but is less
tolerant of low temperatures than the Mexican race (McKellar et al., 1992).
Cultivars have shown tolerance to light frost down to −2°C, but fl owers are
damaged by even light frost (Bower, 1981a).
Night temperatures of 15–20°C and day temperatures of ~20°C are the
most suitable temperatures for fl oral development, pollen-tube growth and
embryo development (Table 7.2). Generally, high humidity, exceeding 50%,
is desirable, especially during fl owering and early fruit set. The Mexican–
Guatemalan hybrids, such as ‘Fuerte’, have shown wider temperature
tolerance to cold than those of the Guatemalan race. Vegetatively, ‘Fuerte’
Table 7.2. Effect of growing temperature (day/night) on vegetative growth and
fl owering of ‘Hass’ avocado (after Chaikiattiyos et al., 1994).
Temperature
Percentage of terminal shoots
(°C)
Panicle Vegetative Dormant
15/10 35 0 65
20/15 20 0 80
25/20 0 100 0
30/25 0 100 0

158 Chapter 7
survives temperatures above −4°C, although the fl owers are damaged and
fruit set can be very poor at 17/12°C (day/night).
Solar radiation and photoperiod
Avocado is a typical C
3
plant, with maximal CO
2
exchange occurring at
20–25°C. The light saturation point for photosynthesis of mature ‘Hass’
avocado trees is 1110 mol quanta/m
2
/s photosynthetic photon fl ux, about
half that at midday in the tropics. Leaves take about 40 days from bud-break
to when they become net exporters of photoassimilates. During these 40 days
leaves may compete with developing fruit for available photoassimilates, and
this competition infl uences fruit retention and fruit development. Day length
is apparently of little importance, as there have not been any published studies
on avocado responding to photoperiod.
Wind
The avocado tree is easily damaged by winds, due to its brittle branches.
Moderately high winds can cause severe damage. If orchards are not located
in naturally sheltered areas, windbreaks are advised.
GENERAL CHARACTERISTICS
Tree
The avocado tree is variable in shape, from tall, upright trees to widely
spreading forms with multiple branches. Trees can attain heights of 15–18 m,
with manageable height being controlled by pruning. Although classifi ed as
an evergreen, some cultivars shed leaves during fl owering, which are replaced
rapidly from terminal shoots. Others shed their leaves gradually, so that
they are never without leaves completely. The dark green leaves are spirally
arranged and variable in size, from 10 to 13 cm wide and from 20 to 25 cm
long, entire, elliptic or ovate to lanceolate (Fig. 7.2).
New growth occurs in fl ushes (Fig. 7.3). Flushes of shoot and root tend to
alternate on a 30–60-day cycle (Ploetz et al., 1991). Root growth continues
throughout the year in subtropical areas, even at a low rate between the
fl ushes, while shoot growth may stop. The period between fl ushes varies
with location (Fig. 7.3) and cultivar. Growth fl ushes in summer tend to be
asynchronous, with only a portion of a tree canopy being involved. Trunk
starch concentration ranged between 4.5 and 6.3% during active tree and
fruit growth. In trees where the fruits are not harvested until full maturity
with 30% dry matter, a trunk starch concentration of 5.3% occurs prior to
fl owering, versus 7.7% in trees harvested earlier (Kaiser and Wolstenholme,
1994).
Avocado 159
(a)
(b)
(c)
3 mm
Fig. 7.2. Avocado leaf, fruit and fl ower. Flower (a) is from the fi rst opening stage
and is female; (b) is the second opening and male, with the anthers semi-erect and
dehisced; and (c) is a fully mature fl ower after dehiscence.
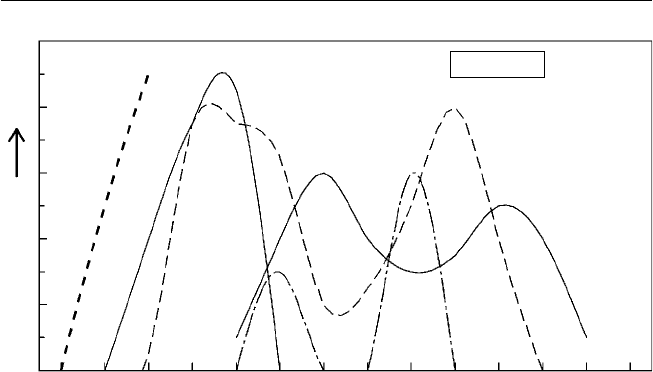
160 Chapter 7
The juvenile period in avocado can be from 5 to 15 years. If girdled in
early autumn 3 years after planting, fl owering and fruit set can be increased
signifi cantly. Since the cultivars ‘Pinkerton’ and ‘Gwen’ have precocious
o spring, juvenility is likely to have a genetic basis, which is modifi ed by
environment (Lavi et al., 1992).
Infl orescence and fl ower
The small, pale green or yellowish-green fl owers are borne on multi-
branched axillary panicles terminating in a shoot bud (Fig. 7.2). One or two
million fl owers may be produced in a single fl owering period, although only
about 200–300 fruit mature (Whiley et al., 1988b). This fl owering leads to
considerable water loss, and the recommendation is to irrigate during this
period. The fl ower is bisexual with nine stamens, six of which form the outer
circle and three in the inner circle. At the base of the inner circle are located a
pair of nectaries, alternating with three staminodes, which also secrete nectar.
Each stamen has four pollen sacs, which release cohesive pollen. The single
pistil contains one carpel and one ovule (Bergh, 1976).
The avocado fl ower has a unique fl owering behaviour (Fig. 7.4), and
all avocado cultivars and seedlings, irrespective of race, fall into one of two
complementary groups, designated ‘A’ and ‘B’ (Table 7.3). Flowers of the
Flower bud
development
Flowering
fruit set
Vegetative
growth
Harvest
Root
growth
Fruit
drop
Fruit
drop
May Jun Jul Aug Sep Oct Nov Dec
Month
Jan
Growth
Feb Mar Apr May Jun
Fig. 7.3. Phenological cycle for bearing cv. ’Fuerte’ in Queensland, Australia,
showing the development and interaction between root, shoot, fl ower and fruit
growth. The cycle will vary with the cultivar and needs to be determined for each
location (redrawn from Whiley et al., 1988a).
Avocado 161
‘A’ class open in the morning for 2–3 h, functioning as females with a white
stigma, while the stamens remain closed and are in a horizontal position.
These fl owers close at approximately noon and reopen the following day
during the afternoon hours for 3–4 h, functioning now as males, with stamens
in a vertical position. The stigmas are no longer functional. Flowers of the ‘B’
class open in the afternoon as females, the stamens remaining closed. These
fl owers close in the evening and reopen the next morning as male fl owers. This
phenomenon is called protogynous, diurnally synchronous dichogamy (Bergh,
1969). The dichogamy is protogynous, as the pistil matures before the stamens
in both classes and all fl owers of a class are synchronized to be functionally
female at one time of the day and functionally male at another time. Looking
at the whole tree with numerous fl owers opening any day during the fl owering
period, varieties of the ‘A’ group are in the female stage during the mornings
and in the male stage during the afternoons and those of the ‘B’ group are
in the female stage in the afternoons and in the male stage in the mornings;
this way they complement each other for fertilization purposes. Temperatures
of 17/12°C and 33/28°C during fl owering time can prevent pollen-tube and
embryo growth, resulting in the production of unfertilized, underdeveloped
fruit. This disruption of fruit set, with endosperm and embryo development
not being observed at low temperatures (17/12°C), is more marked in type
‘B’ fl owers (cv. ‘Fuerte’), where no female fl ower opening occurs (Sedgley and
Grant, 1983). The fl owering cycle is extended by cool temperatures from the
usual 36 and 20 h for types ‘A’ and ‘B’, respectively. Growing temperatures of
25–30°C during the day and night temperatures of 15–20°C are considered
optimum (Table 7.2). A much cooler temperature range during winter will
stimulate fl owering (Whiley, 1984). Type ‘B’ cultivars (‘Fuerte’) are generally
less productive than type ‘A’ (‘Hass’) under cool fl owering conditions.
Time
Type A
Day 1 Day 2
Type B
06:00 12:00 18:00 06:00 12:00 18:00
Fig. 7.4. Protogynous dichogamy pattern of fl ower opening involves two types, A
and B, with both types found in the three ecological races. This behaviour enhances
cross-pollination. These patterns may be disrupted by low temperatures and some
overlap occurs.

162 Chapter 7
Flowering occurs from late autumn to spring, depending upon the cultivar
and prevailing temperatures (Fig. 7.3). West Indian and Mexican cultivars
generally may start to fl ower early, in October or November and October–
December, respectively, north of the equator. The West Indian avocado is
called the summer avocado in Hawaii. Guatemalan cultivars and many
Guatemalan × Mexican hybrids begin to fl ower around March, extending to
May, and are called winter avocado in Hawaii. In the largest ‘Hass’-growing
area in the world, Michoacán, México, the trees can fl ower three or four times
per year; the fi rst, called ‘crazy’ fl owering, occurs in August–September, the
second or ‘advanced’ in October–November; the ‘normal’, which generally is
the heaviest, occurs in December–January and, fi nally, the ‘March’ fl owering,
which takes place at the beginning of spring. These fl owering events may not
occur in all trees, and the intensity of fl owering will vary according to the fruit
set in the other fl owering cycles (Salazar-García, 2000).
Pollination and fruit set
The protogynous, diurnally synchronous dichogamy in avocado fl owers is
the normal behaviour and occurs if warm weather prevails during fl owering.
Synchronous dichogamy of fl owers of the same cultivar restricts self-
pollination and encourages cross-pollination between those of complementary
groups. Exposure to sun, wind and temperature changes can cause variability
in the time of fl oral anthesis, prolonging the open period and time between the
two open periods, and increasing the overlap of female and male opening on a
single tree. However, with moderate day temperatures (25°C) and cool nights
Table 7.3. Classifi cation of selected avocado cultivars according to fl ower types and
climate adaptation.
Type A Type B
Subtropical
Anaheim,Wurtz Bacon, Edranol
Hass, Lamb Hass Fuerte, Ettinger
Gwen, Hazzard Nabal, Millicent
Jalna, Pinkerton Ryan, Sharwil
Reed, Rincon Zutano,Colin V-33
Tropical
Catalina, Collinson Booth 7, Booth 8
Choquette, Fairchild Gripiña No. 2, Hall
Lula, Princesa Itzamna, Kampong
Russell, Semil 34 Linda,
Monroe
Semil 42, Semil 44 Pollock, Prince
Simmonds, Waldin Semil 43, Winslowson

Avocado 163
(20°C), there is su cient overlap of fl ower types to permit self-pollination of
up to 93%. The possibility of self-pollination is enhanced by relatively long
pollen viability. There is also evidence that the stigma remains white and is
still receptive on the second day (Davenport et al., 1994). The avocado can
be insect-pollinated, although self- or wind pollination is possibly the norm
(Davenport et al., 1994). Insects, including honey bees, can carry the sticky
pollen on their bodies and are capable of depositing viable pollen from one
fl ower cycle to another within the same cultivar.
In spite of the fact that some studies have shown relatively high rates of
self-pollination, most investigators have advocated interplanting of pollinator
cultivars of the complementary class in orchards, in order to increase fruit set
(Bower, 1981c). In the case of areas with mild climates, such as Michoacan,
México, there is no need for interplanted pollinator cultivars and most
orchards are made up by solid blocks of ‘Hass’ (Salazar-García, 2000).
Some cultivars are more e ective in increasing fruit set than others.
This may indicate the presence of varying degrees of incompatibility among
cultivars, with some complementary cultivars superior to others. Mexican
cultivars are more e cient pollen providers in California and Israel, while
Guatemalan cultivars are more e ective in Florida, because of greater cold
tolerance of pollen improving the chances in the fertilization process (Gazit,
1976). Under warmer conditions, West Indian hybrids are good as pollen
donors, probably due to their greater tolerance of higher temperatures.
However, fruit set may be a poor measure of fi nal matured fruit, as all cultivars
lose fl owers and developing fruitlets, regardless of pollination.
Following pollination, considerable fruit drop occurs due to poor
pollination and excessive vegetative growth (Wolstenholme and Whiley,
1992). There is probably su cient carbohydrate available from mature leaves
to support the growth of both developing fruitlets and young leaves (Finazzo
et al., 1994). This supply during early fruit development does not limit fruitlet
growth or stimulate fruitlet abscission. However, the lack of an adequate
spring shoot growth, though competitive, will mean that fruit set and early
fruit growth will be dependent upon older leaves and stored carbohydrate,
which may be depleted by fl owering. The later vegetative growth fl ush near
harvest is crucial for fi nal fruit growth, build-up of carbohydrates and the
following period of root growth (Fig. 7.3). This is discussed further with
respect to maturity of fruit at harvest and biennial bearing.
Fruit
The avocado fruit is a one-seeded berry (Fig. 7.2). The single large seed is
composed of two cotyledons enclosing an embryo and is surrounded by a thick
fl eshy mesocarp. The skin varies in thickness up to 6 mm, depending upon the
race (Table 7.1) and has 20,000–30,000 stomata per fruit, less than on a leaf.
164 Chapter 7
The skin colour of the ripe fruit ranges from several shades of green to yellow-
green, from reddish to maroon and from light to dark purple. The buttery fl esh
(mesocarp) is greenish-yellow to bright yellow to cream when ripe. Oil content
ranges from 7.8 to 40.7% on a fresh-weight basis (Kawano et al., 1976). Size
varies from the small fruit of some Mexican types, about 225 g or less, to the
large Guatemalan types, 1.4–2 kg or more (Table 7.1). In shape, the fruit is
usually pyriform to oval and round.
In West Indian and Mexican cultivars, the fruit matures 150–240 days
after anthesis, while Guatemalan cultivars take more than 250 days (Fig. 7.5).
All show a typical sigmoid growth curve (Fig. 7.6). There is little delay before
growth occurs in fruit, seed and pericarp thickness.
CULTIVAR DEVELOPMENT
Cytogenetics and genetics
Persea species generally have a diploid number of 24 chromosomes. Among P.
americana materials examined, Garcia (1975) found one triploid (2n = 36) and
Capac
Mexican
0
0
100
200
300
Fruit mass (g)
400
500
600
70 140
Days from full bloom
210 280
Taylor
Guatemalan
Waldin
West Indian
Fig. 7.5. Increase in fruit mass of three cultivars from different ecological races,
showing the difference in time for fruit to reach maturity and fi nal fruit size relative
to the ecological race (redrawn from Valmayor, 1967).

Avocado 165
a tetraploid (2n = 48). Karyotype in Persea is asymmetric, with chromosomes
ranging in size from 2.3 µm to 6.1 µm.
Garcia and Tsunewaki (1977), using peroxidase isozyme analysis, found
signifi cant variations between Mexican strains, within and between nine
states in Mexico. The three races showed signifi cant variation in their pattern
of activities. The frequency of signifi cant variations within strains of the three
races is high in the Guatemalan (62.5%), intermediate in the West Indian
(50.0%) and relatively low in the Mexican race (39.0%). Isozymes have also
been used as genetic markers to detect di erent genotypes resulting from
natural outcrossing and controlled pollinations (Bergh and Ellstrand, 1986).
A number of molecular approaches have been used as genotypic markers and
assignment of racial composition. E orts to apply these techniques in avocado
breeding programmes are hampered by the long generation time and the
inability to perform controlled pollinations (Violi et al., 2009).
Skin colour, fl owering group and anise scent in the leaves are probably
coded by several loci, having several alleles in each loci (Lavi et al., 1993a). The
juvenile period also has a major genetic component. Quantitative traits, such
as tree size, fl owering intensity, fruit weight, fruit density and harvest duration,
show both additive and non-additive genetic variance (Lavi et al., 1991).
125
100
75
50
25
Fruit
length
Seed
length
Pericarp
thickness
0 50 100
Days from full bloom
Fruit, seed and pericarp (mm)
150 200
0
Fig. 7.6. Rate of total fruit and seed growth and the increase in pericarp thickness of
the West Indian cultivar ‘Waldin’ (redrawn from Valmayor, 1967).

166 Chapter 7
Breeding
Intensive avocado breeding has been conducted over many years in the USA,
Israel, South Africa and other countries. However, until recently, cultivars in
the avocado-growing countries of the world originated as chance seedlings
(Bergh, 1976).
Breeding methods may be by self-pollination or by hybridizing two selected
parental cultivars, the choice depending upon objectives of the breeding
programme. The avocado is highly heterozygous, because of signifi cant cross-
pollination between complementary cultivars, and signifi cant variability
can be obtained among self-pollinated progenies (Lavi et al., 1993b; Violi
et al., 2009). Selfi ng can be achieved by the use of cages with pollinator bees
enclosed within the tree or by using isolated trees of a desired parent cultivar
(Bergh, 1976). Outcrossing is reported to be nil where trees of a cultivar
are separated from other genetic lines by 100 m or more. Variations in daily
weather conditions during the fl owering period may disrupt the diurnal
synchrony of the fl owers, allowing self-pollination.
Cross-pollination is necessary when two or more desirable traits are to be
combined or when traits intermediate between two cultivars are desirable, but
this has proved di cult with avocado. The major cultivars in subtropical areas
are hybrids of the Guatemalan and Mexican races, as the Mexican race is the
source of cold-hardiness. In tropical regions, hybrids of the Guatemalan and
West Indian races prevail.
Selection and evaluation
Breeding objectives may be divided into traits that are universally desired
and those which are regionally specifi c. Tree characteristics sought after in
all growing areas are ease in propagation, vigour, precocity, spreading, short
fruit-maturation period, heavy and regular bearing, and wide adaptability
with resistance to disease and insect pests. Traits specifi c to regions are cold
or heat tolerance and salinity tolerance. Other qualities, such as dwarfi ng or
semi-dwarfi ng, genetic uniformity, freedom from sun-blotch disease, caused by
a viroid, and drought resistance, are also constantly evaluated (Bergh, 1976).
Universal fruit characteristics are resistance to diseases, pests and
blemishes, long tree storage, uniform ripening, minimum fi bres and long shelf-
life (Currier, 1992). Traits that di er due to specifi c climatic conditions or
market preferences are fruit size and shape, skin colour, oil content and chill
tolerance. Market preference is usually based upon consumer familiarity with
certain traits. Fruit size is a good example. Consumers in California generally
prefer a size range of 200–300 g, while in most Latin American countries
larger fruits are preferred.

Avocado 167
Breeding for cold-hardiness is a major objective in the subtropics.
‘Brooksville’, a seedling of the Mexican race, has shown outstanding cold-
hardiness, withstanding artifi cial chilling to −8.5°C. ‘Gainesville’, another
Mexican type, withstands fi eld temperature as low as −9.4°C. The cold-
hardiness of these cultivars has to be combined with genes for dependable
productivity and fruit market acceptance (Knight, 1976).
Avocado rootstock breeding is an important aspect of the total breeding
programme. The avocado root rot, caused by Phytophthora cinnamomi, has
led to testing thousands of seeds and bud-wood from avocado trees that have
resisted the disease over many years and other Persea species collected in their
native habitats. Small-fruited species, such as Persea caerulea (Ruiz y Pavón)
Mez., Persea donnell-smithii Mez., Persea pachypoda Nan and Mart. and Persea
cinerascens Blake, though having strong resistance, are graft-incompatible
with the avocado. Hybridization between the small-fruited Persea species and
avocado has not been successful. California and Florida have been the most
active places doing hybridization, introducing species and varieties from their
native sites and trying existing local or foreign materials in search of better
rootstocks; Israel, South Africa, Australia and lately Chile have also been
working on this important matter.
Rootstocks
In California, several rootstocks have been tried and used, especially for root
rot resistance, with salinity and alkalinity resistance being less important.
After many years of using Mexican seedlings like ‘Topa Topa’ and ‘Lula’, with
the increase of the root rot problem in the 1970s the industry adopted the
rootstock ‘Duke 7’, a Mexican seedling of ‘Duke’ with moderate resistance
to root rot, which became the fi rst commercially successful rootstock that
was propagated vegetatively. Another resistant rootstock, ‘Martín Grande’
(G-775C), a hybrid from P. americana and P. schiedeana, did not perform well
and was discarded. ‘Thomas’, ‘D9’ and ‘Barr Duke’ (all Mexican) have greater
resistance than ‘Duke 7’ and are available (Ben-Ya’acov and Michelson,
1995). The latter three were all found in infected fi elds. In the absence of root
rot, the rootstock can signifi cantly infl uence canopy growth and yield. ‘Duke
7’ and ‘Thomas’ produce signifi cantly more fruit per cubic metre of canopy
than other rootstocks, with ‘D9’ and ‘Martín Grande’ being the poorest
performers (Arpaia et al., 1992). During the last few years ‘Toro Canyon’, of
the Mexican race, has become important; it is more resistant to root rot than
‘Duke 7’ and more tolerant to salts than the Mexican seedling rootstocks; it
also has resistance to Phytophthora citricola. Two releases from the University
of California-Riverside are ‘Zentmyer’ and ‘Uz’ of the Mexican race, which
look very promising. Additionally, California has introduced, and will probably
be using commercially, the ‘Dusa’ and ‘Latas’ (Mexican × Guatemalan)

168 Chapter 7
clonal rootstocks developed in South Africa. ‘Dusa’ has already been released
to nurseries since it is root rot and salinity tolerant. Several dozen selfi ng or
outcrossing seedlings showing exceptional resistance to Phytophthora root rot
are under trials.
South Africa has serious root rot problems, and they followed a path
similar to California in the early years of last century and used Mexican
seedling rootstocks. In the 1950s, because most of their Mexican mother
plants were contaminated with sun blotch, they started using the Guatemalan
‘Edranol’, which had no resistance to root ríot, and later ‘Duke’, and then
after many trials ‘Duke 7’, which is now the standard rootstock, providing
reasonably healthy, productive and uniform trees (Kremer-Köhne and Köhne,
2007). Recent rootstock selections from South Africa, ‘Latas’ (Merensky
I), ‘Dusa’ (Merensky II) and ‘Evstro’ (Merensky III) have great production
potential with ‘Hass’ and tolerance to Phytophthora root rot and salinity. ‘Dusa’
is more root rot tolerant and productive than ‘Duke 7’ and it has been made
available to growers in several countries. Australia uses plants grafted on
seedling rootstocks that are very diverse; this complicates management of the
orchard. They have started a programme to identify superior rootstocks and
are looking for an e cient clonal propagation system. They will start selecting
material from superior trees in the fi eld and will evaluate imported rootstocks.
Chilean breeders used plants grafted mainly on ‘Mexicola’, and other Mexican-
race seedlings are going in the same direction. While in Peru ‘Topa Topa’
seedlings are generally used, and West Indian types imported from Israel are
used in areas with salinity.
A rootstock with resistance to salinity is a focus of research in Israel,
California, Chile, Australia and the north and central coasts of Peru. The
three races di er in their ability to resist the uptake of salts (Table 7.1), in
their ability to retain the toxic salts in the root system without translocating
them to the scion and in their tolerance within their tissues. The West
Indian rootstocks have the highest level of tolerance to salinity, followed by
Guatemalan and Mexican races (Ben-Ya’acov and Michelson, 1995). Israel
has taken two approaches: (i) selection of types that produced a resistant
seedling population; and (ii) selection of individual resistant plants for
vegetative propagation. Unfortunately, the true West Indian and high-salinity-
resistant types are di cult to propagate by cuttings. The cold-tolerant Mexican
‘Toro Canyon’ and ‘Duke 7’ have the best salinity rating; ‘Thomas’ and ‘Martín
Grande’ had intermediate ratings; and ‘Barr Duke’ had the lowest rating. The
recent rootstock selections from South Africa, ‘Latas’, ‘Dusa’ and ‘Evstro’,
show some salinity resistance.
The main problem with clonal rootstocks is that the plants lack a tap
root, and therefore special care has to be taken that water supply is timely
and adequate, especially in the most superfi cial portion of the soil, during
the fi rst year or so, otherwise the plants will su er and many of them will not
recover properly.

Avocado 169
Cultivars
Cultivars from California, and some from Chile, Australia and Israel, are best
suited for subtropical areas or for higher elevations above 800–1000 m in the
tropics; they normally have a higher oil content, which for many consumers
makes them tastier. The cultivars developed in Florida and the Caribbean
Islands are most suited for the tropics and normally have a lower oil content,
and most of them are green when ripe and are therefore called ‘green skins’
in the international trade. Some California and Florida cultivars have become
international (Table 7.4), providing the basis for avocado development in
many countries. ‘Hass’ a Guatemalan type, is the most widely distributed,
grown and exported cultivar, replacing ‘Fuerte’, a Mexican–Guatemalan
hybrid. One or both have dominated the plantings in Australia, Israel, South
Africa, Mexico, Chile, Peru, the Canary Islands and other areas (Table 7.4).
In California, ‘Hass’ has gradually become the dominant cultivar, largely due
to the relatively poor yields and inconsistent bearing of the type ‘B’ cultivar
‘Fuerte’, whose fl owering is disrupted by low temperatures; ‘Zutano’, ‘Bacon’,
‘Gwen’ and ‘Pinkerton’ are other commercial cultivars. In the last years ‘Lamb
Hass’ and ‘Gem’ and a few others have been developed as alternatives or
complements for ‘Hass’. In Mexico ‘Colin V-33’ was selected from a population
of open-pollinated ‘Fuerte’ seedlings; it is a type ‘B’ with very-good-quality
pulp, green-coloured fruit, superior to ‘Fuerte’; it is considered a dwarf plant
and can be used as a dwarfi ng interstock (Barrientos-Priego et al., 2000). In
California, the fruit of most cultivars can be stored on the tree for months after
reaching maturity, depending upon season, so not many cultivars are needed
to market fruit throughout the year. However, this tree storage can lead to
more pronounced biennial bearing.
Florida cultivates a larger number of mostly West Indian and West Indian–
Guatemalan hybrids to extend the market season (Campbell and Malo, 1976).
Cultivars are grouped into early-, mid- and late-season. ‘Pollock’, ‘Waldin’,
‘Simmonds’ and ‘Nadir’ are some early-season types. Some mid-season
Table 7.4. A list of selected cultivars grown in major avocado-growing areas.
California Florida Australia Israel
South
Africa Mexico Spain Chile Peru
Fuerte Pollock Zutano Fuerte Fuerte Fuerte Hass Hass Hass
Hass Simmonds Sharwil Hass Hass Hass Bacon Fuerte Fuerte
Zutano Nadir Bacon Nabal Edranol Bacon Fuerte Negra Nabal
Bacon Booth 8 Fuerte Ettinger Ryan Reed Reed la Cruz
Reed Lula Hass Horshim Criollo Zutano Bacon
Pinkerton Hardee Wurtz (Local) Gwen Edranol
Gwen Ruehle
Choquette
Zutano

170 Chapter 7
cultivars are ‘Booth 7’, ‘Booth 8’, ‘Collinson’, ‘Hall’ and ‘Hickson’, while late-
season cultivars are ‘Lula’, ‘Monroe’, ‘Choquette’, ‘Booth 3’ and a few others
(Table 7.4). A similar situation occurs in Hawaii, where a large number of
cultivars are grown, including introductions from California, Florida, Australia
and Mexico and selections of local origin. ‘Sharwil’, an Australian cultivar, has
become a leading cultivar, with a recent release, ‘Green Gold’, increasing in
acreage. Puerto Rico and the Dominican Republic cultivate some of the Florida
cultivars along with some locally developed cultivars, such as the West Indian-
Guatemalan hybrids ‘Semil’ and ‘Gripiña’.
Mexico grows ‘Fuerte’ and ‘‘Hass’ as the main cultivars, along with other
California and Florida ones. The ‘Criollo’ is mentioned as a selection of the
Mexican race and is well adapted to median ecological areas of Mexico (Diaz-
Avelar, 1979). Besides Mexico, Brazil has developed a substantial industry,
mostly with introduced cultivars, such as ‘Fuerte’, ‘Hass’, ‘Carlsbad’, ‘Corona’,
‘Edranol’, ‘Nabal’ and ‘Ryan’, and cultivars of local origin, such as ‘Solano’,
‘Ouro Verde’, ‘Quintal’ and ‘Fortuna’.
In Australia, ‘Hass’ and ‘Fuerte’ have been planted most frequently,
especially along the coastal areas of northern New South Wales and southern
Queensland. ‘Sharwil’, a local selection (Guatemalan–Mexican hybrid), has
gained considerable popularity, although in some areas it had fl uctuating
yields. Under tropical conditions in the Northern Territory, the Mexican
and Guatemalan cultivars yield poorly (Sedgley et al., 1985). South African
production is largely from ‘Hass’ and other California cultivars. Israel’s
production was initially based on California cultivars, until an intensive
selection programme over the years produced a large number of local
cultivars, such as ‘Ettinger’ and ‘Horshim’.
In Asia, the avocado has not attained the popularity it has in other areas,
although it was introduced into Malaysia over 120 years ago. In Indonesia,
avocado grows well at 200–1000 m on well-drained soils, and trees are
generally propagated by seeds. In the Philippines, many cultivars have been
introduced from the USA since 1903, and avocado is grown in nearly all parts
of the country, although it has not attained the popularity of other fruit.
CULTURAL PRACTICES
Propagation and nursery management
Avocado is primarily propagated commercially by budding or grafting upon
seedling rootstocks. However, the variability of seedling populations with
respect to certain desirable characteristics, such as resistance to Phytophthora
root rot and tolerance to salinity and calcareous soils, has posed problems
(Ben-Ya’acov and Michelson, 1995). Seedling production has largely changed

Avocado 171
from grafting nursery-grown trees to grafting container-grown trees. Seeds
are taken from fruit picked from trees free of sun-blotch and are treated in
a water bath at 49–50°C for 30 min, cooled and surface-dried in a partially
shaded area. Seeds are planted (broad side down) in polyethylene bags, with a
well-draining potting mix. Seeds germinate in about a month. The papery seed
coats should be removed. If seeds are not very fresh, cutting o a piece of seed
tip or doing some vertical cuts at the side of the seed will improve germination
speed and percentage; sometimes a slice of bottom can also be cut o .
Seedlings may be cleft or side-wedge grafted 2–4 weeks after germination.
Scion wood from terminal growth of sun-blotch-free cultivars should be used.
Propagation is usually done in shade houses, preferably with temperature
control if the environment has a wide temperature range. Grafted plants
must be hardened for approximately 2 weeks under full sunlight before fi eld
transplanting. Under open-fi eld conditions in the central coast of Peru, with
13–15°C lowest temperatures in winter, Duarte et al. (1975) reduced by 2
months the time to obtain a grafted plant with seeds sown at the start of
autumn and by 1 month with seeds sown at the end of autumn, by spraying
the seedlings three times with 250 or 500 ppm GA at 2-week intervals,
starting when they had reached 15 cm.
Leafy avocado cuttings of some genotypes under mist consistently rooted
nearly 100% under practically any conditions, while others did not root at
all or rooted with di culty (Kadman and Ben-Ya’acov, 1970b). Generally,
West Indian cultivars with strong resistance to salinity are di cult to root.
Cuttings from mature trees were di cult to root and, in those that rooted
well, they took 4–10 months to root. Cuttings from 1-year-old seedlings show
a higher percentage of rooting in 4–12 weeks. A 50% light intensity in the
intermittent-mist system during the summer is better for rooting than full
sunlight. The anatomy of the avocado stem provides a reason for the di culty
in rooting as the fi bre bundles and the sclereid ring are thicker in the West
Indian types, intermediate for Guatemalan and hybrid types, and least for the
Mexican cultivars.
Air layers and cuttings have been successfully rooted. Variability exists
in ease of rooting air layers between races and even among cultivars of a
race, and the di culty of large-scale production has discouraged commercial
development. Generally, Mexican cultivars root most easily, followed by
Guatemalan and West Indian, whether by air layering or by cuttings. Time
required for rooting of air layers ranged from 146 to 518 days, depending
upon cultivar and the time of the year. Etiolated stems show few or no sclereid
connections between fi bre bundles, suggesting that the sclerenchyma ring may
be acting as a barrier to root emergence (Gomez et al., 1973). Hence, shoots of
most avocado cultivars produced in light do not root nearly as well as shoots
produced in darkness.
Seedlings produced upon scions of the desirable rootstock cultivar are
grafted by tip grafting as close to the base as possible. Shoots of the scion

172 Chapter 7
are allowed to grow and then cut back to near the base. When new buds
show signs of growth, the entire plant is placed in a dark room, with the
temperatures maintained at 21–23°C. When the new shoots reach about
8–10 cm in the dark, the plants are again placed in light, with a tar-paper
collar placed around each etiolated stem and fi lled with vermiculite to
continue exclusion of light from the base of the shoots. Only the tips of the
shoots are exposed to light, in order to produce green leaves. This procedure
is done under shade to prevent sunburn. Shoots are then allowed to grow
until several leaves have matured. The collar is then removed and shoots are
detached for rooting in propagation frames. Rooting hormones have shown no
benefi cial e ects, although some reports from Australia indicate that a 360
o
scrape of the etiolated shoot prior to the application of the potassium salt
of indole butyric acid consistently gave a crown of roots. Rooted shoots are
transplanted into 10 cm peat pots and grown in an enclosed area for further
root and top growth and gradually hardened. These rooted plants are then
transplanted into larger polyethylene bags for more growth and hardening,
then grafted when they attain appropriate size. The side-wedge technique is
usually used, so some terminal foliage on the rootstock is retained until scion
growth begins.
In California, South Africa and to a lesser degree in Spain and Chile, clonal
rootstocks are used and propagated based on the double-grafting–etiolation
method. A large avocado seed such as ‘Lula’ is grown in a container to serve
as a nurse stock. When the nurse seedling that was sown into a planting sleeve
is large enough for grafting, a splice or cleft graft with a tolerant rootstock is
made. A metal girdle is loosely put above the bud or graft union so that it will
eventually girdle the stem; the grafted rootstock is forced to root above the
girdle (Brokaw, 1975). The nurse seed with the grafted tolerant rootstock is
then grown in the dark till the graft is 20–40 cm tall. The container is then
removed from the dark, and sterile rooting medium is supplied to cover the
base of the bud union. Adventitious roots develop from the tolerant rootstock.
Once the rootstock growth hardens o , it is grafted with the desirable scion
variety and grown for 2–3 months. The double-grafted plant is then grown
for several months before planting. Rooting hormone or minor cuts near the
bud union between the tolerant rootstock and the nurse seed can enhance
the rooting success of the rootstock. Once in the fi eld it is assumed that the
rootstock will extend its roots and the nurse seed will be excluded from the
system due to the girdle. In South Africa, an improvement to this method
was developed (de Villiers and Ernst, 2007). This consists of positioning
a 55 ml micro-container on the etiolated shoots coming from the grafted
rootstock. Roots will form in these containers, and after grafting the desired
cultivar on to these shoots, they will be separated from the nurse seedling just
above the nurse graft and taken to the nursery for hardening; after this they
will be transplanted into nursery containers or bags. The advantages of this
improvement are that more than one plant can be obtained from a nurse seed;

Avocado 173
reduced plant cost because of the e ciency of this technique; the root system
of the rootstock is separated from that of the seedling, which assures better
sanitary conditions, and it can be inspected at transplant time; plants with a
better root system and with a more uniform growth in the fi eld are obtained.
Field preparation
Land preparation for avocado does not di er from that for other tree crops.
Development of a drainage system is a prime consideration. Subsoiling or
ripping down to at least 0.5 m or more, preferably running diagonally across
the slope to allow subsurface movement of water, aids drainage. If soil pH
needs adjusting, this could be done during the fi nal stages of land preparation.
Cover crops, such as legumes or grain, can be preplanted a year before orchard
planting to increase organic matter and minimize erosion and root rot.
Transplanting and plant spacing
The use of grafted, container-grown trees has dramatically minimized
transplanting mortality. Polyethylene bags can be removed with all the
soil intact around the roots. Soil in the planting hole should be moist but
not wet. In dry areas, application of water in the holes a few days before
transplanting is advisable to moisten the soil. In the tropics, transplanting
can be done during the dry season, if irrigation is readily available; otherwise,
transplanting should be done at the beginning of the rainy season. In the cool
subtropics, where low winter temperatures can be expected, young plants
may need to be protected or transplanting delayed until the coldest period has
passed. In Peru, some growers prefer to plant the rootstocks in the fi eld and
later graft or bud on to them; this results in a more vigorous growth of the
plant during the initial years.
The cultivar’s natural growth habit (spreading or erect), vigour of the
rootstock, and the environment and soils are major determinants of the
mature tree size, and this infl uences spacing and the continued productivity
of the orchard. Close spacing is sometimes used, with later thinning to obtain
maximum benefi ts per unit area. Initial close spacing can be justifi ed only for
precocious cultivars and is benefi cial only if trees are judiciously thinned when
this becomes necessary. For non-precocious cultivars, the yields obtained
before tree thinning becomes necessary may not justify the additional cost.
Traditional spacings in Florida are 7.5–10.5 m in rows and 7.5–12 m between
rows, which precludes canopy overlap. Narrower spacings of 4.5–6 m in rows
and 6–7.5 m between rows lead to higher yields while the trees are young.
In South Africa, similar narrow spacing of 7.5 m × 7.5 m (177 trees/ha) is
recommended for ‘Fuerte’ and 7 m × 7 m (204 trees/ha) for ‘Hass’, requiring

174 Chapter 7
later tree thinning or topping. At close spacing on the square, thinning is done
by removing the trees in every second diagonal row (Bower, 1981b). In Peru,
distances of 6 m × 4 m and 6 m × 3 m are being used, while in Chile 6 m ×
2 m, 6 m × 3 m and even 3 m × 3 m are being tried. The narrow plant spacing
needs careful formation and maintenance pruning to avoid overcrowding.
Intermediate plants will have to be removed as the canopy starts to overlap.
High-density schemes allow for higher initial yields, but management,
especially pruning, has to be more precise.
Placement of pollinizer trees in the orchard so that an adequate ratio
can be maintained after thinning may need to be considered in some spacing
schemes. The old guideline was one pollinizer for every nine trees. Large
blocks of a single cultivar have shown good production, presumably by self-
pollination, but interplanting pollinizer cultivars undoubtedly increases fruit
set for some cultivars that may not have pollen or are a poor pollen source.
‘Ettinger’ is an excellent pollen source for ‘Hass’ and there is a higher survival
of fruit to maturity.
Irrigation
The avocado can tolerate neither water stress nor excess moisture, especially
when drainage is inadequate. Water stress reduces yields, fruit size and
tree vigour. The soil around the trees should be moist but not wet. Irrigation
frequency depends upon fi eld condition, soil drainage and tree density, as well
as canopy size and prevailing weather conditions and past irrigation records.
Evapotranspiration data and tensiometer readings averaged over a period of
days provide a more accurate means of determining irrigation timing. The
application rate is calculated, taking into account the evapotranspiration
rate since the last irrigation, rainfall, percentage canopy coverage of the
ground and e ciency of water use. Young, non-bearing trees require light,
more frequent irrigation. Older trees can withstand longer intervals between
irrigations but never to a point of water stress. Only 50% of the tree’s
requirements should be given in the middle of the cool season and spring,
in order to favour fl owering rather than vegetative growth. When fruit set is
completed, irrigation reverts to normal amounts during fruit development.
High irrigation rates are necessary during fl owering and may be necessary as
the fruits approach maturity and if the weather is hot and dry (Fig. 7.3). Care
has to be taken not to let the soil dry too much at the time of fl owering and
fruit set, since a marked increase of water in the soil can produce a signifi cant
drop of small fruit. Irrigation is necessary down to at least 60 cm. Total
water applied per year is estimated at about 35–50 ha-cm for mature trees
(Gustafson, 1976).
On level land, less-e cient furrow irrigation or sprinklers can be used,
with micro-irrigation systems (drip or micro-sprinklers) being more e cient in

Avocado 175
water use. Mature trees require eight or more drip emitters around the tree,
providing greater control and higher e ciency of water use (up to 80%). Later
plantings of avocado in California are on the sides of steep hills, which can be
irrigated only by drip irrigation or mini-sprinklers, as in Chile.
Pruning
The growing tips should be pinched o young trees, allowing the development
of a more compact tree. This pinching is continued until the tree is too tall.
Lower limbs are removed only if they interfere with irrigation and fertilization.
Management should be keyed to avoiding vegetative and reproductive growth
occurring at the same time. Since avocado is polyaxial, it must continue
to increase in size to remain productive. Shoot growth in the warm season
occurs in spurts of up to 1 m, which is needed in order to put on leaves
required for fruit growth (Fig. 7.3). Tree trimming aims at a canopy that has
fruit at all heights and reduces the competition for light. Pruning at the bud
ring (several closely spaced buds without subtending leaves), formed at the
conclusion of a shoot growth fl ush, releases more buds and increases shoot
complexity, and hence bearing sites. Cutting below this ring depresses tree
vigour and releases only one bud (Cutting et al., 1994). Bud-ring pruning can
extend the period before trimming is necessary. Pruning should be timed to
the end of the autumn period. Tree growth can also be reduced by spraying
with paclobutrazol, an inhibitor of gibberellin synthesis, though its use is not
approved in some countries. Tree thinning is recommended in South Africa
when 90% of the orchard fl oor is shaded. Topping (stag-horning) is also
practised on healthy trees to rejuvenate a crowded orchard, where the trees
are cut back to 1–3 m from the ground. Tree thinning, topping or stumping is
essential to avoid canopy crowding and the loss of bearing volume in the lower
third or more of the tree. Severe topping to ~3 m means the loss of production
for 2–3 years, while less severe topping to ~5 m can increase fruit yield and the
amount of fruit set in the lower half of the tree (Crane et al., 1992). Cultivars
with upright vigorous growth habits may be severely a ected by topping and
orchards may have to be rejuvenated in sections over several years, by topping
to 5 m. For very high density, the plants should be cylindrically shaped, while
for not so high densities a pyramidal shape is more appropriate.
Fertilization
Fertilizer practices di er in avocado-producing areas, due to di erences in
climate, soil, cultivars and management practices. Numerous attempts have
been made to determine critical foliar nutrient levels, in order to regulate
the application of macro- and microelements. The following ranges of foliar

176 Chapter 7
levels for macronutrients are established: nitrogen (N) 1.6–2.0%; phosphorus
(P) 0.07–0.20%; potassium (K) 0.75–2.0%; calcium (Ca) 1.0–3.0%; and
magnesium (Mg) 0.25–0.50% (Malo, 1976). The ranges for micronutrients
are: iron (Fe) 50–200 ppm; zinc (Zn) 50–150 ppm; and manganese (Mn)
30–70 ppm.
Critical levels have been di cult to establish, due to the highly variable
yields of avocado. Nitrogen seems to be one of the controlling factors in
avocado yields, as this is the only element that has shown a curvilinear
relationship to yields. Maximum production of ‘Fuerte’ is found at a moderate
level of N in the leaves, with reduced yields occurring at levels below and above
the moderate level. In ‘Fuerte’, a range from 1.6 to 2.0% N in the leaves in late
summer is a desirable level in order to maintain high production. Nitrogen
fertilization is not recommended during the cool season, with application
delayed to the summer leaf and root fl ush (Fig. 7.3). This application should
include P and K. A later application of P and K should also occur near the
peak of fruit set (Whiley et al., 1988a).
As for many tree crops, including avocado, a soil pH of 7.0 and above
creates problems with Fe and Zn defi ciencies, refered to as lime-induced
chlorosis. Iron chelate is used to correct Fe defi ciencies. Soil application of
Zn is more e ective in acid soils than in alkaline soils, and foliar application
has not proved successful. In Florida, where defi ciencies of Fe, Zn and Mn
are common, good results are obtained by combining the chelates of these
elements and applying them through the drip-irrigation system. Boron (B)
defi ciency occurs in some soil types, and ‘Sharwil’ appears to be more sensitive
to B defi ciency.
Pest management
Diseases
A number of avocado diseases have been reported from producing areas
around the world (Table 7.5), the most serious being root rot caused by P.
cinnamomi Rands. This is suspected when trees show a gradual decline, with
leaves becoming smaller, yellow-green in colour and shedding. In severe
cases, twig dieback occurs. The destruction of the unsuberized feeder roots is
associated with high soil moisture in poorly drained areas of the fi eld, with P.
cinnamomi thriving under wet soil conditions, especially when the temperature
ranges from 21 to 30°C and with a soil pH of 6.5 (Zentmyer, 1976). Soil
fumigants, fungicides and sanitation have been used, with a research
emphasis on the development of resistant rootstocks, such as ‘Martín Grande’,
‘Thomas’, ‘Barr-Duke’ and ‘D9’ (Gabor et al., 1990). The use of resistant
rootstocks is integrated with hygiene, sanitation and cultural methods (Co ey,
1987). Trunk-injected phosphonate fungicide (Aliette-Fosetyl-Al) is timed to
coincide with the shoot maturation (Fig. 7.3), the phosphonate being carried

Avocado 177
to the mature shoots and then translocated to the roots, peaking in the root
in about 30 days (Whiley et al., 1995). In California, the root-ball of replant
trees should be drenched with a solution of phosphonate at the time of
planting, followed by two to three foliar sprays with the same chemical. This
chemical can also be sprayed on to the foliage of diseased trees as long as
functional leaves are present. If too few functional leaves remain on the tree,
heavy pruning is performed to induce new shoots and leaves before spraying
phosphonate. Phosphonate applications are repeated three or four times a
year. However, the chemical does not eliminate the fungus from the soil and
must be used with management practices.
In Mexico, Phytophthora root rot control is based upon integrated crop
management (ICM). The ICM approach is based upon improving plant vigour,
restoring the equilibrium between the root and foliar systems, increasing the
soil fl ora benefi cial to the avocado and harmful to disease organisms, improved
irrigation and fertilization, reduced fungal and insect attacks and avoiding
management practices that weaken the tree and favour the disease attack.
Table 7.5. Some important diseases of avocado.
Common name Organism
Parts affected,
symptoms
Region or
country
Root rot
Phytophthora
cinnamomi
Feeder roots, tree
decline
Worldwide
Verticillium wilt Verticillium dahlia
(Verticillium albo
atrum)
Wilting of branches,
death of trees
California,
Australia,
Florida
Armillaria root rot
and crown rot
Armillaria mellea
Large roots, gradual
death of trees
California,
Mexico
Sun blotch Sun-blotch viroid Stunted, decumbent
growth, distorted
leaves, yellow or red
streaks on fruit
Many areas
Anthracnose
Colletotrichum
gloeosporioides
Leaves, most serious
on fruit, especially
after harvest
Worldwide
Stem-end rot
Dothiorella aromatica
(Dothiorella gregaria)
Diplodia natalensis
Purplish-brown spots
on fruit surface,
fl esh discoloration,
offensive odour
USA, South
Africa, Israel,
Australia,
parts of South
America,
Caribbean
Cercospora spot Cercospora purpurea
Leaves, young stems,
fruit
Florida, other
areas
Scab
Sphaceloma perseae
Foliage, fruit Humid tropics
and subtropics

178 Chapter 7
The main measures consist of: (i) periodic incorporation of cattle manure
to a depth of 30 cm to keep the organic matter content of the soil around
3–3.5%, checking this periodically; (ii) chemical fertilization through soil and
foliage to keep adequate foliar levels of elements; (iii) rejuvenation pruning of
a ected trees with more than 70% defoliation to restore root/foliage ratios;
(iv) proper irrigation to avoid plant stress, by not allowing soil moisture to
drop below 70% of fi eld capacity; and (v) adequate sanitary controls to reduce
damage by other enemies (Mora et al., 2000). This approach has provided
results comparable to or better than the use of chemicals. Chemicals are still
recommended when tree decline is serious. In Australia, root rot has been
minimized by building up heavy mulch with bagasse, grass or cereal straw.
This reinforces the importance of using organic matter to create a better
environment for plant-friendly organisms and fungus antagonists.
Many of the diseases reported are not necessarily serious, although the
potential for causing heavy losses exists. Avocado scab is considered to be an
important disease of avocado fruit and foliage in Florida (McMillan, 1976).
Among fruit diseases, anthracnose, stem-end rot and avocado scab can cause
serious problems. Anthracnose requires control practices in the fi eld during
fruit development.
Sun blotch, caused by a viroid, is of concern. Trees are stunted, with
cracked bark, necrotic streaks on branches, and white or light green areas on
the fruit. There are no known vectors, and what makes it a potentially serious
problem is the presence of many symptomless carrier trees. The disease is
transmitted by use of seedling rootstocks from such trees, as well as grafting
with scion wood from infected plants. Scion wood from a healthy tree grafted
upon a ‘carrier’ rootstock becomes infected. In the major avocado-growing
countries, indexing techniques have been developed and used to identify
healthy cultivars for scion wood, as well as those needed for rootstocks
(Broadley, 1991).
INSECTS
There are many insect pests reported in avocado orchards, although they do
not usually pose any serious problems (Table 7.6). Occasionally, a sudden
increase of a specifi c insect can cause severe damage. This increase in an
insect pest is often associated with a sudden change in weather conditions.
Avocado red mite can cause signifi cant leaf damage and a reduction in
photosynthesis and transpiration (Fig. 7.7), possibly leading to a reduction in
yield. Insects such as scales, aphids, mealy bugs and various mite species are
also commonly found in orchards, but natural enemies have been shown to
provide satisfactory control.
In some producing areas, fruit fl ies may require some form of disin-
festation procedure for fruit to be exported to some markets. Studies in
Hawaii with ‘Sharwil’ avocado have shown that it is not normally a host to
the Mediterranean fruit fl y (C. capitata), melon fl y (Dacus cucurbitae) and the

Avocado 179
Oriental fruit fl y (D. dorsalis) at the mature green harvest stage. Under dry
conditions, eggs are deposited between the pedicel and the fruit, making
it a host.
Weed control
Young avocado trees are sensitive to herbicides, so weeding around the trees is
usually done manually. Black polyethylene mulch around the plant is e ective
Table 7.6. Some important insect pests of avocado.
Common name Organism
Parts affected,
symptoms Region or country
Mediterranean fruit
fl y
Ceratitis capitata
Fruit Hawaii
Oriental fruit fl y
Dacus dorsalis
Fruit Hawaii
Queensland fruit fl y
Bactrocera tryoni
Fruit Australia
Black coffee twig
borer
Xylosandrus
compactus
Branches, twigs Hawaii
Chinese rose beetle
Adoretus sinicus
Leaves of young
plants
Hawaii
Fuller rose beetle
Pantomorus godmani
Leaves of young
plants
Hawaii
Spotting bug
Amblypelta nitida
Young fruits Australia
Pine-tree thrips
(greenhouse thrips)
Heliothrips
haemorrhoidalis
Fruit scarring California,
Florida,
South Africa,
Hawaii, Canary
Islands (wide
distribution)
Red-banded thrips
Selenothrips
rubrocinctus
Leaves, fruit
scarring
Wide distribution
Fruit and twig borer
Stenoma catenifer
Perforates fruit,
seed, shoot
terminals
Tropics
Seed and fruit borer
Heilipus laurii
Seed and fruit Tropics
Seed borer
Conotrachelus
perseae
Seed and fruit Mexico
Stem borer
Xyleborus spp.
Stems and
branches
Tropics
Red spider mite
Oligonychus yothersi
Leaves, fruit
scarring
Mainly Chile
Scales
Oligonychus perseae
Leaf scarring Mexico
Saissetia and others
Sap suckers Wide distribution
180 Chapter 7
in preventing annual broadleaved weeds but not e ective against perennial
grasses and nut-sedges (Nishimoto and Yee, 1980). In bearing orchards, the
canopy provides enough shade to prevent weed growth. Inter-rows up to
the periphery of the trees may be mowed or controlled with herbicides. Disc
harrowing of avocado orchards is discouraged, as the shallow feeder roots will
be damaged.
HARVESTING AND POSTHARVEST HANDLING
Harvesting and handling
The stage of fruit maturity when harvested can signifi cantly infl uence the
occurrence of biennial bearing in some avocado (Whiley et al., 1992). In
the tropics, later harvesting of ‘Fuerte’ fruit having 30% dry matter results
in pronounced biennial cropping. Results for a cool subtropical location in
South Africa suggest no e ect of later harvesting on subsequent yield (Kaiser
and Wolstenholme, 1994). Some cultivars can be stored on the tree and
harvested according to marketing schedules. The length of time fruit can be
Fig. 7.7. Infl uence of avocado red mites (Oligonychus yothersi McGregor) on leaf
transpiration, photosynthesis and damage (redrawn from Schaffer et al., 1986).

Avocado 181
left on the tree depends on the cultivar and season. However, this ‘on-tree
storage’ can lead to biennial bearing or crop failure in the following year.
Generally, cultivars of the West Indian race have little or no tree-storage time,
and hybrids, particularly Guatemalan × Mexican, have a longer tree-storage
life. Other tree-storage limitations are fruit drop and development of an o -
fl avour or rancidity with over-maturity. A higher yield can also be obtained if
harvesting is staggered, with 50% of fruit harvested with 21% dry matter and
the remainder at 30%.
A major problem is the stage of maturity for harvest, especially for
cultivars that remain green upon ripening. Some avocados will start changing
skin colour and then fall from the tree when mature, and a few lines can begin
ripening on the tree. Other green-coloured avocados develop a yellowish tint
on the stem near the fruit. Maturity of cultivars that normally change skin
colour from green to reddish or purplish, such as ‘Hass’, is easy to ascertain.
Immature fruit, if harvested, take longer to soften and they shrivel upon
storage, with the fl esh becoming ‘rubbery’ rather than buttery. Most countries
with commercial avocado production have developed some sort of standards
for determining maturity. The increase in oil content is correlated signifi cantly
with maturity. As the fruit advances towards maturity, oil content increases,
the level depending upon the cultivar (Barmore, 1976). In California, oil
content was used as a standard of maturity, with a minimum of 8% oil, based
on fresh mass of fruit, exclusive of skin and seed. Oil content as a measure
of maturity was impractical in Florida, due to wide variations in oil content
among and within cultivars. Some cultivars, particularly those belonging to
the West Indian race, never reach the 8% oil content, as in California. Florida
uses minimum fruit weight and diameter as related to number of days from
fruit set, thus establishing the earliest harvest date for each cultivar (Hatton
and Campbell, 1959). Fruits originating from known bloom dates become
progressively smaller in size from the earliest bloom date to the latest (Hatton
and Reeder, 1972).
Moisture content is also used as a measure of maturity, as it does not
involve oil determination, and there is a negative correlation between oil and
moisture content. As oil content increases during maturation, there is a similar
decrease in moisture content. A 10 g sample of fl esh from a total of ten fruits is
collectively grated and spread on an open dish and dried in a microwave oven
for a few minutes. The minimum dry matter percentage for di erent cultivars
ranges from 17 to 25%. For example, for ‘Fuerte’ the minimum dry matter
percentage is 19%, ‘Hass’ 20.8%, and ‘Zutano’ 18.7%. In more mature fruit
the dry matter can reach 30%. After the minimum moisture content has been
met, fruit comparable to the sample fruit can be picked and ripened at room
temperature. If the sample fruits ripen within 8–10 days without shrivelling,
they are considered to be mature, and the grower can proceed to harvest
comparable-sized fruit on the tree. Grade standards frequently set a minimum
dry-matter percentage of around 20% for ‘Fuerte’, 22% for ‘Hass’, 25% for

182 Chapter 7
‘Gwen’ and 19% for ‘Zutano’ (Rainey et al., 1992). This method is rapid and
less time-consuming and does not involve oil determination.
Fruits are harvested by cutting or snapping o the stem at the base of
the fruit, with about 12 mm of the stem attached. Aluminium picking poles
are usually equipped with a cutter and a bag to catch the fruit. Large trees
on suitable terrain can be partially mechanized by the use of a three- or four-
wheeled, self-propelled, hydraulically powered platform, from which pickers
can use short poles. Pallets or bins should be placed under the shade of trees
while waiting to be picked up for transport to the packing plant. This prevents
overheating of fruit, as precooling is not generally practised.
Postharvest treatments
Most packing houses are automated, with all debris removed automatically
and fruit cleaned with roller brushes. Cleaned fruits pass through graders,
where all diseased, injured and defective fruits are removed and fruits are
separated into size lots for packing. Sizing is done by use of drop-roll (diameter)
sizers or by weight sizers. Each producing country has its own grading
standards and size classes. The major quality criteria are size; skin colour;
freedom from wounds, blemishes, insect damage and spray residues, and
absence of disease, physiological disorders and bruising. Standards for Europe
allow for three classes of avocado, based on appearance, defects, tolerances,
uniformity, packaging and marketing. The minimum mass is 125 g and a
range of size from 125 to 1220 g in 14 ranges is used in all classes (Anon.,
1995). Fruits with lower quality go to processing or are used for local sales.
Sizes of packing cartons di er in various countries. They are usually
corrugated paperboard cartons for single- or double-layer packing, ventilated
for good air circulation. Pads or styrofoam trays with cup impressions may
be used to prevent bruising. Prepacked consumer units in ‘clam shells’
(polyethylene containers) and mesh bags are also used. Fruit may be waxed or
wrapped and packed by hand. All fruit in a specifi c size container must weigh
between a specifi ed minimum and maximum range. Cartons are labelled
with fruit number and size range. In California, packed cartons are cooled at
5–15°C, depending on cultivar, before loading into transport vehicles. ‘Hass’
can be stored at 5°C for 3–4 weeks. Longer storage leads to greyish-brown
internal fl esh discoloration and irregular blackening of the skin.
Storage temperature for delaying ripening varies with the cultivar
temperatures of 12.5, 8 and 5°C are used for West Indian, Guatemalan
and Mexican cultivars, respectively. A relative humidity of 80–90% is
recommended. Other storage methods, such as controlled atmosphere (CA)
and hypobaric (low-pressure) storage, have been evaluated. With CA storage
at 2–5% oxygen (O
2
) and up to 10% carbon dioxide (CO
2
) at 5°C and 98–100%

Avocado 183
relative humidity is occasionally used to delay softening and reduce respiration
and ethylene production.
Fruit can be ripened at 25°C or by exposure to 10–100 ppm ethylene at
17–20°C for 12–72 h, depending upon fruit maturity, and then transported
to markets. Following ethylene treatment fruit will ripen in 3–6 days. The
ethylene-treated fruits are marketed locally, as the current market strategy is
to provide ripe fruit for immediate consumption.
UTILIZATION
The avocado is considered a nutrient-dense food (Rainey et al., 1994). It has
the highest fi bre content of any fruit and is a source of food antioxidants
(Bergh, 1990a). Avocado has a high oil content, although Florida cultivars are
lower in oil content compared with cultivars from California. Major fatty acids
are the mono-unsaturated oleic acid, followed by palmitic and linoleic acids.
Palmitoleic acid is found to approximate percentages of linoleic acid, and
values are slightly higher or lower depending upon the cultivar analysed. The
nutrient values of the di erent ecological races also vary, but, in general, it is
a fair to good source of P, provitamin A, ribofl avin and niacin (Table 7.7). The
protein content of 1–2% is considered to be greater than in any other fresh
fruit (Bergh, 1990b). Avocado also increases the diet’s content of antioxidants,
foliates, K and fi bre (Rainey et al., 1994).
Avocado is mainly used fresh in salads, its high fat content combining well
with acid fruit and vegetables, such as pineapple, citrus and tomatoes, or with
acid dressings. A major commercial avocado product is guacamole, used as a
favourite dip with potato chips, tortilla chips and similar products. Avocado
may be used to supply the fat content of frozen desserts, such as ice cream
and sorbets. Miller et al. (1965) provide numerous recipes for various types of
salads, cocktails and desserts. In some places, avocado is eaten as a dessert,
adding sugar to it instead of salt. Shakes and ice creams are also made of the
pulp. Recently there is a growing industry of producing avocado oil, which is a
very healthy product, using subtropical varieties.
Deterioration of fl avour and enzymatic discoloration are serious problems
in the commercial processing of avocado (Ahmed and Barmore, 1980).
Avocado is not conducive to heat processing as it results in an o -fl avour.
High polyphenoloxidase and total phenol contents contribute to the processed
product’s browning potential, with di erences in browning potential occurring
among cultivars. Enzymatic browning of avocado products can be minimized
by processing a cultivar with low polyphenolase activity into an acidifi ed
product containing an antioxidant (e.g. ascorbic acid), packing under N or a
vacuum and storing at low temperatures.

184 Chapter 7
FURTHER READING
Ben-Ya’acov, A. and Michelson, E. (1995) Avocado rootstocks. Horticultural Reviews 17,
381–429.
Bower, J.P. and Cutting, J.C. (1988) Avocado fruit development and ripening physiology.
Horticultural Reviews 10, 229–271.
Téliz, D. (coordinator) (2000) El aguacate y su manejo integrado. Ediciones Mundi Prensa,
México, Madrid, Barcelona (in Spanish).
Whiley, A.W. and Scha er, B. (1994) Avocado. In: Scha er, B. and Andersen, P.C. (eds)
Handbook of Environmental Physiology of Fruit Crops Vol. II: Subtropical and Tropical
Crops. CRC Press, Boca Raton, Florida, pp. 3–35.
Whiley, A.W., Saranah, J.B., Cull, B.W. and Pegg, K.G. (1988). Manage avocado tree
growth cycles for productivity gains. Queensland Agriculture Journal 114, 29–36.
Whiley, A.W., Scha er, B. and Wolstenholme, B.N. (2002) The Avocado: Botany, Production
and Uses. CABI Publishing, Wallingford, UK.
Woolf, A.B., White, A., Arpaia, M.L. and Gross, K.C. (2004) Avocado. In: USDA-ARS
Handbook #66. (http://www.ba.ars.usda.gov/hb66/034avocado.pdf – accessed 7
Feb 2010).
Table 7.7. Proximate analysis of avocado (Wenkam, 1990).
a
Halumanu
West Indian type
Nabal
Guatemalan type
Water (g) 82.8 69.9
Energy (kJ) 431 874
Protein (g) 1.5 1.03
Lipid (g) 9.3 21.8
Carbohydrate (g) 5.7 6.3
Fibre (g) 1.6 2
Ash (g) 0.8 0.9
Minerals
Calcium (mg) 8 77
Iron (mg) 0.5 0.4
Magnesium (mg) – –
Phosphorus (mg) 34 42
Potassium (mg) – –
Sodium (mg) – –
Vitamins
Ascorbic acid (mg) – 5.5
Thiamine (mg) 0.03 0.09
Ribofl avin (mg) 0.09 0.14
Niacin (mg) 1.23 –
Vitamin A (IU) – 802
a
Amounts are per 100 g.

© CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1 185
(R.E. Paull and O. Duarte)
8
BANANA AND PLANTAIN
Banana is a general term that refers to all wild species, landraces and cultivars
belonging to the family Musaceae, genus Musa (Ortiz, 2008). The commercial
banana is a giant, perennial, herbaceous monocotyledon, propagated
vegetatively; it is important in the humid tropical lowlands, with year-round
fruit production, but it can also grow in certain subtropical areas. The fruit
is served as a dessert (banana) or cooked and eaten as a staple (plantain).
The banana (English) has various names: bananier (French), pisang (Malay,
Indonesian), kluai (Thailand), chuoi (Vietnam), xiang jiao (Chinese).
The most important type of fruit commercially is the dessert banana,
which is eaten fresh and makes up most of the international trade of this
genus. The second most important group is the plantain, which is an
important staple food in many countries in Africa, Latin America and South-
east Asia, where extensive areas are grown mainly for local consumption.
There are also the East African highlands and the Asian cooking bananas, and
fi nally the East African beer bananas. In some cases, the same genomic group
can include varieties used as plantains or as dessert bananas.
BOTANY
The genus Musa has more than 50 species, with some of these species having
numerous subspecies. This diversity has lead to the genus being divided into
three sections from the fi ve traditional sections: Musa (2n = 22, incorporating
Rhodochlamys), Callimusa (2n = 20, incorporating Australimusa) and single-
species section Ingentimusa (2n = 28) (Wong et al., 2002). Among these
sections, there are wild, seeded plants and edible clones, with overlapping
geographical distributions. Ensete, the other genus in the family, ranges from
Asia to Africa, while Musa ranges from Africa through Asia to the Pacifi c
(Reynolds, 1951).

186 Chapter 8
Genus Musa
Edible bananas are derived from either Musa acuminata (A) or Musa balbisiana
(B), or a combination of both. Cultivars are diploid or triploid, with some new
tetraploids developed by breeding. Considerable somatic variation has led to a
great range of cultivars. Cultivars are described by their name and genomic
make-up, e.g. ‘Pisang Raja’ AAB, the AAB indicating that it is a hybrid with
two genomes of A and one genome of B. Most dessert bananas are AA or AAA,
with the triploid AAA being the most important in the trade. The di erent
groups and subgroups have somewhat distinct fruit characteristics (Table 8.1).
M. acuminata has a number of morphological characters that separate it
from M. balbisiana. For example, M. acuminata has an open petiolar canal, which
in M. balbisiana is closed. M. acuminata has prominent bract scars, bracts that
Table 8.1. Fruit characteristics of some major cultivars within groups and subgroups.
Group Subgroup Characteristics
AA Sucrier Small fruit (8–12 cm long), thin golden skin, light orange
fi rm fl esh, very sweet, 5–9 hands per bunch, 12–18
fi ngers per hand
Lakatan Medium to large straight fruit (12–18 cm), golden yellow,
fl esh light orange, fi rm, dry, sweet and aromatic, 6–12
hands per bunch, 12–20 fi ngers per hand
AAA Gros Michel Medium to large fruit, thick skin, creamy white fl esh,
fi ne-textured, sweet and aromatic, 8–12 hands/bunch.
Susceptible to Panama disease
Cavendish Medium to large fruit, yellow skin, white to creamy fl esh,
melting, sweet, aromatic, 14–20 hands per bunch,
16–20 fi ngers per hand. Susceptible to Race 4 of Panama
disease
AAB Silk Small to medium yellow fruit (10–15 cm), thin yellow-
orange skin, white fl esh, soft, slightly subacid, 5–9 hands
per bunch, 12–16 fi ngers per hand, skin frequently has
blemishes
Pisang Raja Large fruit (14–20 cm), thick skin, cooking banana, creamy
orange fl esh, coarse texture, 6–9 hands per bunch, 14–16
fi ngers per hand
Plantain Yellow skin, creamy orange fi rm fl esh, few hands per
bunch
ABB Bluggoe Medium to large cooking banana, thick coarse skin turns
brownish-red when ripe, creamy orange fl esh, starchy, 7
hands per bunch
BBB Saba Stout, angular, medium to large cooking banana (10–15
cm), thick yellow skin, creamy white fl esh, fi ne-textured,
8–16 hands per bunch, 12–20 fi ngers per hand

Banana and Plantain 187
are lanceolate and curl, and two regular rows of ovules, compared with four
irregular rows in M. balbisiana. Using and scoring 15 morphological characters
allows the relative contribution of the two species to be determined in hybrid
cultivars. Triploids and tetraploids are larger and more robust than diploids.
Origin and distribution
The primary centre of origin is thought to be Melanesia (Malaysia, Indonesia,
the Philippines, Borneo and Papua New Guinea); M. acuminata Colla. has
seeded fruit. Dessert cultivars were developed from it via parthenocarpy and
sterility, aided by human selection and vegetative propagation. M. balbisiana
Colla. also has a wild, seeded fruit, is more suited to drier areas and occurs
from India to New Guinea and the Philippines, though absent from central
Melanesia (Espino et al., 1992). It was similarly taken into cultivation, with the
selection of natural diploid, triploid and tetraploid hybrids.
ECOLOGY
Soil
Deep friable loams with natural drainage and no soil compaction are preferred.
Soils with poor percolation due to excess of clay or with an excessive amount
of sand should be avoided, as well as soils with high amounts of gravel. Soils
having pH between 4.5 and 7.5 are used, although 5.8–6.5 is recommended.
Soil textures ranging from sands to heavy clays are used. A granular soil
structure is the preferred for better water movement and root growth, with
high organic matter and fertility ensuring high yields. Most exported bananas
are produced on highly fertile alluvial loams. Plantains also do best in this type
of soils, but they will do better than the AAA dessert banana in lower-quality
soils; apparently the ‘B’ in their genome is responsible for this adaptability.
Soil depth should be around 1.0–1.2 m, with no superfi cial water tables or
impermeable layers. Soil drainage is essential since, although the plant prefers
moist soil, it does not tolerate standing water. Flooding for a week will kill most
banana plants (Duarte, 1991). Banana will tolerate some salinity: 300–350
mg/l of chlorine and up to 1500 ppm total salts.
Climate
Rainfall
Bananas require a regular water supply that matches or slightly exceeds the
free-water evaporation rate. Irrigation is essential for high yields if rainfall is
less than evaporation and it also provides the advantage of fertilization via the

188 Chapter 8
irrigation water. Areas with very high rainfall may be too overcast for optimum
photosynthesis, have more disease problems and require extensive drainage.
The plantain group is less susceptible to water stress than dessert bananas
(Belalcazar, 1991), and this is another reason why small farmers grow them
around their houses. Plantains do well with about 30 mm weekly rainfall in
the co ee region of Colombia.
Temperature
A temperature range of 15–38°C occurs in most production areas, with
the optimum temperature being ~27°C. The optimum for dry-matter
accumulation and fruit ripening is about 20°C and for the appearance of
new leaves about 30°C. Growth ceases at 10°C and can lead to ‘choke-
throat’ disorders, where infl orescence emergence is impeded and poor fruit
development occurs. Temperatures less than 15°C can be withstood for short
periods, while temperatures less than 6°C cause severe damage (Turner,
1994), and temperatures below 4°C result in irreversible damage. Frost
causes rapid death. At temperatures above 38°C growth stops and leaf burn
occurs. Plants growing in the subtropics produce fewer leaves per year than
those in the tropics and take longer to produce and develop fruit (Table 8.2).
Sucker emission and development are also slower. Bananas grown at higher
elevations that have lower temperatures tend to produce sweeter fruit, because
of cooler nights. In certain cooler areas, such as the Canary Islands, bananas
are produced in plastic greenhouses.
Plantains and cooking bananas are grown at sea level but also at higher
elevations in Latin America, Asia and Africa. In many cases, they are grown
as subsistence crops and are planted even at 2000 m above sea level in places
where mean minimum temperatures are not below 15°C and the absolute
minimum does not drop below 8°C. Clonal di erences do occur in the ability
to adapt to cooler temperatures. In Colombia, an important producer of
plantains, commercial production occurs from sea level to 1350 m, though
clones such as ‘Harton’ are not planted above 800 m. Lower temperatures
Table 8.2. Phenological differences between cultivars of Cavendish subgroup
ratoon plantation in the humid tropics (Honduras: cv. ‘Grand Nain’, Stover and
Simmonds, 1987) and subtropics (South Africa: cv. ‘Williams’, Robinson and Nel,
1985; Robinson and Human, 1988).
Humid tropics Subtropics
Mean number leaves per month (warm/cool
season)
3.5/2.5 4/0.5
Total leaves per year 40 25
Planting to harvest (months) 9–11 15–20
Harvest to harvest (months) 6–8 11–13
Flowering to harvest (warm/cool season, days) 98–117 120–204

Banana and Plantain 189
do reduce yields signifi cantly, making commercial production uneconomic. It
is estimated that the vegetative cycle is extended by 10 days for every 100 m
increase in elevation (Belalcazar, 1991). At higher elevations, the form of the
fruit bunch changes, with hands becoming more separated.
Wind
Bananas and plantains are very susceptible to wind damage. In all producing
regions, moderate winds between 20 and 50 km/h cause moderate to severe
tearing of the leaves. This leaf-tearing can reduce productivity, but as long
as there is no signifi cant loss of foliar surfaces, it does not normally have a
major e ect. This tearing does reduce plant transpiration when maximum
daily water stress occurs, while net photosynthesis is little a ected (Belalcazar,
1991), giving higher photosynthetate production by unit of water transpired.
This benefi cial e ect may be important during the dry season. The problem
with winds above 50 km/h
is that they cause loss of leaf pieces, break the
pseudostem or uproot the plants, in the latter two cases the crop is lost until
the ratoon suckers complete their cycle. In certain areas of Central America,
a phenomenon called ‘blow down’ is frequent; this is caused by gusts of
high-velocity winds that unexpectedly hit certain areas and break all tall
pseudostems, producing almost the same e ects as a hurricane but in a
limited area.
Light
For dessert bananas, as well as for plantains, solar radiation should be as
high as possible for best growth and yields, although fruit sunburn can occur,
especially if water supply is low; petioles can also be burnt. Shaded or overcast
conditions extend the growth cycle by up to 3 months and reduce bunch size
(Fig. 8.1). For plantains grown under lower radiation levels, the plants tend to
be taller. Plant density plays a role in relation to light, since denser plantings
intercept more light at the top but there will be less radiation at ground level,
reducing sucker production.
Photoperiod
No evidence exists that photoperiod infl uences fl owering. Increasing the
photoperiod from 10 to 14 h increases the rate of new leaf appearance,
probably due to more photosynthesis.
GENERAL CHARACTERISTICS
Plant
This 2–9 m perennial herb has an underground, compressed stem or corm
that is the real stem. The visible part that looks like a stem (pseudostem)
190 Chapter 8
consists of overlapping leaf sheaths. The apical bud of this corm produces the
leaves, and at certain stage it di erentiates into the fl ower bud, which, in the
same way as the leaves, grows up through the centre of the pseudostem until
it emerges at fl owering (Fig. 8.2).
The corm has lateral buds that produce shoots (suckers) near the parent
(Fig. 8.2). These suckers grow and, when old enough, fl ower and bear fruit
and are the basis for the successive ratoon crops that these plants normally
produce for many years. Suckers can be of two types: the ‘sword’ sucker is one
that has very narrow leaves when young and its pseudostem has a conic form;
the other type is the ‘water’ sucker, which has a cylindrical pseudostem and
wide, almost rounded leaves. ‘Water’ suckers are eliminated in the plantation
and normally are not used for propagation, while ‘sword’ suckers are selected
to become the ratoon crop and preferred as propagation material, although
trials with plantain (Belalcazar, 1991) have shown that ‘water’ suckers can
result in an equally productive crop if given proper care when young.
Adventitious roots that arise from the corm form a dense mat and spread
extensively 4–5 m from the parent and down to 75 cm or more. Plantains of
the AAB genome have a shallower root system than export bananas.
The large leaf lamina are 1.5–4 m long by 0.7–1 m wide, with pronounced
midribs and parallel veins (Fig. 8.2). Stomata occur on both surfaces, with
Fig. 8.1. Effect of shading on the bunch weight produced per month (kg/month
and months to harvest). Low light reduces the leaf area produced, and a positive
relationship exists between leaf area of the third mature leaf and fi nal bunch weight:
y = 4.83x – 12.83. r
2
= 0.75, signifi cant at P < 0.05 (after Murray, 1961).
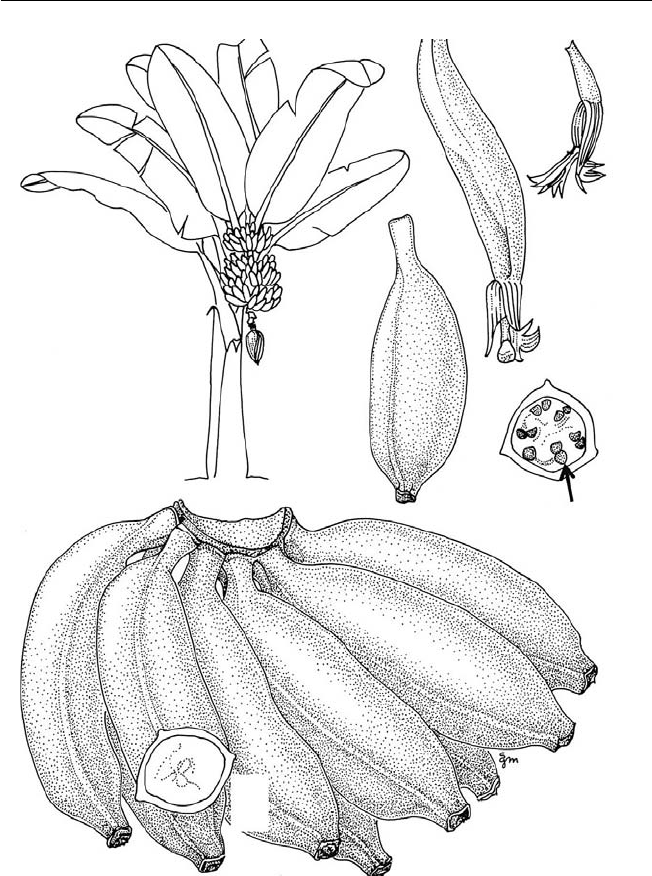
Banana and Plantain 191
(a)
(b)
(c)
(d)
(e)
Seed
Fig. 8.2. Plant, leaves and bunch (a), fl oret (b), young fruit (c), and transverse
section of seedy fruit (d) and the more common seedless fruit (e) of banana. The
hands of seedless fruit are cut from the bunch stem, leaving a portion of the stem
that is called the crown.

192 Chapter 8
three times more on the adaxial surface. The leaf takes 6–8 days to fully unroll
from the tip, and in the tropics the leaves last c. 50 days. Leaves emerging
just before the infl orescence can live up to 150 days or more. Twenty-fi ve to
50 leaves emerge, with 10–15 functional leaves present (total area 25 m
2
)
at infl orescence emergence. The number of leaves at fl owering is positively
correlated with bunch weight (Fig. 8.3); fewer than six to eight leads to
signifi cant reduction in bunch weight. After fl ower emergence, no more leaves
are produced, so the plant should have as many as possible functional leaves
at this time. The main problem with the major leaf disease Sigatoka is that it
reduces the number of functional leaves and their useful area.
Flowers
One terminal infl orescence emerges through the pseudostem and bends
downwards after emergence (Fig. 8.2). The fl owering spike consists of groups
of two rows of appressed fl owers enclosed within large, ovate, reddish bracts
at each node. The bracts refl ex and are shed as the fruit develop. The female
fl owers emerge fi rst and the males at the distal end. Sometimes hermaphrodite
fl owers develop in the middle of the bunch, but they generally abscise.
Flowers at each node are referred to as a hand; each hand has 12–20 fl owers
per node and there are 5–15 hands with female fl owers. The bracts open
sequentially, about one per day. The peduncle continues to elongate up to
1.5 m, terminating in a male bud, which continues to produce male fl owers
enclosed within the bracts. The last hand (false hand) of the female fl owers
Fig. 8.3. The effect of number of functional leaves at fl owering on bunch weight
(redrawn from Turner, 1970).

Banana and Plantain 193
has few fi ngers (fruit) and the rest of the nodes have non-functional pistils.
The infl oresence stem (rachis) is cut below the false hand, where a fi nger is
left attached to maintain a connection with the plant circulatory system and
avoid rachis rotting. The detached rachis removes the male fl owers. In plants
with no false hands at the time of fruit thinning, a fi nger will be left in the last
hand.
The 10 cm female fl owers of cv. ‘Cavendish’ have an inferior ovary of
three united carpels with a short perianth. The perianth consists of fi ve fused
and one free segment, forming a tube around the style and sterile androecium
and three-lobed stigma. Male ‘Cavendish’ fl owers are 6 cm long with fi ve
stamens, which rarely bear fertile pollen.
Pollination and fruit set
Natural pollination
The fruit develops parthenocarpically. The ovules shrivel early and are
recognized as brown specks in the mature fruit along the axial placenta (Fig.
8.2). Very infrequently, seeds are found in mature fruit of edible cultivars,
especially those with a ‘B’ genome. The ‘Cavendish’ group has absolute female
sterility with few viable pollen. The ‘Gros Michel’ (AAA) dessert banana
produces seed once in a while and therefore is used as the female parent in
many crosses. ‘Pisang Awak’ (ABB) can sometimes be very seedy if pollen-
bearing diploids are growing nearby.
Floral induction and fruit set
There are no external symptoms of the start of infl orescence development.
The fl owering stimulus is unknown. Externally, it is not temperature or
photoperiodism, and internally it is not the number of leaves developed,
as the number of leaves is more or less fi xed, depending on cultivars and
environment.
Fruit morphology
The fruit, although it develops from an inferior ovary, is a berry. The exocarp
is made up of the epidermis and the aerenchyma layer, with the fl esh being
the mesocarp (Fig. 8.2). The endocarp is composed of a thin lining next to
the ovarian cavity. The axial placenta has numerous airspaces and ventral
vascular bundles.
Each node of the rachis has a double row of fl owers, forming a cluster
of fruit that is commercially called a ‘hand’, with the individual fruit called a
‘fi nger’. ‘Cavendish’ bananas can have 16 hands per bunch, with up to about
30 fi ngers per hand, and the bunch can weigh up to 70 kg. There are many

194 Chapter 8
di erent sizes and shapes of fruit, with plantains being usually very large
compared to the dessert-type bananas or others. The same is true for external
and pulp colour, which normally vary from cream to slight orange.
Growth and development
Pollen sterility is due to triploidy, while female sterility is due to at least
three complementary dominant genes and modifi er genes. These sterility
genes are found in wild populations and have been selected for fruit edibility.
Parthenocarpy is separate from sterility. For the fi rst fortnight after anthesis
the ovules increase in size (50% over initial), and later they shrivel and ovary
growth slows. Parthenocarpic bananas that have seeds (‘Pisang Awak’ ABB)
show a stimulation of fruit growth, due to the presence of developing seeds.
Pollination can stimulate fruit growth, even without seed development (Israeli
and Lahav, 1986).
There are periclinal and anticlinal divisions from 6 weeks before
infl orescence emergence (anthesis) to 4 weeks after emergence. This division
is followed by cell expansion for 4–12 weeks after emergence. Skin mass
increases rapidly in the fi rst 40 days after fl owering, with the fruit pulp not
beginning to develop until day 40 (Fig. 8.4). Starch accumulation parallels
fi nger length and diameter increases (Lodh et al., 1971). The fruit matures
in the tropics 85–110 days after infl orescence emergence. Fruit development
may take up to 210 days in the cooler subtropics or under overcast conditions
(Table 8.2). Harvest maturity is a commercial stage (three-quarters round),
with the fruit still having some angularity and being only 75% of its potential
maximum size. Export bananas are harvested in the tropics at 14–15 weeks
after fl ower emergence, while plantains are harvested after 11–12 weeks. Fruit
allowed to fully develop to a round shape may show skin splitting. Depending
upon the persistence and viability of the remaining leaves, fully developed fruit
may also show sunburn.
Since fruit number and size decrease from the proximal to the distal
(bottom) hands, fruit thinning is sometimes practised. In most cultivated types,
the bunches bend because of the fruit bunch weight. As the infl orescence
emerges from the pseudostem, it bends towards the sun. Fruits on the inner
whorl of a hand can be 15% smaller than those on the outer whorl. Normally
the last or last two hands that have smaller hands are eliminated if higher fruit
calipers (diameters) are desired. The male infl orescence can be removed soon
after full development of the bunch without damaging the female hands; this
removal reduces the weight and can, in some instances, slightly increase the
caliper of the remaining fruit.
In plantains, there are normally fewer fl owers per bunch, although some
of the new tetraploids have a very heavy load of fruit. Some plantain types do
not have the male part of the infl orescence or it is rudimentary.
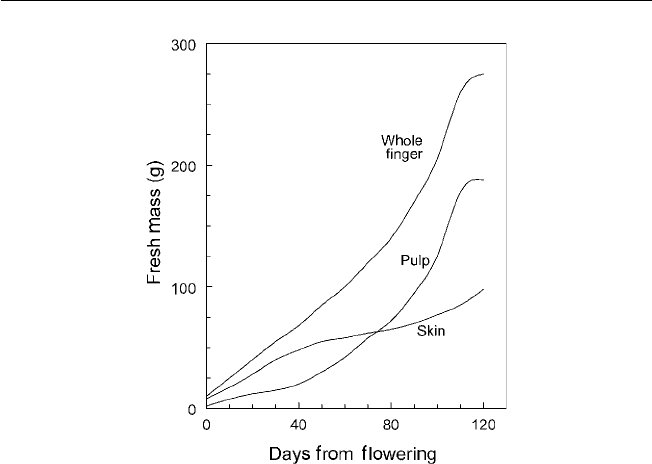
Banana and Plantain 195
CULTIVAR DEVELOPMENT
Genetics and cytogenics
The haploid number is n = 11, with 22, 33 and 44 chromosomes being found
in the diploids, triploids and tetraploids. There are 200–300 clones, of which
more than half are triploids. Triploids are more vigorous, easier to grow and
higher yielding than diploids (Gowen, 1995). Some tetraploids have been
developed that have desirable characteristics such as Sigatoka tolerance and
high yields.
Problems in breeding
The majority of breeding e orts have been directed at the AAA group, because
the group has the highest export potential. However, female sterility and the
low numbers of viable pollen make conventional breeding very di cult. Some
inferior cultivars can, under controlled conditions, produce seeds; however,
it takes 3 years from seed to seed. In the Honduran breeding programme of
the Honduran Agricultural Research Foundation (Fundación Hondureña de
Investigación Agricola, FHIA), the AAA ‘Gros Michel’ dessert banana is used
in many instances as the female parent for crosses, as some fruits have seeds.
Fig. 8.4. Growth of fi nger, pulp and skin fresh mass of ‘Gros Michel’ in the
Caribbean (Simmonds, 1982).

196 Chapter 8
After a cross with ‘Gros Michel’ as female parent, the hybrid seeds are extracted
by ripening large numbers of these fruits derived from the cross, peeling them
and passing the pulp through a strainer that retains the few seeds present.
Considerable breeding e orts and selection of crossed material may
lead to the development and release of cultivars with suitable horticultural
characteristics and increased disease resistance (Gowen, 1995). Apart from
the e orts supported by the International Network for the Improvement
of Bananas and Plantains (INIBAP) (Vuylsteke et al., 1993), there have
been a few e orts to develop better varieties for small and medium-sized
growers serving local markets with local varieties in Brazil, Africa and India.
In Honduras, FHIA has produced a complex pedigree hybrid (FHIA 03), a
‘Bluggoe’ type of cooking banana, resistant to black Sigatoka, ‘Moko’ and
Panama disease, which is planted in East and West Africa, and in Cuba by
small farmers. They have also released ‘FHIA-25’, another AAB cooking
banana, as well as ‘FHIA 21’, a plantain, both are resistant to black Sigatoka.
Selection and evaluation
Considerable e orts have been expended in characterizing various cultivars
under comparable conditions (Rowe and Rosales, 1996). Somaclonal variation
and mutation breeding is widely used. The focus of these programmes is
resistance to black Sigatoka, Fusarium wilt (Panama disease), bunchy-top virus
and nematodes. Gene transfer from unrelated species also o ers considerable
opportunities for improved planting materials. Besides selection for resistance
to the above diseases, other priorities include resistance to the weevil that
bores into the corm, dwarfi sm, tolerance to drought and cold, and improved
bunch yield, harvest index, fruit quality (texture and fl avour) and storability
(Novak, 1992). Similar priorities exist for plantains, with susceptibility to black
Sigatoka and low bunch yield being the major limitations, followed by some of
the same priorities as for dessert bananas. New plantain types have been easier
to produce than dessert bananas and some are being grown extensively in
many tropical areas.
Major cultivars
There are at least 200–300 banana clones in various countries, many
having di erent names in di erent localities (Table 8.3). There are numerous
germplasm collections around the world, including those in Indonesia,
Malaysia, Thailand, the Philippines, India, Honduras, Jamaica, Brazil,
Cameroon and Nigeria. The large number of synonyms for many of the better
cultivars makes for some confusion (Lebot et al., 1993).

Banana and Plantain 197
Table 8.3. Major genomic groups and some cultivars. The seedless diploid (AA) and triploid (AAA) are regarded as desserts, while the
seedless diploid (BB) and triploid (BBB) are cooking bananas. These and the many hybrids are all referred to as Musa spp., followed by
the code and subgroup (SG) name (Cavendish, Gros Michel, plantain, etc.).
AA AAA AAB ABB BBB Other
Sucrier syn. Gros Michel (SG) syn. Silk syn. Pisang Awak (Indo,
Mal), syn.
Saba (Phil), syn Atan (AAAB), AA
Pisang Mas (Mal,
Indo)
Bluefi elds Apple (Hawaii) Ducase (Aust) Cardaba (Phil) Kalamagol (AABB)
Kluai Khai (Thai) Pisang Ambon (Mal) Pisang Rastali (Mal) Katali (Phil) Kluai Hin (Thai) Gold Finger (AAAB)
Amas (Aust) Disu Pisang Raya Serek
(Indo)
Kluai Namwa (Thai) Pisang Nipal (Mal)
Susyakadali (India) Kluai Hom Thong
(Thai)
Latundan (Phil) Pisang Klotok (Indo)
Lakatan (Phil), syn. Cavendish (SG) Woradong Kanpuravalli (India)
Pisang Barangan
(Indo)
Dwarf Cavendish, syn. Cantong Bluggoe, syn.
Pisang Herangan
(Mal)
Chinese (Hawaii) Tundan Pisang Kepok (Indo)
Senorita Canary Banana Lady Finger, syn. Pisang Abu Keling
Dwarf Chinese Pome Pacha
Naadan
Kluai Hak Muk (Thai)
Basrai (India) Pisang Raja (Mal,
Indo)
Nalla Bontha (India)
Governor (West Ind)
syn.
Radja (Phil) Moko (Trinidad)
Enano (Latin Amer) Larp Da Jiao (China)
Giant Cavendish, syn. Houdir Fen Da Jiao (China)
Continued

198 Chapter 8
Table 8.3. Continued.
AA AAA AAB ABB BBB Other
Mons Mari (Old) Mysore (India), syn.
Williams (NSW) Colombo
Grand Nain Poovan (India)
Bongali Johaji (India) Honderawala
(India)
Beijiaw (China) Plantain (SG)
Honchuchu Horn, syn.
Pisang Ambon Putih
(Malay), syn.
Pisang Tanduk
Kluai Horn Dek Mai
(Thai)
Tindok
Ambon (Phil) French, syn.
Pisang Embun Nendran (India)

Banana and Plantain 199
CULTURAL PRACTICES
Propagation and nursery management
Sexual
Seeds are only used in breeding programmes. Many of the important
commercial cultivars are female-sterile.
Asexual
Suckers with their basal corm or entire corms are used as planting material.
A sucker is a lateral shoot that has a basal corm and a short pseudostem that
arises at the base of a plant from the mother corm. Suckers are normally used
by small farmers or when few plants are needed. The di culty with suckers
is their size, which makes handling and transportation di cult. In addition,
disinfection, which is essential to avoid transporting insects, disease and
nematodes, is often ine ective. A young sucker just emerging from the soil or
a large sucker with narrow leaves and a large corm are normally planted with
the remaining roots at the same depth as they were originally, and the excess
leaves will be pruned back.
The banana exporting companies normally use corms that are at the
base of a plant that has not fl owered and that was raised in special nursery
fi elds; these corms will have a weight of 2.5–5 kg. In other cases, 1.6–1.8 m
tall sword suckers that have a diameter of 15–20 cm at 20 cm from the soil
are used. The suckers are dug out and 15–20 cm of the pseudostem retained.
Sometimes large, older corms (bull heads) from plants that have fl owered can
be used for planting if propagation material is scarce. Smaller pieces of corms
can also be used.
The ideal is to disinfect the corms. The corm is pared so no dark spots and
roots are left, and the pared corm is dipped in a mix of a fungicide, nematicide
and insecticide for 5–20 min. The large exporting companies use a hot water
dip at 56–58°C for 15–20 min or 65°C for 12–15 min. If no pesticides
are available or are not allowed, simply paring the corms is helpful to limit
transfer. Corms to be used for planting should never be left overnight on the
ground, they should be either covered tightly or put on a trailer or truck, to
avoid reinfestation by banana weevil.
When propagating material is scarce, several techniques can be used to
increase the number of suckers. A sucker is planted and, before it di erentiates
a fl ower bud, several practices can be used to prevent further growth of the
apex and induce axillary bud growth. One practice is to drive a stake into the
pseudostem at a 45° angle, aiming to destroy the apical meristem, or a coring
tool is used to achieve the same objective. Another technique is to partially cut
the pseudostem before fl owering at about 1.5 m from the ground, so the top
will bend and it will look like a ‘7’, or to cut it down; in other cases the outer

200 Chapter 8
leaf sheaths are pulled down to the bottom of the pseudostem, in order to
expose the axillary buds, and then soil is mounded around the base. All these
practices prevent fl owering, and induce axillary bud growth that develops into
numerous suckers.
For high-density plantain plantings developed in the last decade in Central
America for the ‘Curraré Enano’ (AAB) plantain variety, the corms are graded
by size into three or four grades then planted in di erent areas to ensure fi eld
uniformity. Larger corms, 500–2000 g, are planted directly into furrows in the
fi eld in either a horizontal or vertical orientation. Smaller corms of 200–500
g are planted into plastic bags and kept for 6–8 weeks before grading by size,
with the larger corms planted into the fi eld. The advantage of planting bagged
plants is that no fi eld irrigation and weeding are necessary before planting out
and the growth in plastic bags means that a more uniform stand and no empty
spaces are obtained, as only sprouted suckers from the corm are planted. The
corms are pared and if possible disinfected before planting in the fi eld or bags,
as for dessert bananas.
Tissue culture (in vitro plantlets) allows for rapid multiplication of
uniform, disease-free materials (Loyola Santos et al., 1986). Other advantages
of tissue culture include very high fi eld establishment rates, uniformity of
harvest timing, precocity and high production, at least in the fi rst crop cycle.
These advantages have to be balanced against higher cost, extra care at
multiplication and the transmission of viruses that have not been eliminated.
Somatic variation is the major problem and care in multiplication is necessary
to reduce the incidence to an acceptable level (<3%) (Smith, 1988).
Field preparation
The fi eld should be ripped, ploughed and disced before planting. This
may involve cross-ripping to 1 m to break up any soil compaction. Lime,
phosphorus (P) and potassium (K), as determined by soil analysis, should be
added at this time. Sloping land should be prepared so as to avoid erosion.
Drainage is vital to avoid waterlogging, which can reduce yields. The water
table should be kept below 1.2 m in depth. Extra drainage can be installed
during fi eld preparation via trenches and drains. Drainage preparation can be
a fairly expensive task in the export banana production fi elds, as the fi elds are
expected to remain in production for many years. In Chapter 3, there is a brief
description of these drainage systems.
Transplanting and spacing
A planting hole slightly larger than the material is dug or a furrow made. The
corms are usually covered with 10–15 cm of soil. Planting is scheduled in

Banana and Plantain 201
subtropical areas to meet certain harvest periods in the fi rst crop cycle (Galán
Saúco, 1992). Also in the subtropics, the late summer through to the winter
is avoided, so as not to expose young plants to low temperatures. The concern
in the tropics is to avoid hot weather or a dry season; hence, the best time for
planting is just before the wet season. In hurricane-prone areas, planting is
done before the hurricane or typhoon season, so that the plants are still short
and less prone to being blown over.
Planting densities from 1000 to 3000 plants/ha are used in triangle,
rectangle, single- or double-row cropping patterns. Double rows combine
higher density with a 3.5 m alley for access. The actual density depends upon
cultivar and climate. Higher densities are used in hot, dry localities to generate
the necessary shade and microclimate for maximum yields. The vigour of a
plantation is related to the canopy characteristics, leaf area index and yield.
If only one growth cycle is to be used, a higher density may be planted (3000
plants/ha), for three or more growth cycles; a lower density is recommended
(2000 plants/ha) for highest gross margin.
Export bananas are usually planted in equilateral triangles or in double
rows; in a few cases a square arrangement that is 2.4 m × 2.4 m is used.
The equilateral triangle (hexagonal) system allows for a better use of space;
normally the sides of the triangle are 2.5 or 2.7 m, resulting in 1800–2000
plants/ha. In the double-row system, two closely planted rows are separated
by a wider space from the next double row. The double rows are 1 m apart; the
plants are at 2.25 m in the row and 3.75 m is left between the double rows,
giving a total of 1840 plants/ha. This system allows for the movement of small
equipment in the fi eld and makes harvest easier by allowing a cable system
to be set up parallel to the double-planted rows, from which the bunches are
hung with strings to avoid the pseudostems from falling over because of the
fruit weight and damaging the bunch.
Orientation of double rows N to S or E to W does not signifi cantly e ect
yield (Belalcazar, 1991). More important is to orient rows to avoid erosion in
fi elds planted on a slope and wind damage from the prevailing winds.
High-density annual plantings
In recent years, especially with plantains, a system using high-density planting
and a single harvest developed in Colombia (Belalcazar, 1991) is now being
used in Central America (Lardizabal and Gutierrez, 2006). The fi eld is prepared
and planted in double or single rows in a rectangular arrangement, with 3333
to around 4000 plants/ha. To achieve 3333 plants in the double-row system,
there would be 1.2 m between the twin rows and 1.5 m in rows, with 2.8 m
between the double rows. In single-row arrangement, the plants are 1.50 m
in row and 2.0 m between rows. For 4000 plants/ha, the distances between
double rows and single rows are reduced. When 50–60% of the plants are

202 Chapter 8
harvested, the new planting can be started with corms or plants in bags
planted into the space between the double or single rows; once harvest has
fi nished the old plant stumps are eliminated. The big advantage of this, aside
from higher yields, is that harvest time can be decided in advance, to avoid
harvesting and marketing fruit when market prices are depressed because of
oversupply. Additionally, since plants become more susceptible to Sigatoka
after fl owering, a high-density planting system limits disease incidence in the
fi rst part of the cycle and reduces the need to spray for disease control. The
system is more appropriate for plantains, which normally do not last as long
planted in the fi eld as export bananas, and are normally replanted after two or
three harvest cycles. If a fi xed harvest season is important, the new planting
can be done in a di erent fi eld if the original fi eld is not ready for replanting.
The system has also been successfully implemented for dessert bananas.
Cableway
Cableways are an important innovation in banana harvesting and handling
by the large banana exporters. The cableway greatly reduces mechanical
injury to the fruit during transportation to the packing house. It is also used to
take fertilizers and other materials to the fi elds. When ‘Gros Michel’, a tough-
skinned variety, was replaced because of Panama disease by ‘Valery’ and later
‘Grand Nain’, as both clones have delicate skins, it gave the impetus to use
cableways, as well as the use of packing boxes for export fruit.
Irrigation practices
Irrigation water can be supplied by furrow or over-canopy sprinkler, with
drip irrigation and micro-sprinklers becoming more popular. Irrigation is
used to supplement rainfall and its scheduling requires a calculation of
amount and frequency. This schedule is based on pan evaporation, soil water-
holding capacity, banana root depth, water depletion and crop water use.
The important characteristics of banana are its: (i) high water-loss potential,
associated with its large, broad leaves; (ii) shallow root system – c. 90% of
roots are in the top 300 mm; (iii) poor ability to absorb water from a drying
soil; and (iv) rapid physiological response to water defi cit. Soil water potentials
more negative than −20 to −40 kPa can adversely a ect growth, with relative
impact depending upon local climate – hot and dry (severe impact) to moist
and humid (less impact). In the case of plantains, the same is true; however,
plantains are slightly more tolerant of water scarcity than dessert bananas,
but yield can be depressed by drought.
Micro-sprinkler use has become popular with banana and plantain
exporters in Latin America. Sprinklers are spaced ~11 m from each other,

Banana and Plantain 203
in a square arrangement, on a riser at about 70–80 cm above the soil level.
This system o ers many advantages, the main being the use of less-powerful
pumping equipment than required for the large cannons, which would cover
about a hectare, previously used. The micro-sprinklers, being below the
canopy, do not splash water from one leaf to another and thus prevent the
spread of fungal diseases like Sigatoka. In recent years, drip irrigation has
shown promise, though vandalism from labourers or neighbours who cut the
hoses and steal them is a problem for small producers. Drip irrigation works
very well in well-managed plantations with fi eld security.
Pruning and bunch propping
Other than removal of the male infl orescence, no other vegetative pruning
is normally practised. Withered styles and perianths persisting at the end
of the fruit are usually removed at the packing station after harvest, but are
sometimes removed by hand 8–12 days after the bunch emerges, to reduce
fruit scarring and disease (cigar-end rot). Early removal of one or more hands
from the distal end of the bunch is practised to increase fruit size by reducing
inter-fi nger and hand competition. This hand removal is done by the exporting
companies for dessert bananas and plantains to achieve better calipers (fruit
diameter – size) in the remaining fruit.
Bunches falling from the plant or the whole plant falling over can lead to
considerable bunch damage and can lead to rejection of the a ected fruit for
export. Lodging is due to poor corm anchorage, poor planting material or very
large bunches. The problem is reduced if single or double poles are wedged
against the throat of the plant under the curvature of the bunch peduncle or
twine guys are extended from this same point in the opposite direction of the
fruit bunch and tied to lower positions on nearby plants.
Sucker management and leaf removal
Sucker management is an essential step to remove unwanted suckers
developing from the base of the parent corm and to select a suitable sucker
to produce the ratoon crop. The strategy is to remove suckers that receive
nutrients from the parent plant and which would extend its cycle and reduce
its yield if they remained (Fig. 8.5). This operation is vital in the subtropics.
Desuckering is done by hand by cutting and gouging every 4–8 weeks,
and para n-oil injection so that suckers do not use too many of the resources
available to the parent (Fig. 8.5). Selection of ratoon suckers is critical to
maintaining yield, production and appropriate plant spacing. A sucker on the
most open side is usually selected as the daughter plant and left as the follower,
taking into consideration fi eld spacing. The plants ‘walk’ since the ratoon
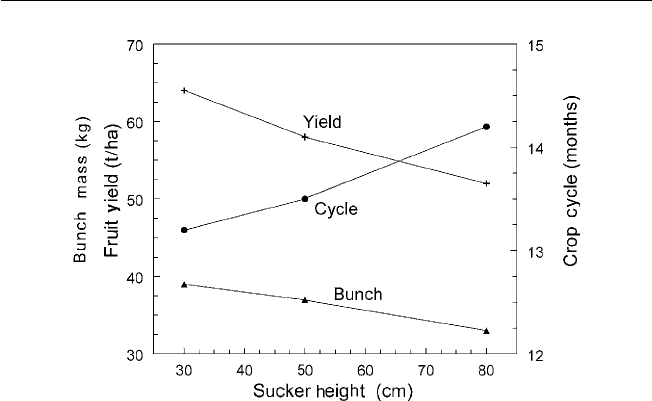
204 Chapter 8
comes at the side of the original plant; therefore care has to be taken that all
plants ‘walk’ in the same direction by carefully selecting the ratoon suckers
in order to keep the original distances between plants. Normally a machete is
used for this operation and care should be taken to disinfect it before every new
plant, to avoid transmission of bacterial diseases such as ‘Moko’.
Removal of dead leaves is practised to reduce disease spread and to prevent
senescent leaves from hanging over suckers and reducing light and to prevent
fruit scarring. At least six to eight healthy leaves should remain on the plant
at fl owering to ensure maximum bunch development (Fig. 8.3). Plants with
severe leaf removal or damage (e.g. as a result of insect feeding) have reduced
bunch weights. The green life of the harvested banana is also reduced by
leaf loss.
Fertilization
Regular fertilization practices are followed on large commercial plantations
(15% of total world production) to maintain optimum productivity, while
in the less-intensively cultivated plantation less supplementary fertilization
occurs (Martin-Prevel, 1990). Defi ciency symptoms have been described for
leaf blade and petioles. Mineral analysis of banana plants has been used and
Fig. 8.5. Effect of the amount of unwanted sucker growth of ‘Williams’ banana
before removal, on remaining sucker (follower) bunch mass, annual yield in the
fi rst ratoon crop and the crop cycle duration between the fi rst and second ratoon.
Unwanted suckers were removed when they were 30 cm, 50 cm and 80 cm high,
leaving one following sucker per plant mat. The leaf area of the 80 cm sucker was
39 times greater than the 30 cm sucker (redrawn from Robinson and Nel, 1990).

Banana and Plantain 205
ranges of defi ciency and adequacy suggested. For plant growth and fruit
production, large amounts of nutrients are required. These nutrients come
from the soil and decaying plant material, and the remainder comes from
applied organic matter and fertilizer. The amount of nutrients removed by the
harvest of cv. ‘Cavendish’ fresh fruit with a yield of 50 t/ha/year includes 189
kg/ha nitrogen (N), 29 kg/ha P, 778 kg/ha K and 101 kg/ha calcium (Ca).
As a proportion of the total nutrients taken up by the banana plant, this is
equivalent to 49% of the N, 56% of the P, 54% of the K and 45% of the Ca.
These total amounts and proportions are about half of those for plantain
(Table 8.4). Most of the data have been derived from experimentation with
bananas produced for export, especially from varieties in the ‘Cavendish’
subgroup. Cultivar and subgroup di erences have been recorded, with the
optimum nutrient supply not being the same for all cultivars. Seasonal
infl uences on fertilization are much greater on bananas growing in
the subtropics, where temperature probably has the greatest infl uence.
Intercropping needs should also be considered.
The large N and K requirements are modifi ed by the local soil nutrient
concentrations. The rate of application depends on climate, soil type, variety,
management practices and yield. Since vegetative and reproductive stages of
development are found in one fi eld at the same time, and as the initial stages
of fruit infl orescence development are crucial for fi nal yield, a constant supply
of nutrients is essential for high yield. If the pseudostem is left standing
after bunch harvest, up to 40% of the nutrients (especially N, P and K) can
be removed by the following ratoon sucker (Fig. 8.6) and increase the bunch
weight of this next generation. Delaying fertilization can have a signifi cant
impact on yield, reducing it by 40–50% or more; a 3-month delay makes it
di cult for the plants to recover.
Because of the extensive root system, fertilizer should be applied away
from the pseudostem, mostly on the side of the next ratoon. Solid fertilizer is
applied three to four times per year, more frequently if there is high rainfall,
for this a 60 cm semicircular strip of fertilizer 30 cm wide is broadcast 30–40
cm in front of the ratoon sucker. Fertilizer applied through irrigation water
(fertigation) is more e cient and gives better control of application time and
rate to meet demands. Frequency of fertilizer application is increased when
fertigation is used monthly, weekly or continuously, especially if drip irrigation
is used. Foliar application is also used.
Numerous forms of fertilizers have been tested. If the di erent forms
meet the management strategy and crop needs and do not lead to excessive
runo or leaching, most are suitable. The most common forms of nitrogen
are ammonium nitrate (NH
4
NO
3
), ammonium sulfate (NH
4
)
2
SO
4
and urea; it
should be applied three to six times per year. For phosphorus, simple or triple
superphosphate is usually applied once a year due to slow P mobility, at rates
of 40–60 kg of P
2
O
5
/ha/year, while, for potassium, potassium chloride (KCl),
potassium sulfate (K
2
SO
4
) and potassium nitrate (KNO
3
) are used, the sulfate
206 Chapter 8
Table 8.4. The quantity of nutrients in banana and plantain with yields of 50 t/ha or 30 t/ha, respectively, from 2400 plants/ha and
bunch masses of 25 kg and 15 kg (Lahav and Turner, 1983), and critical nutrient concentrations in the lamina of the third-youngest leaf
of the vegetative plants (Stover and Simmonds, 1987).
Nutrient Fresh fruit (kg/ha) Remaining in plant (kg/ha)
Proportion removed in
fruit (%)
Leaf lamina critical
concentration in
bananaBanana Plantain Banana Plantain Banana Plantain
N 189 76 199 189 49 29 2.4%
P 29 11 23 16 56 41 0.15%
K 778 243 660 945 54 20 3–3.5%
Ca 101 9 126 149 45 6 0.45%
Mg 49 11 76 53 39 17 0.2–0.22%
S 23 9 50 19 32 32 n/a
Mn 0.5 0.2 12 7 4 3 60–70 ppm
Fe 0.9 0.4 5 3 15 11 60–70 ppm
N, nitrogen; P, phosphorus; K, potassium; Ca, calcium; Mg, magnesium; S, sulfur; Mn, manganese; Fe, iron; n/a, not available.
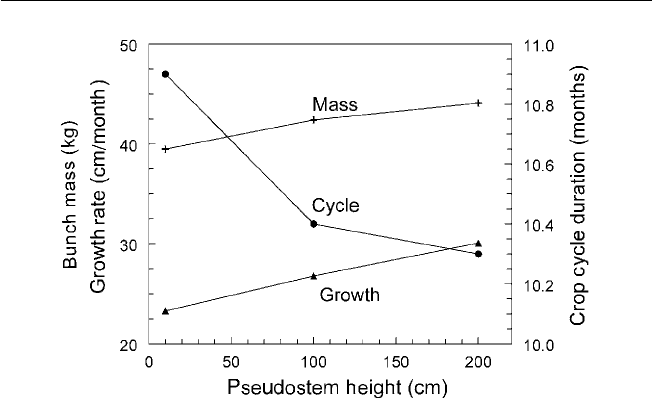
Banana and Plantain 207
form adds sulfur to the soil, the rates vary widely. For example, using KNO
3
at
100 kg/ha in South Africa is suitable where minimum leaching occurs, while
250 kg/ha is needed in Israel and up to 600 kg/ha is used in other countries
with high runo , leaching and yields. Under less-intensive cultivation,
organic matter plus 0.25 kg each of urea and KCl per mat (production unit)
supplement is applied every 3 months. In some areas, no K is recommended,
e.g. the Sula Valley of Honduras and the Jordan Valley of Israel.
If some calcium or magnesium defi ciency is observed, dolomitic rock,
or magnesium sulfate, calcium carbonate or magnesium oxide can be used.
For sulfur defi ciency normally a sulfate fertilizer will solve the problem. For
minor elements foliar application is preferably to soil applications of the
defi cient element.
In the high-density annual planting being used in some places in Latin
America for plantains, if no drip irrigation is in place, research has shown that
two dry fertilizations of N and K per cycle are enough, with 30–40% of the
total yearly amount applied when the plants have developed the fi rst leaf in the
fi eld, and the other 60–70% when they have developed ten leaves; applications
are applied around the single pseudostem (Belalcazar, 1991). Phosphorus is
applied at planting time.
Fig. 8.6. The effect of cutting height of pseudostem at primary bunch harvest on the
growth rate of the follower plant in the month after cutting, crop cycle in months
to harvest of the follower, and the weight of the follower bunch (after Daniells and
O’Farrell, 1987).
208 Chapter 8
Bunch covers
Polyethylene bunch covers (30–40 μm thick) are almost universally used
to improve yield and maintain fruit quality (Fig. 8.7). Some covers have a
pesticide impregnated into them to reduce thrips or mite damage. The covers
produce a warmer microclimate around the bunch, which can accelerate
fruit development; they also prevent fi ngers inside the bunch covers from
being chafed by leaves and covered by dust and pesticides; the cover can also
discourage some insects from entering. The higher temperature and humidity
generated inside the bunch cover are helpful in subtropical areas, though
not required. For tropical areas, perforated covers are used for aeration and
cooling. Covers are applied after the bracts have fallen and should hang 15 cm
below the distal hand. Thinner covers are used in the tropics, where wind is
less of a problem. The banana exporting companies are also using protective
pads between developing hands, so that no bruising or chafi ng occurs
to the fruit.
Fig. 8.7. Bunch covers provide protection of developing fruit from pathogens and
insect damage. The coloured plastic hanging from the bottom of the bunch indicates
date of emergence and is used to indicate harvesting (a). Cableways are used in
large plantations to transport bunches of fruit to the packing sheds to minimize
mechanical injury during handling (b).

Banana and Plantain 209
Disease and pest management
Diseases
Fruit diseases can cause severe problems if not controlled (Table 8.5). The
most virulent disease of banana and plantain is black Sigatoka, which is
found worldwide, except for subtropical areas or tropical areas where it does
not rain and in the Caribbean. Sigatoka disease has two forms: the yellow,
caused by Mycosphaerella musicola and this form is more prevalent in cooler
areas, and black Sigatoka or black leaf streak, which is a much more virulent
type caused by Mycosphaerella fi jiensis. Both alter the photosynthesizing
capacity of the leaf because they produce necrosis that can extend to the
whole leaf area, and at the same time there is an induction for premature
ripening of the fruit because of more ethylene being released by the infected
leaves, thus reducing calipers and yields. Control in intensive production
systems relies on the use of fungicides, which have to be rotated and are
very expensive, increasing the production costs signifi cantly. Therefore, this
approach may not provide a long-term solution for small growers and others
serving local markets.
One way to reduce fungicide costs, which in intensive production systems
can account for 25–30% of the production costs, is to use disease forecasting,
which is based on the evaluation of climatic conditions and calculating
the stage of evolution of the disease (SED). The idea is to spray as soon as it
is considered necessary with a strong systemic fungicide mixed with pure
mineral oil and rotate products with di erent modes of action to reduce
fungicide resistance development. This approach has reduced the 40–60
applications per year to 12–14. Biofungicides have been tried, but in high
disease pressure areas they do not work well, but they can be combined with
contact fungicides, allowing reduction of the rates that are presently used.
Eliminating the necrotic leaves or parts of the leaf is another practice to reduce
dispersion of conidia and ascospores. Breeding for resistance is essential, using
natural resistance in other banana varieties, or biotechnology to transfer
resistance will probably provide a long-term solution.
Fusarium wilt (Panama disease) has caused severe disruption of banana
and plantain production where susceptible cultivars, such as ‘Gros Michel’,
are grown; fortunately, resistant genotypes do exist, but the fungus can
also mutate and eventually a ect them. The two other major diseases are
the bacterial disease ‘Moko’ and the viral bunchy-top disease. For ‘Moko’
prevention is the best strategy, consisting of isolating and eradicating any
infected plant and its neighbours and having tools used for cutting in the
plant, especially the machetes used for desuckering, disinfected after each cut
with Vanodine™ or a similar product.
Preharvest diseases, including fruit freckle (Phyllostictina musarum) and
speckle (Deightoniella torulosa), can cause fruit skin spotting. ‘Cigar-end rot’ is
associated with Verticillium theobromae and Trachyspharea frutigena, infecting

210 Chapter 8
Table 8.5. Principal diseases of banana, causal organisms, distribution, varietal susceptibility and symptoms.
Disease Causal organism Distribution Varietal susceptibility Symptoms
Bacterial
Moko
Pseudomonas
solanacearum:
only distinct
strains of race 2
attack bananas
and plantains
Central and South
America, southern
Caribbean, Philippines,
Indonesia
Cavendish AAA
susceptible, Bluggoe
ABB susceptible, Horn
AAB resistant, Pelipita
ABB resistant
Root: progressive transient yellowing,
older leaves fi rst, necrosis and collapse
of base petiole, younger leaf panels
fl accid and necrotic, premature bunch
development, discolored vascular
bundles cream to brown or black
in all parts of corm, roots, suckers.
Fruit: male bud blackens and shrivels,
vascular discoloration extends up fruit,
bunches blacken and rot and then it
progresses down pseudostem
Blood disease Possibly related to
Moko
Indonesia Bluggoe ABB susceptible Similar symptoms to Moko leaf yellowing
but more conspicuous, reddish tinge
to discolored vascular bundles, infects
fruit via style
Rhizome rot
Erwinia
chrysanthemi
Worldwide Certain AAA and AA
varieties susceptible
incl. Cavendish
Rotting of corms, plant falls over. Soft,
pale brown or yellow rot in outer
cortical tissue. Entry through wounds,
especially in wet conditions
Fungal
Panama disease
(Fusarium wilt)
Fusarium
oxysporum fsp.
cubense
Race :1 worldwide,
Race 2: widely
distributed, Race 3:
Central America,
Race 4: Canary Islands,
Taiwan, Australia,
South Africa
Gros Michel, some
Cavendish varieties
and some plantains
Progressive yellowing from the oldest
leaves, longitudinal splitting lower
portion of the outer leaf sheaths,
wilting and buckling of leaves at the
petiole base. Initial site of infection
feeder roots. Sometimes confused with
Moko disease

Banana and Plantain 211
Disease Causal organism Distribution Varietal susceptibility Symptoms
Sigatoka leaf spots
Black Sigatoka –
Black leaf streak
Mycosphaerella
fijiensis
Paracercospora
fijiensis
Worldwide, except
some islands in the
Caribbean and
subtropical areas
Cavendish and most
other commercial
varieties are susceptible.
Some resistance in
M. acuminata and AA
diploids
Dark leaf spots on lower leaf surface,
which enlarge and coalesce, causes
signifi cant reduction in leaf area and
yield, and premature fruit ripening
Yellow Sigatoka
Mycosphaerella
musicola
Worldwide, more
common higher
elevations cooler areas
Less serious than black
Sigotoka, plantains
(AAB) resistant.
Starts as pale yellow streaks on the upper
leaf surface, they enlarge and coalesce
Viral
Banana bunchy-
top virus
(BBTV)
Vector banana
aphid
South-east Asia,
Australia, India,
Central Africa, Pacifi c
Gros Michel AAA
susceptible, Cavendish
AAA susceptible, Saba
BBB tolerant
Infected leaves become stunted and
chlorotic at the margins. Bunching of
leaves at the apex. No fruit production,
suckers infected
Banana mosaic Cucumber mosaic
virus. Many aphids
as vectors
Worldwide Cavendish AAA
susceptible, Horn AAB
susceptible
Sharply defi ned interveinal chlorosis of
leaves can lead to rotting heart leaf and
cylinder. Stunted plants
Banana streak
virus (BSV)
Vector mealy bugs,
infected planting
material
Worldwide Cavendish AAA
susceptible, Mysore
AAB susceptible
Yellow streaks, continuous or broken,
running across the leaf blade. More
pronounced in warm weather

212 Chapter 8
the fl ower perianth and slowly developing along with fruit growth. The end of
the fruit darkens and later is covered with spores looking like a lit cigar. The
disease is found in Latin America, West Africa, Egypt and Australia. The pulp
develops as a dry rot. Removal of pistil and perianth 8–12 days after bunch
emergence and polyethylene bunch covers help to reduce incidence.
A complex of di erent organisms is known to cause the most serious
worldwide postharvest disease – crown rot. The wound caused when the
bunch is dehanded is colonized by the organisms, the rot spreading into the
pedicels during shipping and sometimes into the pulp. A number of organisms
have been found, with the actual complex varying with region; most common
isolates have been Colletotrichum musae, Fusarium spp., V. theobromae, B.
theobromae and Ceratocystis paradoxa. Spores are carried by wind or rain splash;
however, dehanding knives and delatexing tanks may be bigger sources. Good
dehanding practices, with clean cuts using sharp knives, and sanitation of
packing sheds and areas are essential. Exporters use alum and a fungicide to
try to control this infection.
Anthracnose on the fruit skin and neck rot are both caused by C. musae
and can be a serious problem when the fruit becomes overripe. Sanitation and
postharvest fungicide are used for control.
Insect pests and nematodes
Insect pests are generally of minor importance (Table 8.6). Occasionally,
severe fruit scarring can occur. Borers are a problem where control practices,
such as planting clean material and insecticide schedules, are not followed.
The continuous nature of banana production makes pests such as nematodes
more important (Table 8.6).
The main insect problem in many areas of the world is the black weevil
(Cosmopolites sordidus). The weevil lays eggs in the corm, and the larvae
coming from them bore into the corm, causing extensive corm rotting because
of secondary infection. The damage to the roots and the expansion of the
rotting to most of the corm leads to weakening of the plant, which lowers
production, and frequently toppling of the plant with total loss of the bunch.
The main control measures should be paring and disinfecting the corms
before planting, eliminating residues of old plants before a new planting,
fallows and the use of traps with pheromone or pseudostem piece traps; both
attract the adults. In the pheromone traps, the weevils are drowned and in the
pseudostem piece traps they can just be hand-collected or can be poisoned if
an insecticide is added to the piece.
Nematodes can severely limit production, with the extent of the problem
varying with cultivar, soil conditions, type of nematode and plant vigour.
Nematicides are used for fruit destined for export and are applied every 4–6
months, but they are expensive, their use has to be continued and many of
them are being banned by the importing countries. More economic measures
include: improved fallow to eliminate old material, done in combination with

Banana and Plantain 213
Table 8.6. Major pests of banana.
Pest Latin name Spread Susceptibility Symptoms
Banana borer/
weevil
Cosmopolites
sordidus
Worldwide No clones resistant Burrows into rhizome, network
of tunnels, lays eggs at base of
pseudostem, adults (12 mm long) feed
on pseudostem, nocturnal
Bunch pests
Thrips
Chaetanaphothrips
spp. red rust thrip
Central America, South
America, Asia, Australia
Rust blemish to fruit skin, especially
between fi ngers of immature fruit
Frankliniella spp.
South-east Asia, Australia, Corky scab on developing fruit
Flower thrips Caribbean, Latin America
Scab moth
Nacoleia octasuma
Australia, Pacifi c Islands Brown scabs on developing fruit
Nematodes
Burrowing
nematode
Radopholus similis
Most areas except East
Africa, Israel, Canary
Islands, Egypt and
Taiwan
AAA more tolerant
than AAB
Elongated blade, lesions on root
Lesion nematode
Pratylenchus
goodleyi
African highlands, Canary
Islands
Cavendish
susceptible
Similar to R. similis
Pratylenchus
coffeae
Tropics AAB more commonly
associated
Root-knot
nematodes
Meloidogyne spp.
Worldwide Deformation and stunting of roots. Gall
formation

214 Chapter 8
an injection of herbicide into the pseudostems, resulting in the nematode
population being signifi cantly reduced; use of water isolation ditches so no
nematodes enter the fi eld through water coming from neighbouring fi elds;
use of healthy plant material – initially it could be tissue-cultured although
it is very expensive; use of tolerant or, hopefully in the near future, resistant
varieties and the reintroduction of biodiversity with new and di erent green
manure or cover crops, manure incorporation and other practices (Rísede et
al., 2010).
Weed management
Weeds are a major problem during stand establishment before canopy closure
occurs. Cultivation needs to be carefully carried out so as not to damage the
surface feeder roots, using hand-hoeing around the plants. Herbicides can
be used once the banana canopy is su ciently high to avoid contact with the
leaves. Mulching and intercropping during early stand development can be
used. Failure to weed can lead to severe yield decline. It is very important to
keep the area around the new plants clean during the initial weeks until they
develop and then continue to clean until the shade of the canopies will reduce
the weed problem.
Orchard protection – windbreaks
Banana plants tolerate wind up to 30 m/s. Higher speeds lead to tearing of leaf
laminas. Windbreaks may be necessary if prevailing winds tear leaves into
strips less than 5 cm wide (Turner, 1994). The need for a windbreak must be
balanced against the e ects of shading, the area needed, competition for water
and nutrients, and the limited protection on the leeward side. Plant propping is
carried out to reduce the impact of winds or the excessive weight of the bunch.
HARVESTING AND POSTHARVEST HANDLING
Harvesting
Individual fi ngers increase in weight and begin to lose angularity in cross
section during maturation. Fruits for export are harvested while still green
at 75% maturity with some angularity, 10–14 weeks after fl ower emergence
in the tropics and up to 9 months in the cool subtropical areas. The idea is to
export only fruit that is not over-aged and that has a minimum caliper. The age
of the fruit is established by tying a coloured ribbon to the bunch cover at the
time the cover is put in place, so the di erent ribbon colours indicate the week

Banana and Plantain 215
the bunch emerged. The maturity is checked by determining the caliper of the
middle fi nger of the outer whorl of the second hand. When the caliper reading
is between 31 and 41 mm in diameter, the bunch is ready to harvest. If the
caliper reading is less than the 31–41 mm minimum, the fi nger is rechecked
the next week, and if after the week the caliper is still too small, the bunch
will be harvested and fruit sent to the local market. In this way, over-aged
fruit are not exported. If over-aged fruit are included in export fruit shipment,
it is possible that they will start to ripen in transit and induce ripening of the
whole shipment.
Small growers tend to harvest bunches when more than three-quarters
mature with little angularity and at fully rounded stage. More mature fruit
have a shorter postharvest life and are more liable to be sunburnt and split.
Other criteria used to judge maturity include drying of leaves, drying of stylar
ends and days from bunch emergence. The days from bunch emergence can
vary from 7 to 24 weeks, depending upon cultivar, season, crop management
and environment. Other measures include pulp to peel ratio and skin fi rmness.
The bunch is removed from the plant by cutting a notch in the pseudostem
while supporting the bunch with a pole and slowly lowering it on to the
shoulder pad of a harvester. The stem is then fully cut, leaving a 300 mm
peduncle. The bunches are transported to the packing shed on padded trailers
or on an overhead cable system. Dehanding can be performed in the fi eld, with
the hands transported to the packing shed on padded trailers. Care is essential
in these steps to avoid any mechanical injury that would reduce fruit quality.
Plantains in the tropics are harvested for export about 11 weeks after
fl ower emergence, and colour ribbons are again used as a guide to establish
bunch age. Plantain maturity standards vary widely and lead to considerable
variation in product quality.
Postharvest treatments
Bunch covers are removed in the fi eld or after the bunches reach the covered
packing shed. Bunches must be protected from exposure to direct sunlight to
avoid sunburn injury during transportation and at the packing-shed holding
area. Hands are removed with a sharp, curved knife or curved chisel, leaving
part of the crown attached. The hands, irrespective of whether dehanding
takes place in the fi eld or in the packing shed, are placed in clean water to
remove dirt and latex exuding from the cut crown; this should last a minimum
of 20 min. Care is needed to avoid the build-up of fungal spores in the water of
the wash tank, by frequent changes and the use of chlorine.
The hands are removed from the tank and sometimes cut into clusters of
adjacent fi ngers, with defective fi ngers being removed. The clusters are placed
in trays that pass through a fungicide-treatment spray or dip on a conveyor.
The conveyor then passes the trays to a packing station, where weight

216 Chapter 8
adjustment occurs and the fruits are packed into cardboard cartons, with a
thin plastic liner to prevent chafi ng injury and water loss. Hands or clusters
are tightly packed to avoid movement during shipping. Carton sizes vary
from 12 to 20 kg, with Central American cartons holding 18.1 kg of fruit.
Cartons may then be cooled to 13°C before shipping to market. In small local
operations, bunches may be sold, with dehanding taking place upon sale to
the consumer. Alternatively, hands may be shipped to market in a number of
di erent containers. Export dessert bananas, because of the regular nature of
the hands and curved nature of the fi ngers, can be easily packed. Many other
cultivars are not as easily packed, as the fi ngers are not arranged in a regular
manner, e.g. ‘Pisang Mas’ (AA).
Green bananas are shipped at 13–14°C to delay ripening. Lower
temperature can lead to chilling injury, whose symptoms include a dull, grey
skin colour, poor ripening, poor conversion of starch to sugar, poor fl avour
development and susceptibility to decay. Symptom development is dependent
upon exposure temperature and time, and susceptibility depends mostly on
the cultivar. Maturity, prior growing conditions and previous temperature
exposure may also be factors. ‘Dwarf Cavendish’ shows injury after 20 days at
11°C and ‘Lacatan’ after 12 days at 14°C. Plantains, such as ‘Pisang Awak’,
are less susceptible to chilling injury than dessert types.
Marketing
Market quality standards vary widely, with export bananas having the most
stringent standards. In large measure, this is related to consumer preferences
and the condition of the bananas received at the market. In western
supermarkets, unblemished fruits are preferred, even required, while in
markets in tropical areas postharvest handling is very abusive and fruits with
blemishes are normal, with degree of fruit ripeness being a deciding factor.
Export quality standards are applied before and after shipping and
include: blemishes and fruit shape, fi nger length and diameter, cluster size
and arrangement, and carton weight. A minimum fi nger length on a hand is
203 mm for export from Central to South America, with the whole hand being
culled if one fi nger does not meet this standard. When hands are cut into
clusters, the cull fi ngers are carefully cut out. The diameter for ‘Cavendish’
fi ngers ranges from 31 to 41 mm. Fingers with obvious blemishes are culled.
Such standards can lead to cull rates of up to one-third, with culled fruit being
sold locally, processed or dumped.
Banana fruits are very susceptible to both abrasion and impact injury.
The major marketing problem of export bananas is mechanical injury,
which shows itself as black sunken areas on the skin after ripening. The
thin plastic liner in export cartons minimizes chafi ng damage to fi ngers that
rub against the side of the carton during handling. Latex allowed to dry on

Banana and Plantain 217
the skin oxidizes as brown and black stains and can lead to downgrading
of fruit. Other concerns are diseases, particularly crown rot, which may
a ect the whole carton and promote uneven fruit ripening. Because of weak
pedicels, the fi ngers of some cultivars fall from the hand during ripening,
exposing the pulp.
Plantains have a tougher skin than bananas and so there is less danger
of causing harm to them by improper handling; in general they should be
treated the same way as bananas, but in most instances they use less elaborate
systems for harvest, transport to the packing house and packing than for
bananas.
Plantain boxes weigh 22.6 kg. Alternatively, green plantains are partially
ripened, peeled and frozen, with a heat treatment to minimize enzymatic
browning reactions.
Ripening
Dessert bananas are allowed to ripen and are chiefl y eaten raw when they
have low starch, high sugar and developed fl avour. Plantains have high starch
content and are eaten when green or ripe after boiling, frying or roasting. The
conversion of starch to sugar during ripening of plantain-type bananas is less
complete than in dessert bananas (Fig. 8.8).
The ripening process for the export dessert-type bananas is normally
carried out by specialists under controlled conditions just before distribution
and marketing to consumers. When bananas are allowed to ripen naturally,
it is di cult to predict when fruit will be ready to eat. Under controlled
ripening conditions, ethylene is supplied from compressed gas cylinders,
ethylene generators or ethylene-generating chemicals, such as ethephon.
Commercially, bananas are treated with about 100 ppm ethylene for about
24 h under controlled temperature and humidity conditions and ventilation
to prevent carbon dioxide (CO
2
) build-up. Newer systems pressurize the
room to allow uniform ethylene distribution and temperature. Temperature
control allows fruit to be ripened on a specifi c schedule to colour stage 3 for
distribution in from 4 days at 19°C to 10 days at 14.5°C. The humidity control
has a signifi cant impact on the fi nal skin colour developed and fl esh softening.
In local markets, fruits are ripened by covering with a tarpaulin or cloth
after inserting a packet of calcium carbide (0.3 g/l) to generate acetylene.
Acetylene is a 100 times less e ective analogue of ethylene. A prolifi c ethylene-
producing ripening fruit, such as avocado, can also be used as an ethylene
source, as can burning of incense sticks or young leaves of a number of trees,
such as gliricidia (Gliricidia sepium Stend) (Acedo and Bautista, 1991).
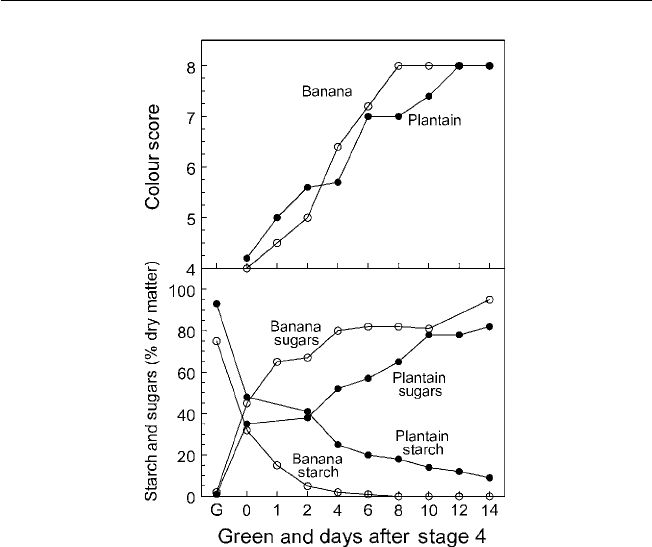
218 Chapter 8
UTILIZATION
The main carbohydrate di erence between bananas and plantains is that
bananas are less starchy than plantains (Fig. 8.8). Bananas had 1% starch
when fully ripe and 0% when overripe, while plantains had 9% starch when
fully ripe and 3% when overripe (Marriot el al., 1981). The same bananas
had 23% sugar when ripe or overripe while plantains had 20% sugar when
ripe and 27% when overripe. The moisture content of ripe bananas is ~80%,
versus ~65% for plantains. Bananas are at their best when yellow, plantain
when black.
The fl avour, texture, convenience, ease of eating and nutritional values
have made dessert bananas very popular (Baldry et al., 1981). Banana is a
useful source of vitamins A, C and B6 and has about twice the concentration
of K compared with other ripe fruit (Table 8.7). Plantain as a staple provides
considerable energy and protein, although the diet needs to be supplemented.
In West and Central Africa, people derive about one-quarter of their energy
requirements from plantains (Wainwright, 1992).
Bananas are used in special diets where ease of digestibility, low fat, no
cholesterol, minerals (high K, low sodium (Na)) and vitamin contents are
Fig. 8.8. Change in dessert bananas’ and plantains’ colour score (a), and starch and
total sugars (b) (redrawn from Marriot et al., 1981).

Banana and Plantain 219
required. The fruit does not cause digestive disturbance; it readily neutralizes
free acid in the stomach and does not give rise to uric acid. These special
diets are used for babies, the elderly and patients with stomach problems,
gout and arthritis.
Only a very minor proportion of the total world banana production is
processed. The procedures used include canning as slices, drying as slices or
fl akes, freezing of juice, extraction, frying and fermentation. Puree is canned
or frozen and used in baby foods, baking and drinks. Banana essence is a clear
colourless liquid used in desserts, juices and drinks. Dried ripe fruit can be
made into fl our. The lack of acidity makes processing di cult, because of the
need for pasteurization. A year-round supply of fresh fruit makes preservation
less economic. A beer is brewed from plantains, also called beer bananas, and
consumed in Uganda and Tanzania.
Plantains can be eaten green or ripe. Green plantains can be peeled and
cooked or cooked and mashed and mixed with bacon or pork meat pieces or
boiled and mashed and made into balls that are eaten in soups. The green
Table 8.7. Proximate fruit composition of banana and plantain in 100 g edible
portion (Wenkam, 1990). Differences in mineral content can be due to cultivar,
fertilization, water supply and environment.
Williams (AAA) Brazilian (AAB) Plantain (BB)
Water (g) 71.3 79.2 64.1
Energy (kJ) 418 301 523
Protein (g) 1.08 1.75 1.28
Lipid (g) 0.13 0.18 0.03
Carbohydrate (g) 26.56 18.03 33.39
Fibre (g) 0.11 0.25 0.43
Ash (g) 0.9 0.82 0.87
Minerals
Calcium (mg) 5 2 4
Iron (mg) 0.49 0.35 0.54
Magnesium (mg) 40 26 35
Phosphorus (mg) 18 13 21
Potassium (mg) 494 302 393
Sodium (mg) 1 1 3
Vitamins
Ascorbic acid (mg) 5.1 8 17.5
Thiamine (mg) 0.044 0.026 0.038
Ribofl avin (mg) 0.045 0.041 0.064
Niacin (mg) 0.69 0.61 0.43
Vitamin A (IU) 88 82 31

220 Chapter 8
fruit can also be cut into round or long slices that are deep fried and eaten as a
snack. In some cases, thick slices about 10 mm wide are cut, partially fried and
then expanded by pounding them, after which the frying is completed; these
slices, called ‘patacones’ or ‘tostones’, are very popular in Latin America and
are eaten as a side order with meat and other food preparations. Plantains are
also left to ripen completely until their peel turns black, at this point they are
usually sweet (Fig. 8.8); after this they are peeled and sliced or halved and fried
or baked to be eaten with the main course. Alternatively, the ripe fruits are
baked as a very nice dessert with added sugar, cream or butter, and cinnamon.
Corms, shoots and male buds are also eaten as a starch source or
vegetable. Immediately after harvest, the pseudostem still has considerable
starch reserves. The male bud, with the outer fi brous bracts removed, is boiled
in South-east Asia and eaten as a vegetable, after several changes of water to
remove astringency. Banana leaves are used as food wrappers for steaming.
FURTHER READING
Gowen, S. (ed.) (1995) Bananas and Plantains. Chapman and Hall, London.
Hassan. A. and Pantastico, F.B. (eds) (1990) Banana – Fruit Development, Postharvest
Physiology, Handling and Marketing in ASEAN. ASEAN Food Handling Bureau,
Kuala Lumpur, Malaysia.
Robinson, J.C. (1996) Bananas and Plantains. Crop Production Science in Horticulture,
CAB International, Wallingford, UK.
Simmonds, N.W. (1982) Bananas, 2nd edition. Tropical Agricultural Series, Longman,
London.
Soto, M.S. (1992) Bananos (in Spanish). Lithography Imprenta LIL, San José, Costa
Rica.
Turner, D.W. (1994) Bananas and plantains. In: Scha er, B. and Andersen, P.C. (eds)
Handbook of Environmental Physiology of Fruit Crops. Vol. II, Subtropical and Tropical
Crops. CRC Press, Boca Raton, Florida, pp. 37–64.
Turner, D.W. (1997) Bananas and plantains. In: Mitra, S.K. (ed.) Postharvest Physiology
and Storage of Tropical and Subtropical Fruits. CAB International, Wallingford. UK,
pp. 47–83.

© CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1 221
(R.E. Paull and O. Duarte)
9
LITCHI AND LONGAN
Litchi (Litchi chinensis Sonn.) and longan (Dimocarpus longan (Lour.) Steud) are
members of the Sapindaceae. These sub-tropical and tropical trees, respectively,
are grown widely in the tropics. The fruit is marketed fresh, dried, canned and
as juice.
BOTANY
The Sapindaceae is composed of around 150 genera and 2000 species of trees,
shrubs, and a few herbs and vines, usually monoecious, distributed widely in
the warm tropics and subtropics. The common family name soapberry refers
to the tree (Sapindus saponaria L.), a native of tropical America producing a
fruit containing a soap-like substance (37% saponin). The majority of species
are native to Asia, with a few species in South America, Africa and Australia.
Among the numerous genera, four related genera and fi ve species are of
interest to the fruit horticulturist:
1. Blighia sapida Koenig, the Jamaican akee, is native to Guinea and is com-
monly grown in the Caribbean Islands, particularly in Jamaica. The brightly
coloured yellow to red fruit has thick walls in three parts, each containing a
white nut-fl avoured pulp with a shiny black seed attached at the tip. Pulp from
immature or overripe fruit and the pink raphe attaching the aril to the seed are
poisonous. When the pods open naturally, the pulp can be fried or boiled. When
fried, it resembles scrambled eggs.
2. Dimocarpus longan (Lour.) Steud., longan, lungan, langngan, dragon eye
(English), lengkeng (Malaysia, Indonesia), longanier, oeil de dragon (French),
lamyai pa (Thailand), or nhan (Vietnam) is reported to have originated
in north-eastern India, Burma or southern China in the Yunan province.
Commonly called dragon eye, the trees attain heights of 9–12 m under suitable
environments and it is more vigorous than the litchi (Menzel et al., 1988b).
The longan is generally grown in similar areas to that of litchi in China and
Thailand but climatic requirements are less exacting.

222 Chapter 9
3. Litchi chinensis Sonn., ssp. chinensis, the litchi, is a subtropical species
indigenous to south China. It is principally cultivated in Guangdong, Fujian,
Guangxi and Sichuan provinces. Gro (1921) discusses in detail the origin of
the names litchi and longan. The Chinese characters for litchi convey the idea
that the fruit of the litchi must be removed from the tree by means of knives
with the twigs attached. Many cultivar names are pronunciations of various
southern Chinese dialects and spellings, which has led to some confusion. The
most recent Chinese descriptions use the spelling litchi and national dialect
name (Mandarin – common language). Other spellings and common names
are lichee, litchee, leechee, lychee, lin-chi (Thailand), laici (Malaysia), lici, litsi
(Indonesia), letsias (Philippines), li-chi or lizhi (China), vai, cayvai, or tu hu
(Vietnam).
There are two subspecies; one is found in the Philippines (ssp.
philippinensis) and the other in Indonesia (ssp. javanensis). The Philippine
subspecies has a long, oval-shaped fruit with thorn-like protuberances
that split when ripe, exposing an inedible aril, partially covering the seed.
The Indonesian subspecies is similar to the Chinese species and crops
more regularly in hot equatorial areas, though it has not been exploited
commercially.
4. Nephelium lappaceum L., the rambutan (Indonesia, Malaysia, Philippines,
English), litchi chevelu (French), and ngoh and phruan (Thailand), and
Nephelium ramboutan-ake Bl., the pulasan, ngo-khonsan (Thailand) and
kapalasan (Indonesia) are both native to the Malaysian peninsula and are now
distributed widely in South-east Asia. This species will be covered in Volume 2.
ORIGIN AND DISTRIBUTION
The cultivation of litchi in China antedates the beginning of the Christian era
according to Chinese writings going back to 1766 (Storey, 1973). Early
distribution into other tropical and subtropical regions occurred during the
16th and 17th centuries. The litchi was introduced into India in 1798 and has
developed into a signifi cant industry. Litchi and longan reached Europe during
the early part of the 19th century and are mentioned as having reached
Trinidad before 1880, with a few trees being grown in Florida as early as 1883
(Gro , 1921). The fi rst litchi tree is said to have been brought to Hawaii about
the year 1873 by Mr Ching Chock, a Chinese merchant, and was planted
on the property of Mr Chun Afong and became known as the ‘Afong’ tree,
identifi ed as Gui Wei (Kwai Mi), later re-identifi ed as similar to ‘Da Zao’. The
o ce of Foreign Seed and Plant Introduction in the United States, Department
of Agriculture began introducing litchi plants in 1907. The late Professor
G.W. Gro , who resided in south China almost continuously from l907 to l94l
as Dean of the College of Agriculture, Lingnan University, Guangzhou, China,
was responsible for the introduction of a large number of cultivars into the

Litchi and Longan 223
USA. His contributions number 17 out of the 25 cultivars and clones now
established in Hawaii. Today, the litchi is found in most countries of Latin
America, the Caribbean, India, Mauritius, Africa, Australia, Israel, the Canary
Islands, Madeira Islands, and many other countries.
ECOLOGY
The litchi and longan are evergreen subtropical plants, indigenous to areas
where cool, dry winters and warm, wet and humid summers prevail. Such
conditions are found mostly in subtropical regions and at higher elevations
in the tropics. In Guangzhou, summers are known for their rains and high
humidity (>80%) during night and day. The winter is cool and dry, and frost is
rare. Litchi is described as a water-loving, low-elevation plant, which is planted
largely along the banks and dykes in the Pearl River delta in Guangdong.
However, there are upland cultivars (mountain litchi) grown on hillsides or in
orchard systems with very little tillage after the trees mature.
Soil
Observations indicate that the litchi and longan thrive on a wide variety of soil
types as long as drainage is good enough to prevent waterlogging. Satisfactory
litchi growth occurs in India and Florida in neutral to slightly alkaline soils,
with free lime present. In south China, the litchi soils are alluvial silty loams
to clays in the Pearl River delta or laterite soils on the hills. Longan thrives best
on deep clay loams, and both species prefer a slightly acid (pH 5.0–6.5) soil.
Climate
Rainfall and moisture
High rainfall and humidity induce good growth in litchi and longan, with
litchi being able to resist fl oods. Roots of litchi trees covered with water for 8
days showed no deleterious e ects, even when fruiting. In China and Thailand,
litchis are also grown on banks with high water tables beneath them.
Ample annual rainfall is considered to be around 1250–2000 mm for
litchi and 1500–3000 mm for longan. When rainfall is lower, irrigation is
essential. A dry autumn and winter is important to prevent litchi vegetative
growth (Fig. 9.1), which is essential to good fl owering (Nakata and Watanabe,
1966). In south China, litchi anthesis occurs during the rainy season
(130–375 mm/month) and when relative humidity is higher than 80%. Too
much rain during anthesis, however, can reduce fl ower opening and insect
activities needed for pollination. A major problem of litchi culture in Hawaii,
with the exception of the Kona district of Hawaii, is that rainfall comes in
late autumn and winter, making it di cult to control vegetative fl ushing.
Irrigation after a period of soil water stress is reported to aid in longan
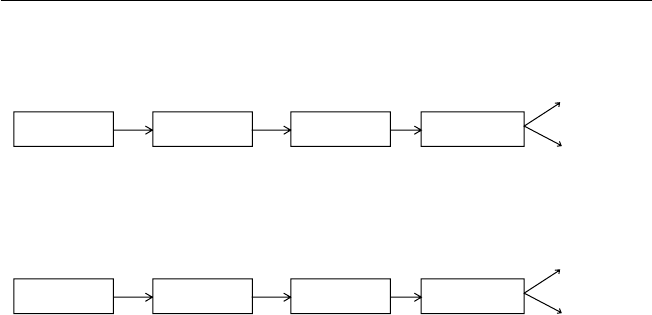
224 Chapter 9
fl owering (Fig. 9.1), even though the tree fl owers profusely in areas with high
water tables. Both crops require adequate moisture, from rainfall or irrigation,
during fruit set and growth.
Temperature
Young litchi and longan trees are severely damaged or even killed at −2
to −3°C, with dormant and mature trees having more tolerance to low
temperatures. Periodic cold is required for fl owering, though in Guangzhou,
air temperatures rarely drop below 0°C. The total duration of relatively low
temperatures rather than the frequency or time of occurrence during a critical
period seems to be the determining factor (Fig. 9.1). When trees are deeply
rooted, low air temperature is more important than soil moisture (Menzel et
al., 1989). There is considerable cultivar di erence in response to temperature
(Menzel et al., 1989). In a test involving seven cultivars, all cultivars showed
panicle emergence after 6 weeks at 15/10°C or at 20/15°C; however, there
are di erences in the degrees of fl owering between the cultivars at 20/15°C
(Table 9.1). There is no fl owering at 25/20°C or 30/25°C. Temperature also
infl uences the time from emergence to anthesis: 14–16 weeks at 20/15°C,
versus 6–8 weeks at 15/10°C. The greatest number of infl orescences
per branch occurs if low temperatures are maintained until anthesis. If
temperatures are increased from varying the period at 15/10°C to 30/25°C
after emergence and before anthesis, there is a reduced number of female
fl owers and some fl oral buds revert to vegetative growth (Fig. 9.2). Pollination
is optimum at about 19–22°C and occurs in 5–7 days. At lower temperatures,
pollen tube growth is strongly inhibited. Hot, dry conditions may reduce
Litchi
Longan
Harvest Leaf Growth Rest
Floral Induction
and development
Temperature >25 °C
High water, high N
Temperature <25 °C
Low water and N
Temperature <25 °C
Low water and N, or sodium chlorate
Temperature >25 °C
High water, high N
Girdling
Remove vegetative shoots
Pruning
old panicle removal
fertilize and Irrigate
Fertilize
Irrigate
Temperature <25 °C
Temperature >15 °C
or <10 weeks
Temperature >22 °C
or <8 weeks
8–10 weeks
Flowering
No flowering
Irrigate
Irrigate
Temperature 15 °C
Temperature 15–22 °C
10 weeks
Infloresence
with flowers
only
Infloresences
with one or more
leaves or revision
to vegetative shoot
Harvest Leaf growth Rest
Floral Induction
and development
Fig. 9.1. Fruiting cycle of litchi (after Menzel and Simpson, 1994) and longan as
affected by temperature, nitrogen fertilization and water availability. Litchi leaf low
nitrogen levels need to be in the range of 1.75–1.8%. There can be more than one
fl ush of leaf growth during the year. Longan can be induced to fl ower with a sodium
chlorate treatment.
Litchi and Longan 225
yields, as poor fruit set occurs at 33°C. In hot equatorial areas, litchi grows
vegetatively and seldom fl owers.
Longan is less demanding than litchi for fl owering, requiring 15–22°C
for 2–3 months for fl ower induction (Fig. 9.1). The exact chilling and cultivar
di erences in this requirement are not known. In hot equatorial areas, longan
grows vegetatively and seldom fl owers. Flowers can be induced by a chemical
treatment discussed below.
Light intensity and photoperiod
Litchi is a day-neutral plant, and fl oral initiation is associated with starch
content of leaves and stems. Shading, such as when litchi trees begin to crowd
each other in an orchard, leads to a decline in production, due to smaller
panicles and a decrease in the number of panicles on a tree (Lin et al., 1991).
Shading to 50% of full sunlight causes a 40% reduction in the panicles formed
and a 65% reduction in panicle length. There is little information on the
response of longan to varying light levels; it is thought to be day neutral.
GENERAL CHARACTERISTICS
Tree
The litchi is a spreading evergreen tree, growing to 10–12 m under favourable
conditions. It is generally considered to be a slow grower with a long life. A
Chinese writer, Wen Hsun Chen, said that at the time of his writing (before
1924), the cultivar ‘Chenzi’ (Chen Family Purple, ‘Brewster’) was already
300 years old and still thriving (Gro , 1921). Depending upon cultivar, trees
may be broad with low-hanging branches or have upright branches and a
compact, rounded head. Branches are brittle and even large limbs are easily
broken by winds.
Table 9.1. Effect of temperature and cultivar on percentage of litchi terminal
branches fl owering. Trees at 25/20°C and 30/25°C did not fl ower (Menzel and
Simpson, 1988). Panicles can be of two types; one has only fl owers, while the other
has a mixture of leaves and fl owers, and is referred to as a leafy panicle.
Cultivar
Vegetative shoot
Branches fl owering %
Leafy panicles Leafl ess panicles Flowering panicles
15/10°C 20/15°C 15/10°C 20/15°C 15/10°C 20/15°C 15/10°C 20/15°C
Da zao 0 50 93 50 7 0 100 50
Bengal 0 49 71 32 29 9 100 41
Shui dong 0 58 39 3 61 28 100 31
Gui wei Pink 0 20 55 69 46 8 100 77
Gui wei Red 0 80 7 3 93 5 100 8
Salathiel 0 17 0 20 100 58 100 78
Huai zhi 0 4 0 14 100 81 100 95
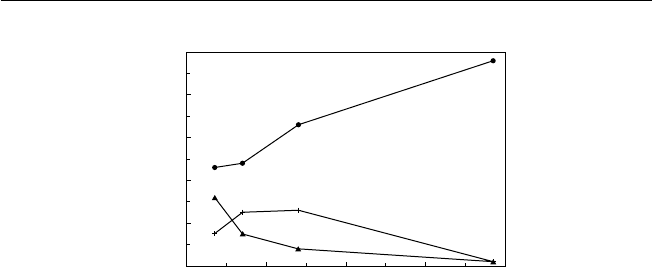
226 Chapter 9
Leaves are arranged alternately, pinnately compound with two to fi ve
pairs of leafl ets arranged in opposite positions or slightly obliquely along the
rachis (Fig. 9.3). The leafl ets are elliptical to lanceolate, 2.5–6.4 cm wide,
7.6–17 cm long, and deep green. Young, emerging fl ushes range from pale
green to pinkish to a copperish red. Growth fl ushes can occur several times a
year and are vegetative fl ushes of several leaves in summer and can be fl oral
in winter. Vegetative fl ushing is encouraged immediately after harvest in
summer by fertilizing and irrigating (Fig. 9.1). The fl oral fl ush is a terminal
infl orescence. A close relationship exists between number of leaves associated
with a fl owering panicle and number of fruits on that panicle (Fig. 9.4). Hence,
a minimum number of two leaves per fruit is required.
The longan tree can grow to 20 m and has either a spreading or erect
habit. The compound longan leaves are around 25 cm long with 6–12 leafl ets,
alternate or nearly opposite, oblong, 5–15 cm long with blunt or pointed
ends (Fig. 9.5). The leaves are dark glossy green on the upper side and pale
green underneath.
Flowers
The small litchi fl owers are 3.0–6.0 mm in length when fully expanded (Fig.
9.3), and borne in profusion on leafl ess or leafy, well-branched terminal
panicles. In the northern hemisphere, appearance of panicles can begin
as early as November to as late as April, depending upon cultivar and
environmental conditions. Flowers are yellow-green or brownish-yellow,
apetalous with small valvate sepals (Fig. 9.3), a fl eshy disc and up to eight
stamens. Among the numerous, apetalous fl owers on the panicle, three sex
types are described for litchi. These sex types are designated Types I, II and III,
0
0
20
40
Percentage
60
80
100
40
Leafy panicles
Leafless panicles
Vegetative shoots
Days at 15/10°C after panicle emergence
80 120 160
Fig. 9.2. Effect of post-panicle emergence temperature response to 15/10°C before
transfer to 30/25°C on ‘Gui wei’ litchi panicle development (after Menzel and
Simpson, 1991).
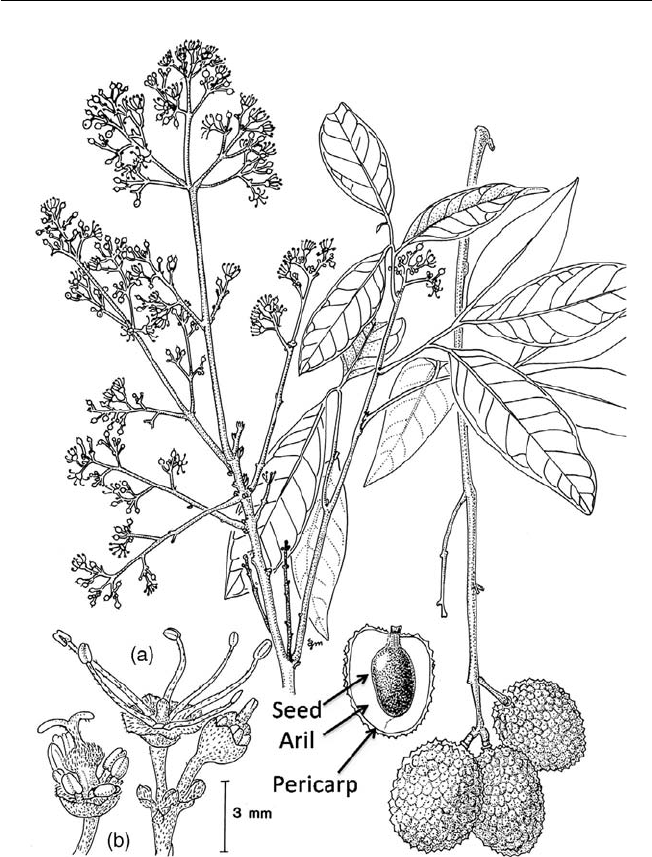
Litchi and Longan 227
based on the chronological order of development. Type I is morphologically
and functionally a male (Fig. 9.3), possessing six to eight stamens producing
an abundance of pollen and lacking ovule di erentiation. Type II fl ower is
morphologically a hermaphrodite functioning mostly as female, with a well-
Fig. 9.3. Leaf, fl ower and fruit of Litchi chinensis. (a) male fl ower with reduced pistil
and six stamens, and (b) ‘hermaphrodite’ fl ower, which functions as a female with a
well-developed pistil (two carpels and a bilobed stigma and six to eight stamens that
do not dehisce (sterile)). From Nakasone and Paull (1998).
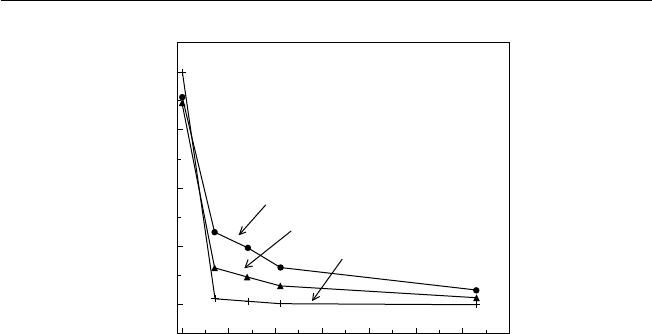
228 Chapter 9
developed two-carpel pistil and a two-lobed stigma (Fig. 9.3). The fi ve to
eight stamens do not dehisce normally and have little viable pollen. Type III
fl owers function as a male, though they are morphologically a hermaphrodite
with a rudimentary pistil lacking style and stigma. The six to eight stamens
produce numerous viable pollen grains. Approximately two-thirds of the
fl owers on a panicle are composed of Type III and only about 20% of the
fl owers are potentially fruit-producing (Type II). Observations indicate that
the ratio of functional females (Type II) to functionally staminate fl owers
(Types I and III) varies with cultivar (Table 9.2), with 10–30% being female
(Limangkura, 1966). Similar sex types occur in longan (Lian and Chen,
1965), of small, greenish-yellow to brownish, self-incompatible fl owers
having fi ve sepals and fi ve petals (Fig. 9.5). Insects are the major pollinators
(Alexander et al., 1982).
Litchi fl oral anthesis occurs in cycles of sex types (Mustard, 1954), with a
period of about 10 days during which only functional male fl owers are open.
This is followed by a transitional period of 2–3 days when both staminate
and female (Type II) fl owers open, then 2 days of only female fl owers. This is
followed by 2–3 days of transitional types, and fi nally male fl owers for a period
of 7–10 days. The number of days to complete each of the cycles appears
to di er somewhat among infl orescences and between cultivars and with
environmental conditions. Infl orescences produced around the same time
generally produce the same sex types. It has been observed that the initial
fl owers are all functionally male in one season, and in the following season
initial fl owers at one site may be all functionally female and at another site
all functionally male. Thus, the infl orescence may begin fl oral anthesis at any
sexual phase of the cycle, going through the above sequence. The expression
0
0
20
40
100 leaves per panicle
50 leaves per panicle
2 leaves per panicle
60
80
10 20 30
Days from full bloom
Fruits per panicle
40 50 60 70
Fig. 9.4. Effect of leaf number on the litchi panicle branch to number of fruit set per
panicle for cultivar ‘H-1224’ (after Yuan and Huang, 1988).
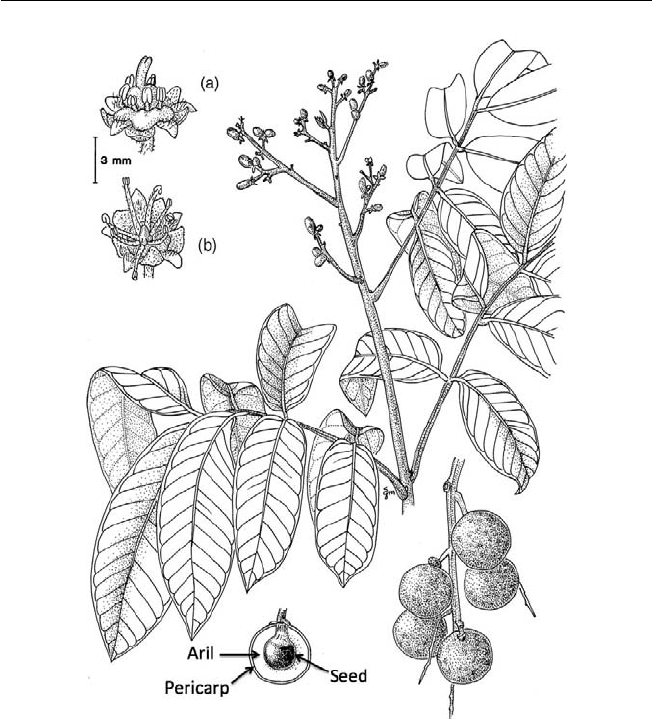
Litchi and Longan 229
of sex type may be related to temperature, with lower temperatures leading to
a higher percentage of Type II fl owers.
Longan fl owers are small, greenish-yellow to brownish, and self-
compatible with fi ve sepals and fi ve petals. Longan has the same three di erent
fl ower types as in litchi: staminate fl ower (Type I), hermaphrodite fl ower
functionally as ‘female’ (Type II) and hermaphrodite fl ower functionally as
‘male.’ Longan shows a similar progression of fl ower types under normal
conditions: fi rst functionally male, then hermaphrodite and fi nally male.
However, the Type II may bloom fi rst under some cultural management
Fig. 9.5. Leaf, fl ower and fruit of Dimocarpus longan. (a) female fl ower with two
carpels and a bilobed stigma and stamens on short fi laments, which do not dehisce,
and (b) male fl ower showing eight hairy stamens with bilobed anthers (from
Nakasone and Paull 1998).
230 Chapter 9
practices or di erent climatic conditions. An overlap of the types occurs on
one tree since not all panicles open at the same time.
Pollination and fruit set
Natural pollination
Controlled pollination studies have shown the presence of a relatively high
degree of self-sterility in litchi. Limangkura (1966) self-pollinated litchi fl owers
and produced 4.2% set, while cross-pollinated fl owers yielded 6.9% set. Litchi
infl orescences caged to prevent insect visitation had 0.026–0.105% fruit set as
compared to 0.17–11.25% fruit set on infl orescences open to insect visitation
(Pandey and Yadava, 1970). Bees (Apis and Mellifera spp.) make up 98–99%
of the total insect visitors to the fl owers, mostly during the morning hours,
when nectar secretion occurs. The stigmas of litchi fl owers are receptive to
pollination for 72 h from the time it divides into the two lobes, and the anthers
remain functional up to 1–3 days after anthesis.
Controlled pollination studies in longan have shown the presence of a
relatively high degree of self-incompatibility. Bees (Apis and Mellifera spp.) are
the major pollinators and comprised 98–99% of the total insect visitors to
the fl owers. The visits occur mostly during the morning hours, when nectar
secretion occurs. Too much rain during anthesis can reduce fl ower opening
and the insect activity needed for pollination. Irrigation after a period of
soil water stress is reported to aid in fl owering, even though the tree fl owers
profusely in areas with high water tables.
Floral induction, fruit set and chemicals
Growth regulators and other horticultural techniques have been tried by many
researchers to overcome the problem of irregular litchi fl owering, to enhance
fruit set and to reduce seed size (Menzel, 1983; Joubert, 1986). Sodium
naphthalene acetate (SNA) at 200 and 400 ppm promoted blossoming in
‘Chenzi’ only when climatic conditions favoured vegetative growth. The
inhibition of vegetative growth is a requisite for fl oral initiation, and SNA
mimics low temperatures that inhibit growth. There is no increase in fl oral
Table 9.2. Distribution of the three sex types of fl owers per panicle of two litchi
cultivars (Limangkura, 1966).
Cultivar
Flowers per
panicle
Functional fl ower type (%)
Potential fruiting
fl owers (%)I II III
Groff 964 14 20 66 20
Hei ye 1042 22 19 59 19

Litchi and Longan 231
initiation and yields by SNA treatment during years with dry autumn months
and on trees that yielded heavily during the previous season.
A combination of ethephon and kinetin is more e ective in reducing
litchi shoot length and inducing high proportions of fl oral buds than either
chemical alone (Chen and Ku, 1988), while unsprayed shoots remain
vegetative. Killing winter fl ushes by the use of chemicals to promote litchi
fl owering is also practised. A 25% urea solution, ethephon (1500 mg/l),
maleic hydrazide (0.125%) and DNOC (dinitro-orthocresol) at 0.1–0.5% forces
the young winter leaf fl ush to abscise, with subsequent emergence of axillary
fl owering. A 0.5% urea spray applied to the late-summer growth fl ush causes
the soft leaves to turn green, mature quickly and produce fl owers in the spring.
The higher the concentration of ethephon applied at the time when trees
approached fl owering, the lower the percentage of fl owers produced.
Girdling (cincturing) branches or the trunk in late summer increases
fl owering and fruiting (40–800%) in litchi trees that would have fl owered
poorly in the spring. If no pronounced growth fl ush has occurred in the 6
months before the fl oral initiation period, or the trees are in poor condition
or have had a late-autumn vegetative fl ush, they have increased fl owering
(Menzel and Simpson, 1987). Trees to be girdled should be fertilized
immediately after harvest and irrigated to induce fl ushing and then girdled in
late summer when the leaves have become fully matured (Fig. 9.1). Girdling
of limbs and trunks of trees that start fl ushing in late summer has no signifi -
cant e ect on trees that would have fl owered profusely on their own. Early
recovery from girdling is associated with vegetative fl ushing during winter
(Menzel and Paxton, 1986b).
Retention of litchi fruit after setting is a universal problem. Fruit
development is punctuated by four phases of abscission (Fig. 9.6), the fi rst
occurring within 15 days of anthesis, in which more than 92% of pollinated
fl owers drop due to endosperm degeneration and subsequent embryo abortion.
The second period of abscission is possibly due to degeneration of the embryo
sacs. Phase IV does not occur in fruits that have aborted seeds. There are many
causes of fruit drop, including unfavourable climatic conditions and nutrient
defi ciencies. Spraying newly set fruit twice at 15-day intervals with 35–100 ppm
of 2,4,5-T and NAA progressively reduces fruit drop, improves fruit size and
even hastens ripening (Prasad and Jauhari, 1963). Lower concentrations also
reduce litchi fruit splitting, another serious problem in many areas. Growth
regulators (GA 100 ppm, NAA 20 ppm, 2,4,5-T 10 ppm, chlormequate 250
ppm) sprayed on litchi cv. ‘Rose Scented’ at the pea stage had reduced fruit
drop (Khan et al., 1976). Spraying ‘Early Seedless’ and ‘Calcuttia’ with 20
ppm IAA enhances fruit set; 50 ppm GA-3 increases fruit retention and 100
ppm GA-3 improves fruit weight, with a combination of IAA and GA-3 being
suggested (Singh and Lal, 1980).
Reduction of seed size or the production of seedless fruit has also been an
objective in the use of growth regulators. Kadman and Gazit (1970), using
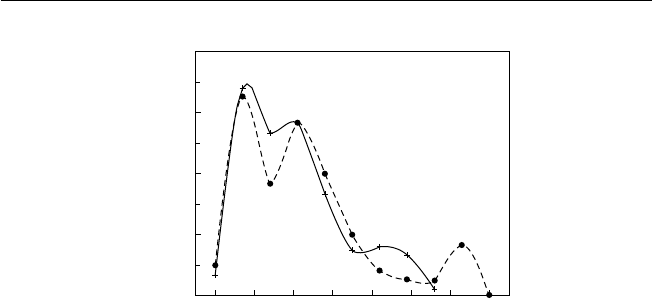
232 Chapter 9
2,4,5-TP (trichlorophenoxypropionic acid), prevented fruit drop to a higher
degree and resulted in more than 75% of the litchi fruit having small seeds.
However, if the infl orescence is fi rst treated with 2,4,5-TP and then sprayed
with a combination of 2,4,5-TP and gibberellic acid, fruits are 50–100%
larger than those treated only once, and 90–100% of the fruits are seedless.
The development of forced fl ower initiation in longan by the use of
potassium chlorate (Fig. 9.7) has extended the production area and also
assured more regular yielding (Yen et al., 2001). However, signifi cant
variations in fl owering application methods have been reported. The variation
is due to variety, developmental status of plants at forcing, soil conditions
and climate (Yen et al., 2005). Potassium chlorate is applied as a soil drench
(200–1000g per tree), either as the salt or in solution is more e ective than
as a leaf spray (1–2%), injection (1–5%) or trunk girdling (300–600 g).
Treatment with chlorate leads to fl ower induction and the appearance of
fl ower buds in about 40 days, though it can induce leaf chlorosis and drop.
Common bleach decomposes, especially at high temperatures to chlorate and
a level of ~4 g/l has been detected (Matsumoto et al., 2007). Trees treated with
7.6 l of household bleach (~30 g chlorate) were induced to fl ower (Fig. 9.7),
and bleach was more e ective than 250 g of chlorate. The greater e ectiveness
of bleach suggests potentially rapid decomposition in the soil to chlorate. The
e ectiveness of bleach supports an earlier observation that water released
from soybean shoot factories induced longan to fl ower. Bleach is used to
sterilize the seeds before germination and to wash down the equipment.
The rapid induction of fl owers by chlorate means that a longan grower
can take orders for fruit to be delivered 6 months later under good conditions.
0
0102030
Days after full bloom
Relative abscission rate (%/day)
40 50 60 70
3
6
9
Huai Zhi
I II III IV
H-1224
12
Fig. 9.6. Litchi fruit abscission rate, taken as a mean over 2 years, occurs in at least
four stages (I–IV). Normal-seeded ‘Huai zhi’ (+) harvested 75–80 days after full
bloom and aborted-seeded ‘H-1224’ (·) harvested at 70–75 days after full bloom
(after Yuan and Huang, 1988).
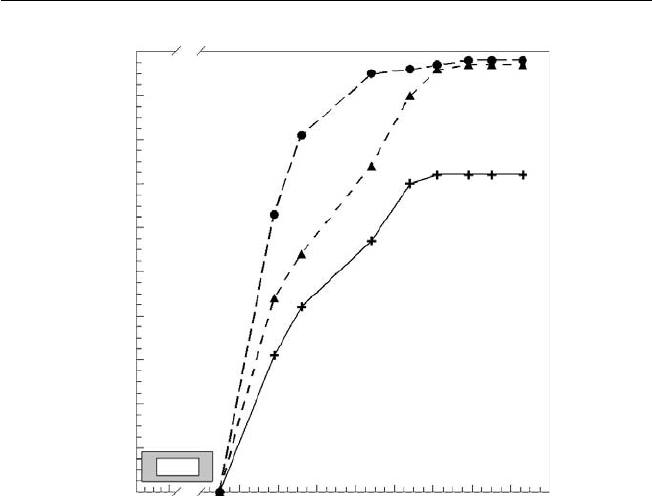
Litchi and Longan 233
Another advantage of this treatment is that blocks of trees can be forced to
fl ower sequentially to produce fruit almost year-round. Fruit quality is more
a ected by cultural management rather than potassium chlorate fl ower
induction. The application of potassium chlorate has changed the pruning
methods. Heavy pruning after harvest is now used to control tree size, followed
by potassium chlorate application.
Fruit
Litchi fruit takes from 80 to 112 days to reach maturity from anthesis,
depending upon the cultivar and environment (Fig. 9.8). Litchi aril growth
begins about 25 days after anthesis and the aril growth then parallels total
fruit growth. The fruits show a dramatic increase in total sugars from about
3 to 16–20% during the rapid growth period of the aril. Photosynthesis in the
leaves behind the fruit cluster is more important than photosynthesis in older,
shaded leaves or stored starch in the stem (Hieke et al., 2002). The tart fl avour
0
0
10
20
30
40
50
60
70
80
90
100
5
No
flowers
30 40 50 60
50 g
KCIO
3
250 g
KCIO
3
Bleach
Days from treatment
Flowering terminals (%)
70 80 90 100 110 120
Fig. 9.7. Induction of longan fl owering with potassium chlorate and bleach
(redrawn from Matsumoto et al., 2007). No fl owers were observed for the fi rst 35
days.
234 Chapter 9
of immature litchi fruit is due to high organic acids. The increase in Brix/acid
ratio as fruits mature is due to the decrease in organic acids and increase in
sugars (Paull et al., 1984), contributing signifi cantly to the litchi fl avour. Peel
anthocyanins increase and chlorophyll decreases during this same late growth
phase. Cultivar di erences do not allow peel colour to be an accurate measure
of maturity. Late-season cultivars show a longer initial growth phase than the
early and mid-season cultivars.
The size and shape of the litchi fruit are characteristic for di erent
cultivars. ‘Chenzi’ has fruit averaging 23 g; other cultivars’ fruits can weigh
more than 30 g. The ratio of edible aril to seed is more important than large
fruit size. Most cultivars have medium to large seeds, occasionally producing
0
0
2
4
6
Mass (g)
8
10
12
20
(a)
40
Days from full bloom
60 80
Seed
Skin
Aril
Total
100
0
0
2
4
6
Mass (g)
8
10
20
(b)
40
Days from full bloom
60 80
Pericarp
Seed
Aril
Total
100 120
Fig. 9.8. The fruit growth pattern of (a) litchi cultivar ‘Groff’ fruit (after Paull et al.,
1984) and (b) longan cultivar ‘Dong bi’ fruit (after Ke, 1990).

Litchi and Longan 235
fruit with small, shrivelled seeds. The Hawaiian cultivar ‘Gro ’ has perhaps
the smallest-sized fruit (approximately 12.5 g), but produces small, shrivelled
seeds, ‘chicken tongue’, in about 95% of the fruit, greatly increasing the
edible portion to 50–70%. In cultivars with shrivelled seeds, little embryo
development occurs, and the seed growth that does occur stops 30–40 days
from anthesis.
Usually only one of the two locules in the litchi and longan ovary develops
to form a normal fruit. The other aborts and remains at the base of the normal
fruit. Occasionally, both carpels develop equally to form two normal fruit.
The aril tissue in litchi and longan develops from just above the degenerating
obturator and is formed by an outgrowth of the funiculus (Huang et al., 1983).
Aril growth parallels embryo development, though embryo development is not
required for litchi aril growth. If embryo development is arrested during the
liquid endosperm stage and when little embryo growth has occurred, the fi nal
fruit is fi lled with aril and a small aborted seed, called ‘chicken tongue’ (Huang
and Qiu, 1987). When no endosperm development takes place, no embryo or
aril development occurs and you get a ‘hollow’ fruit. Both of these conditions
relate to fertilization disorders. Aril development is not uniform and thus
overlaps at the chalazal end of the seed. The aril is therefore free from the seed
in both litchi and longan.
Longan fruit are brownish, globose to ovoid, 1.5–2.0 cm in diameter; the
skin is thin without protuberances and dull green when developing; the aril
is white, translucent and sweet, with an aromatic spiciness. The fruits show
a single sigmoid growth curve, with the major portion of seed development
occurring between 50 and 70 days from anthesis, reaching maturity in 100
days. During early development the seed is the major sink in longan fruit, and
aril growth occurs only after the seed has attained full size and maximum
weight, about 50 days after anthesis (Fig. 9.8b). Growth of aril accounts for
most of the increase of fruit weight during late development and reaches
60–70% of the fruit fresh weight. Growth periods were signifi cantly di erent
among longan varieties (from 140 to 200 days) and are not correlated to fi nal
fruit size.
Sugar accumulation in the longan aril begins after the main period of
seed development, 70–100 days post-anthesis (Ke, 1990). The fruits can
reach 25% total soluble solids and are ready for harvest when the total soluble
solids reach at least 17–20% and suitable fruit size (above 8–10 g). Sugar
accumulation in the aril begins after the main period of seed development,
70–100 days post-anthesis. The fruits show a dramatic increase in total sugars,
from about 3 to 16–20% during the rapid growth period. The phenomenon is
called ‘desweeting’ in China and Taiwan. The rate of desweeting varies with
cultivar. Fruits of slow-desweeting types can be retained on the tree longer,
and are better for adjustment of harvest time. The soluble solids of the cultivar
‘October’ in Taiwan can be maintained above 20% for 50 days.

236 Chapter 9
CULTIVAR DEVELOPMENT
Cytogenetics and genetics
There are suggestions that the litchi may have been derived from more than
one wild progenitor. Haploid chromosome numbers of 14, 15, 16 and, rarely,
17 have been reported, with diploid numbers of 28, 30 or 32. The diploid
number for longan is 2n = 30. It is possible to generate intergeneric hybrids
using litchi as the female parent with longan. The hybrid plants are similar to
the maternal parent with smaller leaves.
Breeding and selection
Like most single-seeded fruit, the litchi and longan do not lend themselves to
breeding by a controlled pollination system between two selected parents
(Zee et al., 1998). The highly heterozygous nature of the species means that
planting open-pollinated seeds derived from within a cultivar collection is
the usual procedure used in new cultivar development. For example, the
Hawaii litchi cultivar ‘Kaimana’ is a selected seedling of open-pollinated seeds
of ‘Hei Ye’.
Litchi genetic diversity is indicated by the large number of cultivars in
China and India, and these provide the basis for the development of new
cultivars. Singh and Singh (1954) reported that the 33 or so cultivars grown
in India are all selected from seedling populations derived from introduced
Chinese cultivars.
It is di cult to identify longan cultivars by skin colour alone. Useful
criteria for identifi cation include the retention of total soluble solids after
harvest, crispness and smoothness of the fl esh, and taste (Table 9.3).
Selection and evaluation
Galán Saúco (1989) has presented a detailed list of characteristics for a litchi
cultivar:
• Fruit: large, with small or shrivelled seed and a high proportion of edible
aril; bright red skin colour; long shelf-life and ability to retain skin colour
under storage conditions; fi rm fl esh with acceptable sugar/acid ratio; and
resistance to diseases.
• Tree: vigorous; precocious; regular and high yielding; resistant to water
stress, wind, soil salinity, diseases and insects; and adapted to regular
fl owering under warmer temperatures in the tropics.

Litchi and Longan 237
Table 9.3. Fruit characteristics of selected litchi and longan cultivars.
Cropping regularity
Cultivar Synonyms Season Vigour
Fruit
quality
Fruit
wt (g)
Edible
% TSS
Flesh
colour
Skin
colour
Seed
size Tropics Subtropics
Litchi
Da zao Tai So early vigorous fair 24 72–77 15–18 milky white bright
red
large regular irregular
Hei ye Hak Ip,
Blackleaf,
Groff
mid moderate good 19 78 16–20 milky white dull red large irregular irregular
Huai zhi Wai chee late dwarf good 21 72 15–18 milky white dull red large irregular regular
Gui wei Kwai May (Mi),
Bosworth
mid vigorous very good 17 70–83 18–21 white light red mostly
small
irregular irregular
No mi ci No Mai Chee mid to late vigorous excellent 25 82–86 18–21 milky white bright
red
small irregular irregular
San yue hong Sum Yee
Hong
early medium fair 40 62–68 15–20 milky white bright
red
large
Chen zi Chen Family
Purple,
Brewster
early vigorous good 20 54–58 15–18 waxy white deep red large
medium
irregular irregular
Longan
Kohala mid spicy 15 regular
Sao-an-liao mid 15 62 13 dark
brown
small
Duan ru mid 13 14
Yang tao ye Carambola
leaf
mid 16 11
Fen ke mid crispy 11 66 20 white dark
yellow
medium
Continued

238 Chapter 9
Table 9.3. Continued.
Cropping regularity
Cultivar Synonyms Season Vigour
Fruit
quality
Fruit
wt (g)
Edible
% TSS
Flesh
colour
Skin
colour
Seed
size Tropics Subtropics
Daw good regular irregular
Chompoo excellent regular irregular
Biew Kiew excellent irregular
Fuyan Lucky eye late vigorous good 12 67 15 transparent
crispy
dark
brown
small regular
Wuyan Black round mid vigorous good 17 71 14 white light
brown
large regular
Shi xia Shek Yip, Shi
Yuan
mid vigorous excellent 10 69 19 white crispy dark
brown
small regular

Litchi and Longan 239
Sometimes a selection is elevated to a cultivar status by satisfying only a few
of the above characteristics (Table 9.3). Such a selection is ‘Gro ’, by virtue
of consistent bearing over a 7-year period, producing fl owers usually several
weeks later than other Chinese cultivars grown in Hawaii and producing
aborted seeds in about 95% of the fruit. Average fruit size is 12.5 g, smaller
than other Chinese cultivars grown in Hawaii, but there is approximately 20%
more edible aril for the same weight of fruit (Storey et al., 1953).
Breeding for adaptation of cultivars to the higher temperature conditions
in the warm tropics is an important criterion (Menzel, 1983). Menzel and
Paxton (1986a), using glasshouses at high temperatures (30°C day/25°C
night) to evaluate litchi seedlings for low vigour, found this to be a useful
technique for initial screening prior to fi eld evaluation of adapted genotypes
that would fl ower and fruit under warm conditions. The rate of fl ushing in
the glasshouse at high temperatures agreed with the relative vigour and
consistency of fl owering of the parent cultivars in the fi eld.
Similar criteria have been developed for longan (Table 9.3). Longan fruit
criteria include retention of total soluble solids after harvest, crisp and smooth
fl esh, good taste, uniform size and high aril content.
Cultivars
According to Gro (1921), Chinese nurserymen recognized the di culties in
perpetuating the desirable characteristics of highly regarded cultivars under
conditions other than those in which the fruit originated. The environment
profoundly infl uences cultivar characteristics, and this may explain the large
number of cultivars, with 74 cultivars mentioned in Wu Ying Kuei’s list of very
ancient origin. Among the many known cultivars in China, the cultivars ‘San
yue hong’ (‘Sum Yee Hong’), ‘Shui dong’ (‘Souey Tung’), ‘Fe zi xiao’ (‘Fay Zee
Siu’), ‘Hei ye’ (‘Haak Yip’), ‘Gui wei’ (‘Kwai May’), ‘No mi ci’ (‘No Mai Chee’)
and ‘Huai zhi’ (‘Wai Chee’) in Mandarin, with the Cantonese in brackets,
are most widely grown (Table 9.4). Sixty-seven cultivars and selections are
described for Guangdong, with 26 of these being major commercial cultivars
(Anon., 1985). ‘Brewster’ (‘Chen Zi’, ‘Chen Family Purple’) trees are vigorous,
large and one of the faster-growing cultivars. ‘Hei Ye’ has been observed to be
a slow grower, low by comparison to cultivars such as ‘Chen Zi’ and ‘Gui Wei’
of the same age but becomes spreading.
Cultivars in Thailand are ‘Da zao’, ‘Hei ye’, ‘Huai zhi’ and ‘Hong huey’
(syn. ‘Mau mong’) (Table 9.4). Other cultivars are ‘Kim cheng’, ‘Hong thai’,
‘Ohia’ (syn. ‘Ouw’, ‘Hei ye’, ‘Baidum’), ‘Gui wei’, ‘Gro ’, ‘No mi ci’, ‘Chen zi’
and ‘Mauritius’. In Australia, the cultivars most often planted are ‘Da zao’,
‘Bengal’, ‘Hei ye’, ‘Huai zhi’ and ‘No mi ci’. In the USA, Florida cultivates
primarily ‘Brewster’, ‘Mauritius’ and ‘Sweet Cli ’. The ‘Mauritius’ in Hawaii
is di erent from the Florida ‘Mauritius’. In Hawaii, most of the plantings

240 Chapter 9
consist of ‘Da zao’, ‘Gro ’, ‘Hei ye’ and ‘Brewster’. In subtropical South Africa,
particularly in the Transvaal Lowveld, the Soutpansberg and Natal Coast
areas, ‘Mauritius’ is the principal commercial cultivar and is a synonym of
‘Gui Wei’. The genetic diversity has led to many synonyms and confusion,
along with mislabelling of di erent cultivars, with isozyme analysis and
molecular markers now beginning to separate the di erent lines (Aradhya et
al., 1995; Viruel and Hormaza, 2004).
Table 9.4. Major litchi and longan cultivars grown in different countries (after
Menzel and Simpson, 1990); name in national Chinese pronunciation unless
otherwise indicated (*).
Country
Main growing
area Major litchi cultivars Major longan cultivars
China Guangdong Huai zhi, Hei ye, Sun
yue hong, Gui wei,
No mi ci
Shixia, Chuliang
Guangxi Huaizhi, Heiye, Dazao,
Sanyuehong, Guiwei,
Nomici
Fujian Shui dong, Hei ye, Da
zao, Chen zi, Fu yan,
Wu yuan, Shi xia
Fuyan, Wulongling,
Chike
Taiwan Taichung Heiye, Nomici,
Yuhebao, Huaizhi,
Sakengzong,
Sanyuehong
Chienliou*, Yangtaoye,
Saoandiao, Duanru,
Fenke
Thailand Chiangmai,
Lamphum,
Fang
Da zao, Huai zhi,
Hei ye
E-Daw*, Chompoo*
(Seechompoo), Haew*,
Biew kiew*, Dang*,
Baidom*
India Bihar State Shahi*, Rose Scented*,
China*
Madagascar Wet coastal belt Da zao
South Africa Transvaal –
Lowveld
Da zao
Reunion Da zao
Mauritius Da zao
Australia Eastern coastal
strip
Da zao, Bengal*,
Huaizhi, Gui wei,
Salathiel*
USA Hawaii Da zao, Kaimana*, Hei
ye
Kohala*, Ilao*, Wai*,
Sweeney*
Florida Brewster* (Chen zi) Kohala*, Chompoo*,
Homestead #1* & #2*

Litchi and Longan 241
Although more than 400 longan cultivars have been developed in
China, less than 10 are economically important (Chian et al., 1996; Liu and
Ma, 2001). More than 200 clonal cultivars are maintained at the National
Longan Germplasm Repository at the Fruit Research Institute, Fujian.
More than 50 cultivars have been identifi ed in Taiwan, though less than 5
cultivars are widely cultivated (Yen and Chang, 1991). In Thailand, ‘E-Daw’
accounts for more than 90% of longan production (Subhadrabandhu and
Yapwattanaphun, 2001). There are about 20 cultivars of longan in Australia,
mostly introduced from China, Thailand and the USA. A longan clone
‘Kohala’ has been released in Hawaii and displays prolifi c vegetative growth
and produces heavy crops in Hawaii as well at Homestead, Florida. There are
numerous longans, with a wide range of characteristics (Table 9.3). Molecular
markers are being developed to identify longan cultivars.
CULTURAL PRACTICES
Propagation
Sexual
Seed propagation is used to produce litchi seedlings from selected cultivars for
breeding and selection studies and for production of rootstocks for grafting.
Litchi seeds remain viable in the fruit for about a month after harvest with a
moisture content of 45%; viability is soon lost within 4–5 days once removed
from the fruit and the moisture content falls below 27%. Seeds packed in moist
sphagnum moss may be shipped, though seeds frequently germinate in the
packing within a week. Germination and seedling development is improved
by the use of the large seeds and mycorrhizal soil for germination. Seeds
germinate within 4–10 days in soil, sand, vermiculite, perlite, peat, wood
shavings or mixtures of these materials, with adequate moisture and aeration.
Seedlings are not used for new planting as they have a long juvenile phase of
up to 10 years.
Longan seeds are also recalcitrant and quickly lose their viability after
removal from the fruit. Similar to litchi, longan seedlings have a long juvenile
period of greater than 8 years.
Asexual
Litchi and longan are usually propagated by air-layering, the method used by
Chinese propagators for centuries. Moist peat or sphagnum moss is wrapped
around the girdled area of a branch and held in place with plastic sheets
cut to about 25–30 cm
2
, with the ends fastened with string or some taping
material, such as plastic electrician’s tape. Su cient roots are formed in 8–10
weeks, and the success rate is nearly 100%. There is no need to apply growth

242 Chapter 9
regulators to enhance rooting, though some recommend a lanolin paste with
250 ppm IBA, 100 ppm 2,4,5-T, and IAA or IBA at 500 ppm.
Cuttings with two to three leaves from girdled and ungirdled branches of
‘Brewster’, ‘Gui Wei’ and ‘Hei Ye’, treated with the sodium salt of NAA, IBA
and their mixtures and misted or not in a polyethylene enclosure give poor
results. Others have obtained 93% rooting and 100% survival by using green,
woody cuttings dipped in 5000 ppm IBA and rooted under intermittent mist.
The variable and often low rate of success in litchi grafting is attributed
to stock–scion incompatibility, poor cambial contact, grafting at the wrong
physiological stage and poor management after graftage (Menzel, 1985).
Graft incompatibility is suggested when only 20% of girdled ‘Brewster’ scion
and 10% of ‘Gui wei’ are successful on rootstocks of the same cultivars.
Histological studies of the graft unions between ‘Hei ye’ and the above two
cultivars show a slow rate of proliferation of callus tissues. Currently, there
is generally a lack of knowledge on the graft compatibility between litchi
cultivars. Proper culture of rootstocks and preparation of scion-wood (girdling
for starch accumulation), proper stage of maturity of scion-wood, appropriate
post-graft environment, particularly high humidity, and other factors
contribute to more consistent success in grafting.
Grafting is used for longan, with semi-hard terminals as scions. The scions
are cleft-grafted into a node subtended by a leaf in Australia. This grafting is
done on to 12–18-month-old rootstocks. In China, whip grafting, side grafting
and budding are used. Top-grafting is used to top work large trees.
Field preparation
Deep ripping may be necessary if the soil is compacted. Liming to pH 5.0–5.5
should be carried out and manure incorporated before planting. Litchi
trees tolerate poor drainage but do not grow well in standing water. Surface
drainage should be installed if such conditions exist. If nematodes are
expected to be a problem, fumigation should be carried out. Longans require
well-drained alluvial soils for good development.
Transplanting and spacing
Sun-hardened trees (6–12 months old) can be planted out at any time of year
if there is su cient moisture available and there is no chance of frost. Light
mulching (100 mm) after fertilization is benefi cial for young litchi trees.
Seedlings should start to produce fruit in 6–10 years and asexually propagated
trees in 3–5 years.
Spacings of 5–12 m, depending upon cultivar size, are used. ‘Gui wei’
is a very vigorous tree, ‘Hei ye’ being moderate, while ‘No mi ci’ is a smaller

Litchi and Longan 243
cultivar. In Florida, litchi are planted at 7 m × 4 m, with regular mechanical
pruning to avoid overcrowding (Campbell and Knight, 1987), and in
subtropical areas 6 m × 6 m spacing is possible (Galán Saúco, 1990).
Windbreaks are necessary for young and mature trees for both crops. This
is essential because trees propagated by air-layering do not have a tap root and
are easily uprooted by winds.
Irrigation
Regular irrigation is essential during the growth of young trees. During dry
periods irrigation may be needed at 2- or 3-week intervals. Moisture control
is applied when the trees reach fl owering age. In China, litchis are grown
along canals, on dykes around paddy fi elds and around village ponds, and
no irrigation is practised. Moisture should be withheld during autumn and
winter to discourage vegetative growth and promote fl owering (Fig. 9.1).
High soil moisture tension for 6 months, beginning mid-summer, or even for
3 months, beginning in late summer, promotes fl oral initiation and fruit set
by inhibiting emergence of vegetative fl ushes (Nakata and Suehisa, 1969).
Once the infl orescence has appeared and throughout fruit development,
adequate moisture is necessary. Irrigation should be terminated a few weeks
before harvesting. Moisture stress under non-irrigated situations can cause
developing fruit to ripen prematurely, even before the aril has fully developed.
Fruit growth will resume if water is provided, leading to fruit splitting. Copious
irrigation immediately after harvest is desirable to encourage new shoot
growth (Fig. 9.1). In areas with dry summers, irrigation is necessary, followed
by limb girdling in late summer to discourage further vegetative fl ushing and
to accumulate carbohydrates in the trees during the autumn months (Nakata,
1953). This practice is necessary in areas where climatic conditions do not
enforce a natural period of tree dormancy in the winter.
Pruning
Less research has been done on the need for and e ectiveness of pruning
longan trees than litchi. During the fi rst 2–3 years, all side shoots up to
0.8–1.0m should be removed from litchi, as these will form low-hanging
branches, which interfere with cultivation. In bearing litchi trees, even
branches 1.5 m high will bend to the ground when laden with fruit. In
Australia, the main terminals of 3–4-year-old trees are headed back in the
spring (prior to fl ushing) in vigorous cultivars, to create a more compact tree
with many terminals, thus increasing the potential bearing surface (Menzel
et al., 1988a). Approximately 16 cm is pruned from the tip, and from each
pruned branch an average of three new terminals are produced. A pruning

244 Chapter 9
system is practised in Taiwan to maintain small tree size (<2 m), to obtain a
wine-glass shape, to encourage regular fl owering and for ease of management
and harvesting (Yen, 1995). An inverted cone approach is also used: wide at
the bottom and narrow at the top (3–4 m high), with mechanical trimming
to maintain shape. Tree thinning is also practised after harvest, to open the
canopy for light penetration, which is benefi cial for infl orescence formation
(Lin et al., 1991). Light pruning sometimes encourages several weak fl ushes
and results in poor fl owering. Severe heading-back of a tree is harmful to
future infl orescence formation. Little or no pruning is done in India and
yields are said to be heavier than with the pruning practised in Taiwan.
Canopy thinning after harvest is done in India. Panicle thinning or pruning is
sometimes practised. Girdling is practised in October to inhibit new fl ushes; if
too severe the next year’s growth will be reduced (Yen, 1995).
Fertilization
Fertilization practices of commercial litchi (Table 9.5) and longan orchards
di er due to di erences in climate, soil, availability of di erent kinds of
organic and inorganic fertilizers and other factors. The Chinese practice
for litchi on alluvial or red basaltic soils provides 300–400 g of urea, 100 g
superphosphate and 25–50 kg animal manure to large trees just after
harvest, but not later than July. At blossoming, 100 g urea/tree or mixed
NPK is applied. To prevent fruit drop, trees are sprayed with 0.1–0.2% urea;
sometimes 0.2% magnesium sulfate is added.
A light fertilization application should be given with care immediately
after fi eld transplanting, due to the sensitivity of litchi roots to fertilizer burns.
Heavy applications should be delayed a year or after one or two growth
fl ushes. One-year-old trees are given ~30 g urea/plant/month. Also, 30 g of
a high-analysis mixed fertilizer such as 15-4-11 is given every 3 months with
Table 9.5. General guide for litchi fertilization.
Year Fertilizer formation Time of application Amount (g/tree)
First year 14-14-14 At planting time 125
14-14-14 At 4 months 125
14-14-14 At 8 months 125
14-14-14 At 12 months 125
Second year 14-14-14 Once/4 months 250
Third year 14-14-14 Once/3 months 455
Fourth year 14-14-14 Once/3 months 455
Fifth year 10-20-20 Appearance of fl owers
(Feb, Mar)
1000–1500
14-14-14 After harvest (May–June) 1000–1500

Litchi and Longan 245
the urea. Fertilizer in subtropical areas is withheld during cold months to
prevent fl ushing.
In Australia, general recommendations for 5-year-old trees are: 150 g
urea, 300 g single superphosphate and 150–200 g potassium sulfate per
application. These amounts are increased by 20–30% each year until year 15,
when each application rate increases to 1200 g urea, 1200 g superphosphate
and 600–800 g potassium sulfate. No fertilizer is applied to bearing trees in
the spring, to prevent vegetative fl ushing in the autumn, in order to increase
the prospect of good fl owering. Three applications of a complete fertilizer,
such as triple-14 at 114 g per tree the fi rst year and increasing to 227 g per
application in the second year, are used in Hawaii. In the third year, two or
three applications at 454 g per application are applied. For bearing trees a
general rule of thumb of 0.90 kg of a complete fertilizer for every 2.54 cm
of trunk diameter is given upon completion of harvest to encourage summer
fl ush. An application in late winter or early spring before fl oral initiation has
begun can result in a vegetative fl ush rather than a fl ower fl ush. The main
fertilization is applied immediately after harvest in the summer (Fig. 9.1),
followed by irrigation and the annual limb girdling.
Critical nutrient periods are before fl owering and fruit set, several weeks
after fruit set and after harvest. Litchi leaf nitrogen should be ca. 1%. Leaf
samples for analysis are taken twice a year, once at the end of harvest and
the second at fl owering, with only P and K applied during fruit set to avoid
vegetative growth from N application. Minor elements are applied once a year
at the end of the cold period.
Erratic bearing
Litchi trees 7–8 years old can produce up to 45 kg. Potential yields for 10-year-
old trees are 50–70 kg, 15-year-old trees at 100 kg, and 20–24-year-old trees
at 150–180 kg (Campbell and Knight, 1987). Occasionally, yields can be as
high as 500 kg in very old trees. Apparently, potential yields can continue to
increase with older trees, as has been demonstrated in China.
The litchi can be a heavy bearer when conditions are favourable for
fruiting; however, considerable year-to-year variation in yields occurs wherever
grown (Fig. 9.9). Erratic fl owering can be due to unfavourable weather
conditions, and even if fl ower production is consistent, fruit set problems
contribute to variable yields (Chapman, 1984). Cultivars are very particular
about their climactic requirement (Table 9.1); trees propagated from good
bearing trees and planted in another location are known to have produced
fruit for a few years and later ceased to produce for years.
Fruit bearing is dependent upon a number of crucial phases, which need
to be optimized throughout the year (Fig. 9.1). The infl uence of cultivar,
pruning and tree management needs to be integrated. Both species need to be
246 Chapter 9
stimulated to leaf growth after harvest; this is followed by a period of rest, then
fl oral induction and development. These fl ushes are induced by fertilization
and rainfall or irrigation (Yen, 1995). There can be more than one fl ush of
leaves during the 5 months after litchi and longan harvest, with leaves being
forced to mature and growth restricted after this phase. While mature leaves
are essential for fl owering, the presence of young, expanding foliage in the
cool season is inhibitory to infl orescence formation; if removed, fl owering
capacity increases (Lin et al., 1991). In autumn, young, expanding leaves
can be removed mechanically or with a low rate of ethephon (1500 ppm); a
higher rate will abscise more mature leaves. Tree girdling in late summer can
be used to limit new shoot growth. The conditions for fl oral induction, besides
tree condition, are temperature and water stress for litchi and temperature and
possible nitrogen stress for longan. The terminal nature of the infl orescence
inhibits further main axis development, with lateral shoots on branches that
have fruited continuing further shoot growth, with only 22% that produce
fl owers in the following season.
Poor synchronization of fl ushing and fl owering within an orchard
increases management problems. Cultivars vary in their requirements for
induction, leading to early- and late-season bearers.
Pest management
Diseases
A number of disease organisms infecting litchi fruit are listed in the literature,
but none are considered to be serious and all are of a postharvest nature.
Aspergillus spp., Botryodiplodia theobromae, C. gloeosporioides (anthracnose),
Cylindrocarpon tonkinense, Pestalotia sp., Penicillium and Alternaria can cause
1970
0
20
40
60
80
100
1973 1976 1979 1982
Year
Yield (kg / tree)
1985 1988 1991
Fig. 9.9. Change in litchi production at Beilu, Guangxi from 1971. Improved
management practices were used after 1981, which led to increased production and
reduced, but did not eliminate, year-to-year variation in yield per tree (Mo, 1992).

Litchi and Longan 247
some postharvest problems. Anthracnose is a problem in Florida, and the
cultivar ‘Mauritius’ is especially susceptible, the fungus a ecting young fruit
and causing fruit drop. Many of these fruit fungal infections are secondary in
nature, as the organisms enter the fruit only when the fruit skin is damaged.
Puncture sites from fruit fl y oviposition are excellent points of entry.
A serious disease of longan in China and Hong Kong is witches’ broom,
caused by a virus. The virus causes serious deformity of the infl orescence
(Liu and Ma, 2001). It is transmitted by vegetative propagation and is seed-
transmitted.
Nematodes have not been reported as a serious threat to litchi cultivation.
However, tree decline in Natal and Transvaal is associated with two species of
nematode. Roots become ‘stubby’ and brown in colour, and secondary feeder
root development is inhibited.
INSECTS
Insects are of greater concern (Table 9.6) in litchi production than diseases.
The erinose mite (Aceria litchii) is probably the most universal, having been
reported from many widely scattered areas. It attacks the young leafl ets,
producing a thick growth of light brown to reddish-brown, felt-like hair on
the undersurface. The worm-like mite is not visible to the naked eye and is very
specifi c to litchi. Occurrence of this mite coincides with periods of new growth,
regardless of season or locality, and in severe cases new shoots on the entire
Table 9.6. Insect pests of litchi.
Common name Organism Parts affected Region
Erinose mite
Aceria litchii (Eriophyes
litchii)
Foliage universal
Leaf miner
Conopomorpha sinensis
Foliage India and Asia
Rose beetle
Adoratus sinicus
Foliage of
young
plants
Hawaii
Trunk borer
Anoplophora macularia
Trunk Taiwan
Bark borer
Salagena sp.
Bark/wood South Africa
Shot hole borer
Xyleborus fornicatus
Branches India
Conopomorpha sinensis
Fruit China
Litchi moth
Argyroploce peltastica
Fruit South Africa
Macadamia nut borer
Cryptophlebia ombrodelta
Fruit Australia
Litchi stink bug
Tessartoma papillosa
Fruit China
Mediterranean fruit fl y
Ceratitis capitata
Fruit South Africa
Natal fl y
Ceratitis rosa
Fruit South Africa
Oriental fruit fl y
Dacus dorsalis
Fruit Hawaii

248 Chapter 9
tree can be a ected. It is e ectively controlled by sprays of wettable sulfur at
2.3 kg per 378 l of water. It does not infest other relatives in Sapindaceae.
In Taiwan, a trunk borer (Anoplophora macularia) can cause death
of trees within a few months by boring into the trunk and damaging the
conducting tissues. The litchi stink bug can cause serious damage to fruit;
the bug is e ectively controlled by a parasite, Anastatus sp. In Australia, the
macadamia nutborer (Cryptophlebia ombrodelta) is considered a serious fruit-
damaging insect.
Mediterranean fruit fl y (C. capitata) does not attack either species, except
where the fruit skin has been broken by other means and the pulp is exposed.
The Oriental fruit fl y (D. dorsalis) does infest these fruit, causing punctures,
which are often the focus of entry of fungal organisms that cause fermentation
and decomposition. Other fruit fl ies can be serious pests in other countries.
Other pests are birds and fruit bats. In Hawaii, the White-eye (Zosterops
palpebrosus) and the bulbul (Pycnopus cafer) can cause severe damage as fruits
approach maturity. Fruit bats (Pteropus sp.) or fl ying foxes have caused severe
losses in South Africa and Australia.
Weed control
Weed control is most important from the time of fi eld transplanting up to 3
or 4 years old. As trees grow and expand horizontally, there is a decreasing
amount of weed growth underneath the canopy, due to shading. Use of
polyethylene mulch about a metre square around the plant at transplant
time reduces weed growth near the plant. Litchi are reported to be adversely
a ected by organic mulches around the base.
HARVESTING AND POSTHARVEST HANDLING
Harvesting
Fruits are ready for harvest when they attain full red colour. Fruits harvested
before full maturity are acid and do not ripen further or improve in fl avour.
These are non-climacteric fruit and do not respond to ethylene. Litchi and
longan are harvested by cutting or breaking o the entire panicle with the
cluster of fruit. The practice is to remove the panicle together with a variable
portion of last year’s wood in many countries, to enhance the production of a
higher number of terminals that may fl ower next spring (Galán Saúco, 1989).
Panicles without fruit are also cut, as the presence of old panicles is reported
to delay the next fl ush. In small orchards, bamboo or aluminum tree-pruning
poles and tall ladders are used. In large orchards, mechanical ‘cherry picker’
platforms greatly facilitate harvesting. Harvesting fruit clusters on older,

Litchi and Longan 249
large trees is di cult and time-consuming. The trend of the last 20 years is
to maintain tree height at less than 3 m. This height allows all management
practices and harvesting to be carried out from the ground, which is faster and
more e cient.
Harvesting is not done in the afternoon in China, as fruit colour is said to
become dull more rapidly, or on rainy days, as wet fruit is said to break down
rapidly. Panicles of fruit are packed in straw baskets or cartons containing
15–20 kg of fruit. Green leaves are also packed with the fruit, to reduce
damage in transit and assist in providing moisture and preserving fruit colour.
Harvested fruit are never exposed to sun, to prevent rapid drying.
Longan fruit maturity is judged by fruit shape, skin colour and the fl avour
of each cultivar. Most fruit can be picked from a tree with one harvest, unless
multiple fl owerings have occurred. No defi nite harvest index exists for longan,
but growers usually note the changes in skin appearance: mature fruit develop
a smooth and darker skin. Fruits are clipped from the stem, as hand removal
often leads to some of the skin being removed. One-piece fi breboard crates,
either 4.5 kg or 2.25 kg, with a plastic liner, are used, if not already packed in
polystyrene punnets.
Postharvest treatments
The major problem in marketing these two fresh fruits is the rapid skin
browning, which makes them unattractive, although the aril remains edible
(Paull and Chen, 1987). In many Asian countries fruits are sold still attached
to the panicle in bunches, while in western countries individual fruits in
punnets are marketed. Much of the postharvest research has been directed
towards the prevention of pericarp browning in storage and marketing of
fresh fruit. The fi eld skin colour of both litchi and longan can be retained by
a sulfur dioxide acid treatment applied before insect disinfestation. All longan
exported from Thailand are so treated, as are much of the litchi from some
other countries. The sulfur dioxide treatment is not approved in the USA.
Retention of litchi red pericarp colour for longer periods has been
demonstrated by wrapping fruit in PVC fi lm or bagging in polyethylene bags
and placing in storage temperatures of 0–10°C (Paull and Chen, 1987).
Commercial quantities (5.4 kg) of litchi and longan packaged in large
polyethylene bags and twisted closed, or individual fruits in punnets placed
in cardboard cartons and sealed are best stored at 2°C for up to 32 days. At
the retail level, all fruit not on display should be kept under refrigeration,
preferably as close as possible to 0–2°C. If surface transportation is used
instead of air freight, properly packaged fruit should be shipped at 0–2°C. Skin
colour and fl avour of fruit is retained for about a month.
Grade standards are similar for both species and are generally set between
the shipper and buyer. Small, poorly coloured, immature or damaged fruit

250 Chapter 9
are culled. Similar mechanical injury to litchi tubercles leads to a site for skin
browning. Soluble solids should be greater than 15% and acid levels low.
Longan pre-cooling is carried out by room or forced air cooling. Longan
in plastic baskets can be hydrocooled, though hydrocooled longan should not
be treated with sulfur dioxide. Sulfur dioxide fumigation damages hydrocooled
fruit skin, producing brown spots on both the inner and outer skin surface and
greater SO
2
residues remain on the fruit. Sulfur dioxide treatment of fruit to be
sold as fresh is not approved in the USA and restricted in Europe.
The storage recommendation for longan is 4–7°C and 90–95% RH. Fruit
can be held for 2–3 weeks, though the skin losses it yellowish coloration and
becomes brown. At lower temperatures, there is a rapid loss of eating quality.
At storage temperature less than 5°C, fruits develop a slight o -fl avour after
about 1 week. Peel colour of longan stored at 0°C turns dark brown, while
SO
2
-fumigated longan remain yellowish-brown. The dark brown peel of
longan that develops at very low temperatures is regarded as chilling injury.
When fruits are stored above 10°C, postharvest disease is of concern.
Controlled atmosphere studies have been reported, though modifi ed
atmosphere storage in 0.03 mm polyethylene bags has been tested for 7 days
at room temperature, then 35 days at 4°C. A modifi ed atmosphere of 1–3%
oxygen delays browning and maintains soluble solids and vitamin C content
in litchi. With 1% O
2
treatment and at carbon dioxide greater than 10–13%
an o -fl avour develops and a build-up of ethanol occurs.
Irradiation studies have shown that litchi and longan for export can be
irradiated at 250 Grays. Hot–cold disinfestation protocols and cold treatment
for fruit fl y disinfestation can be used for litchi and longan (Paull et al., 1995).
UTILIZATION
Important producing areas are China, India, Thailand, Taiwan, South Africa,
Mauritius and Australia. Proximate composition of litchi and longan indicates
they are a poor source of calcium, iron, thiamine and ribofl avin and a fair
source of phosphorus, and do not have provitamin A. They are a good source
of niacin and ascorbic acid (Table 9.7). Both fruits are usually eaten fresh
and can be preserved in a variety of ways. Dried litchi and longan, popularly
known as a ‘nut’, and canning are the most common. Peeled litchi fruit may
be canned in a 40% syrup, containing 0.2% citric acid. Hand-peeling of the
skin is the usual method, though a hot-lye dip and mechanical peeling are
used. The canned litchi, if fresh fruit are not available, are added to rare and
dainty dishes, and restaurants serve delicious litchi dishes with meat or with
syrup dressings.

Litchi and Longan 251
Fresh litchi packed in polyethylene bags can be quick-frozen and stored
for more than a year and still be edible, with the skin colour and fl avour
reasonably well preserved. Fruit can be sun-dried in approximately 20 days;
two-thirds of the moisture is removed. Oven-drying can be used to shorten
the drying time, but care must be taken to use relatively low temperatures
of around 40°C, as higher temperatures tend to caramelize the sugars and
produce a burnt fl avour and bitterness.
FURTHER READING
Galán Saúco, V. (1989) Litchi Cultivation (Menini, U.G. FAO Coordinator). FAO Plant
Production and Plant Protection Paper No. 83. FAO, Rome, Italy (In Spanish).
136pp.
Menzel, C.M. (1983) The control of fl oral initiation in lychee: a review. Scientia
Horticulturae 21, 201–215.
Menzel, C.M. and Waite, G.K. (2005) Litchi and Longan. CABI Publishing, Wallingford,
UK.
Menzel, C.M., Watson, B.J. and Simpson, D.R. (1988) Longans – a place in Queensland’s
horticulture? Queensland Agriculture Journal 115, 251–265.
Table 9.7. Proximate composition of 100 g edible portion of litchi (‘Gui Wei’) and
longan (Wills et al., 1986; Morton, 1987; Wenkam, 1990). The edible portion of
litchi and longan is ca. 72%.
Litchi Longan
Water (g) 77.6 83.5
Energy (kJ) 335 –
Protein (g) 0.94 0.84
Lipid (g) 0.29 0.44
Carbohydrate (g) 20.77 14.9
Fibre (g) 0.16 0.32
Ash (g) 0.37 0.43
Minerals
Calcium (mg) 4 9
Iron (mg) 0.37 0.4
Magnesium (mg) 16 –
Phosphorus (mg) 35 36
Potassium (mg) 255 170
Sodium (mg) 7 3
Vitamins
Ascorbic acid (mg) 40.2 42
Thiamin (mg) 0.035 –
Ribofl avin (mg) 0.084 0.05
Niacin (mg) 1.91 –
Vitamin A (IU) 0 –

252 © CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1
(R.E. Paull and O. Duarte)
10
MANGO
Mango (Mangifera indica L.) is also known as manga (Tamil), mangga (the
Philippines, Malaysia, Indonesia) and manguier (French). It is one of the
best-known and most widely cultivated tropical fruit species, with production
occurring in most countries in the tropics and subtropics. The fruit is marketed
fresh, dried and as a juice, and is used as a source of fl avours, fragrances and
colourants.
BOTANY
Mango (M. indica L.) belongs to the family Anacardiaceae, also known as the
cashew family, with about 75 genera and 700 species, mostly tropical, with
some subtropical and temperate species. Common nut-bearing species include
Anacardium occidentale L., cashew nut, and Pistacia vera L., pistachio nut. Other
related fresh fruits are in the genus Spondias: the yellow mombin or hog plum
(Spondias mombin L.), the ambarella or June plum (Spondias cyhterea or Spondias
dulcis Forst.), and the purple mombin (Spondias purpurea L.). Another species
belonging to this family is the marula (Sclerocarya birrea spp. ca ra Sand) of
Africa.
The genus Mangifera consists of 69 species but not all bear edible fruit. The
mango fruit is large, fl eshy and sometimes fi brous (Fig. 10.1). Other species in
the genus bearing edible fruit include Mangifera altissima Blanco, Mangifera
caesia Jack., Mangifera foetida Lour., Mangifera lagenifera Gri ., Mangifera odorata
Gri ., Mangifera zeylanica (BL) Hooker and Mangifera sylvatica Roxb.
Origin and distribution
The mango originated in the Indo-Burma region and has been cultivated
in India for more than 4000 years. This fruit is intimately associated with
the Hindu religion and there are numerous ancient Sanskrit poems praising
Mango 253
(a)
(b)
3 mm
Fig. 10.1. Leaf, fl owering panicle – (a) male fl ower with one functional stamen and
four smaller, non-functional stamens with no ovary and (b) hermaphrodite fl ower
with a large ovary with a single stigma and a single large stamen – and fruit of
mango.

254 Chapter 10
the blossoms and fruit (Singh, 1960). Indian traders and Buddhist priests
probably introduced the mango into Malaysia and other east Asian countries
during the 4th or 5th century and to the Philippines between 1400
and 1450. The Portuguese, the fi rst Europeans to establish trade routes
with India, transported the mango to East Africa and Brazil. Spanish traders
took the mango from the Philippines to the west coast of Mexico before the
English arrived in the Hawaiian Islands in 1778. The mango was introduced
into Hawaii from the west coast of Mexico between 1800 and 1820, with
credit being given to Don Francisco de Paula Marín, a Spanish horticulturist.
Apparently, the Brazilian introductions were spread to Barbados and to other
islands in the Caribbean area. Mango is now found in all tropical areas, as
well as many subtropical regions of the world, attesting to its wide range of
adaptability.
ECOLOGY
Soil
The tree is not exacting with regard to soil, although fl at alluvial soils with
pH 5.5–7 and a soil depth of at least 1 m are preferred. Freely drained oxisol
soils, such as the deep volcanic soils in Java and the Philippines, are favoured.
It can grow in 40 cm-deep soils in Florida (Malo, 1972), 75 cm in South
Africa (Mostert and Abercrombie, 1998) or 80 cm in the Canary Islands
(Galán Saúco, 2009). A hard layer or hardpan in the soil profi le can limit
root penetration and needs to be broken by subsoiling. In fertile soil, minimal
nutritional problems can be expected. Exchangeable aluminium, which
can be toxic, should be less than 30 ppm, and the tree is sensitive to saline
conditions. Calcareous soil with a high pH and salinity problems limit mango
development, although in Israel, mango is grown in very sandy soils, as well as
in calcareous soils (>38% CaCO
3
) with a pH close to 9 (Whiley and Scha er,
1997). Suitable rootstocks have been selected and used in areas where these
soil types exist, as well as in saline soils.
Climate
Rainfall
Mangoes are very drought-tolerant with a deep root system, which enables it
to capture water and nutrients from deep in the soil profi le. The tolerance of
drought is also associated with the leaves and their latiferous cells remaining
turgid through an internal osmotic adjustment (Scha er et al., 1994). The tree
can withstand occasional fl ooding and it is seen growing and producing fruit
on the banks between fl ooded rice paddies. Good rainfall distribution is crucial
Mango 255
for fl owering and fruit set, rather than total rainfall. A dry period is necessary
for reliable mango production, as it promotes fl ower induction and improves
fl owering intensity and synchrony, especially if the dry period coincides with
cool weather (Fig.10.2). In tropical high-rainfall areas, fl owering is more
erratic and yields are low, with excessive vegetative growth. Flowers are very
susceptible to the disease anthracnose, particularly when rainfall occurs
during fl owering, and low rainfall is preferred at this stage. Irrigation must be
applied regularly to prevent water stress during early fruit development when
cell division occurs and to produce a vegetative fl ush after fruit harvest.
Temperature
Mango can be grown to 1200 m in the tropics, although the best production
occurs at less than 800 m. A temperature around 33°C seems to be the ideal
for fl owering and fruit maturation and between 25 and 27° for vegetative
growth (Galán Saúco, 2009). The tree can endure up to 48°C during fruit
development if su cient irrigation is available, though at 40°C some leaf
damage can occur. Pollen viability declines if it develops at higher than 35°C
or below 15°C (Fig. 10.3). The lower limit for vegetative growth is 15°C
(Whiley et al., 1988), with the damage minimum being 1–2°C, and the tree
has no ability to acclimatize. Frost can severely damage or kill young trees,
with older trees being able to endure −4°C for a few hours with limited damage
(Crane and Campbell, 1991).
Fig. 10.2. Relationship between xylem water potential in the 6 weeks prior to ‘Nam
Dok Mai’ panicle growth and the percentage of terminals fl owering: y = −121.83 –
286.7x, r
2
= 0.52 (Pongsonboon, 1991, cited by Schaffer et al., 1994).
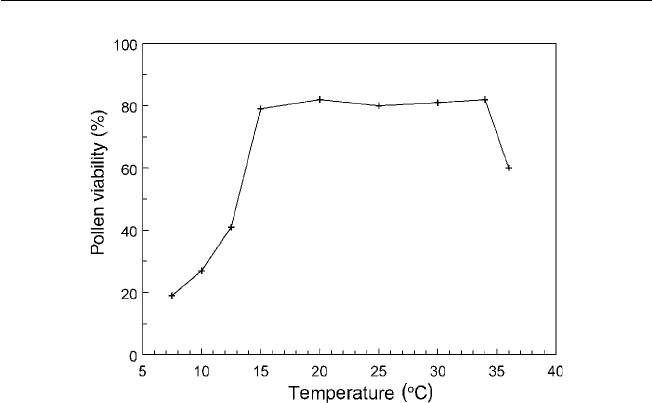
256 Chapter 10
Temperature plays a key role in mango fl owering, and the response varies
with cultivar. For fl ower induction, the ideal seems to be around 10–15°C. The
cooler temperatures in the subtropics are normally followed by fl owering, with
temperatures ~5°C inducing more male fl owers on the infl orescence. Panicle
growth does occur at 12.5°C, when no vegetative shoots are produced (Scha er
et al., 1994). However, low temperatures (<16°C) lead to fl ower deformation,
loss of pollen germination (Fig. 10.3) and slow pollen-tube growth and induce
ovule abortion, with the production of seedless fruit (Young and Sauls, 1979).
For each 125 m increase in elevation and each degree of latitude north or
south of the equator, fl owering is delayed by 4 days.
Light
Shading can prevent or delay fl ower-bud formation, and a higher percentage
of perfect fl owers occur on the side of the tree receiving direct sun. Mango
trees have a signifi cant number of shaded leaves and pruning can increase
light penetration to these leaves, but how this infl uences yield is unknown;
more light may result in larger fruit. Fruits also have a brighter red skin colour
under higher light (Scha er et al., 1994).
Photoperiod
Photoperiod seems to play no role in fl ower induction (Nuñez Elisea and
Davenport, 1995). Flowering occurs at all photoperiods from 10 to 14 h at
an inductive temperature of 18°C day and 10°C night. The failure of biennial
cultivars to fl ower in the ‘o season’ in the subtropics and fl owering occurring
at the equator several times a year indicate a day-neutral habit.
Fig. 10.3. Effect of temperature during pollen development on mature pollen
viability (Issarakraisila et al., 1993).

Mango 257
WIND
Yield is reduced if strong winds occur during fl owering and early fruiting;
however, the critical threshold for wind exposure is unknown. In windy areas,
windbreaks should be installed to improve yields and reduce fruit damage.
Dry winds during fl owering in the subtropics can cause the loss of fl owers,
and sometimes a second fl owering will occur with better fruit set because
temperatures are more favourable (Galán Saúco, 2009)
GENERAL CHARACTERISTICS
Tree
The mango is an evergreen, symmetrical tree ranging in height from 8 to
30 m, bearing leathery, simple leaves in a compact canopy. The leaves are
alternate, elliptic or spear-shaped, spirally located and fairly leathery, and
10–40 cm long (Fig. 10.1). The leaves persist on the tree for up to 4–5 years
before being shed. Leaves of young fl ushes are usually copper-red or purplish,
gradually turning to dark green.
Vegetative growth occurs in terminal fl ushes of the same branch (Fig.
10.4); each fl ush generates 10–12 new leaves with 1–3 fl ushes a year. The
number, frequency and length of these fl ushes per year depend upon cultivar,
temperature, tree age, current fruit load and previous cropping history. More
fl ushes occur in the tropics than in the subtropics. The strong dominance of
the terminal bud prevents lateral buds from emerging. When shoot elongation
slows and stops, a state of dormancy occurs in the apex until the terminal
leaves have matured, and the shoot is then ready for the next vegetative
growth fl ush or fl owering. Reproductive fl ushes will occur after extended
periods of shoot rest in the low-latitude tropics, aided by a dry period, or
during cool winter months in the higher-latitude tropics and subtropics (Fig.
10.4b) (Davenport, 2003). It is common to see di erent parts of a tree fl ushing
at di erent times, especially in the hot tropics. Biennial or irregular fl owering
has been observed in several cultivars in Hawaii; vegetative fl ushes occurring
during the June–July period are more conducive to fl owering the following
spring than fl ushes that occur earlier or later (Nakasone et al., 1955).
The roots form a dense and strong system distributed in the fi rst 2–2.5 m
and having a main tap root that can penetrate 6–8 m. If the water table rises,
a second root system will form above the main system.
Flowers
The induced reproductive terminal buds of the stem produce a large, branched
panicle (Fig. 10.1) with 300–3000 fl owers, depending upon the cultivar. The
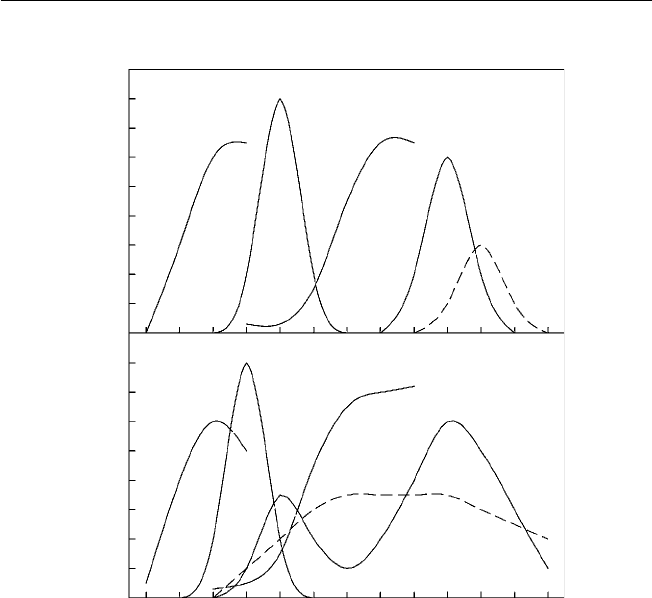
258 Chapter 10
fl owers reach full bloom in as little as 25–30 days after initiation. The panicles
can be 6–40 cm long and 3–25 cm wide, with a pink or purple, sometimes
greenish-yellow, rachis. Surrounding the terminal bud are smaller buds (sub-
apical buds), which are morphologically lateral buds compressed into the
apical position, subtending the terminal bud. Apical dominance disappears
with the destruction of the apical infl orescence (Nakasone et al., 1955).
The panicle consists of male (Fig 10.1a) and perfect hermaphrodite
fl owers (Fig. 10.1b). Perfect fl owers consist of four or fi ve calyx lobes and as
many free petals (Fig. 10.1b). The centre of the fl ower is occupied by a circular
disc (nectary) divided into four or fi ve segments. The one-celled ovary with one
obliquely protruding stigma is attached to this disc. There is usually only one
functional stamen; the other four are reduced to abortive structures, called
staminodes. The male fl ower (Fig 10.1a) is essentially the same, except for the
Flower
buds
(a)
(b)
Flower
buds
Flowering and
fruit set
Flowering and
fruit set
Fruit
growth
Fruit
growth
Vegetative
flush
Vegetative
flush
Root
flush
Root
flush
1234567
Month of cycle
Development Development
8 9 10 11 12 13
Fig. 10.4. Mango phenological cycle, having synchronous growth and fl owering in
(a) the subtropics (after Cull, 1991), and (b) the tropics, with asynchronous growth
and fl owering and a long juvenile phase (after Galán Saúco, 1996).

Mango 259
absence of an ovary. Petals are pinkish-white, with several yellow ridges on the
inner surface. The percentage of perfect fl owers on a panicle ranges from 1.25
to 81%, with a strong varietal di erence in fl ower-sex expression (Table 10.1).
The percentage of perfect fl owers is higher in the apical zone of the panicle
than in the basal and central portions; climate can also a ect the percentage,
as can the position of the panicle in the canopy. Late-season panicles and
those formed in the interior of the tree usually have a larger number of perfect
fl owers (Adlan, 1965). Flower and infl orescence colour and fl ower scent vary
among cultivars.
A good crop of fruit is obtained when only a small percentage of the
fl owers are pollinated. Lack of fruit set is attributed to: (i) lack of fertile pollen;
(ii) poor pollen-tube growth; (iii) failure of ovule fertilization; (iv) failure of
pistil or ovules to develop; (v) abortions of embryo sac, embryo or endosperm;
(vi) anthracnose disease of the fl owers; and (vii) other physical factors
(Scha er et al., 1994). Anthracnose disease is a major problem and is more
prevalent if rainfall occurs during fl owering. The disease will eventually a ect
the whole panicle, leading to fl ower and young fruit shed.
Pollination and fruit set
Natural pollination
About 65% of the fl owers open before 6 a.m. and the rest open during the day.
Anther dehiscence occurs within an hour after anthesis, with a maximum
between 9 a.m. and noon. Eighty per cent of the pollen is viable (Fig. 10.3).
Stigmas are receptive from 1 day before anthesis until 2 days after, with the
day of anthesis being the optimum. Mango cultivars are considered to be self-
fertile, but self-incompatibility has been reported. Self-pollination produces
0–1.68% set, while cross-pollination produces 6.2–23.4%, but there are clear
cultivar di erences. The pollen grains fall on the base of the ovary and the
nectary discs rather than the stigma; hence pollination agents are required.
Insects, including bees, wasps and fl ies, are the principal pollinating agents, as
indicated by the sticky pollen, secretion of nectar, colourful corolla and fl ower
Table 10.1. Percentage of hermaphroditic and male fl owers on three cultivars
grown in Hawaii (Adlan, 1965).
Sex Cultivar
Fairchild Zill No. 9 Total
Hermaphrodite 396 787 556 1739
Male 1660 1153 104 2917
Total 2056 1940 660 4656
% hermaphrodite 19.20 40.50 84.24
260 Chapter 10
scent. Flies seem to be the main pollination agents, with bees and other insects
contributing. Wind may play a small role. Pesticide use during fl owering
a ects insect pollination, pollen germination and ovule fertilization.
Polyembryony
Subtropical Indian cultivars and their derivatives, characterized by roundish,
colourful fruit and susceptibility to anthracnose, are largely monoembryonic
(Fig. 10.5a). Cultivars classifi ed in the Indochina–Philippine group from South-
east Asia are largely polyembryonic, relatively resistant to anthracnose and
often lack bright colour (Campbell, 1961). Polyembryony is the condition in
which several genetically identical embryos develop from the nucellar tissue of
the ovary and often suppress zygotic embryo development (Fig. 10.5b). These
polyembryous fruits are typically more elongated than round (Fig. 10.6).
(a) (b)
Fig. 10.5. Monoembryony (a) and polyembryony (b) in mango seedlings. In
polyembryony, several genetically identical embryos can be formed from the
nucellar tissue and often suppress the zygotic embryo.
Mango 261
Polyembryonic cultivars are generally believed to achieve higher fruit set,
since apomictic embryos take over the functions of the aborted zygotic embryos
and allow normal fruit development. Zygote abortion in monoembryonic
cultivars stops further fruit development and usually leads to fruit drop. A
small number of embryo-aborted fruit do develop into commercial-sized fruit.
Seedless fruit are reported in ‘Harders’, ‘Tommy Atkins’ and ‘Momi-K’ (Galán
Saúco, 1979), possibly due to embryo abortion taking place after growth-
promoting substances required for fruit development have been produced in
the endosperm.
Floral induction and fruit set
Biennial bearing and young fruit drop are major problems in commercial
mango production. Shoot biochemical constituents thought to be related to
fl owering of regular- and irregular-bearing cultivars under di erent climatic
conditions are not consistent. This has led to much research on overcoming
the dependence upon environmental signals for fl ower initiation using
di erent cultivars, using di erent management strategies and chemical sprays
(Table 10.2). This will be discussed in more detail under cultural practices.
Both the number of hermaphroditic fl owers per panicle and the fruit-
bearing potential can be increased following application of 100 ppm of
Ventral
shoulder
(a) (b)
Ventral
shoulder
Dorsal
shoulder
Dorsal
shoulder
Dorsal
side
Axis
Length
stem to apex
Nak
Nak or
stigmatic point
Major
diameter
Major
diameter
Apex
Depression
about stem
Fig. 10.6. Morphological characteristics of the mango fruit (Pope, 1929).
Indochina–Philippine fruit are more elongated (a) and the Indian cultivars are more
rounded (b).

262 Chapter 10
Table 10.2. Chemicals for manipulating fl owering or increasing fruit set and retention in mango (Galán Saúco, 1996).
Area Chemical Application time Dosage (foliar sprays) Action
Subtropics GA
3
Prior to fl ower differentiation
(beginning of winter)
100 mg/l Delays fl owering (repeated use
during winter will eliminate
fl owering)
Cyclohexamide Flowering 0.25 g/l Destroys apical panicles
Dinoseb Flowering 0.5 ml/l Destroys apical panicles
Pentachlorophenol Flowering 5.0 g/l Destroys apical panicles
Ethephon Full-fl ower stage 800 mg/l Destroys apical panicles
Hydrogen cyanide Beginning of fl owering season 0.4% (cv. Haden)
0.6% (cv. Keitt)
Destroys apical panicles
Tropics NH
4
NO
3
KNO
3
End of autumn 20 g/l
40 g/l
Interrupts fl ower dormancy
Paclobutrazol Any time during fruit bud stage or
even at the end of autumn
2.5–5.0 g a.i./tree
(soil drench)
Reduction of vegetative growth and
fl ower induction; increases fruit set
General Polyamines:
spermine
putrescine
KNO
3
Prior to anthesis or at full bloom
Full bloom
10
−1
M
10
−4
M
2–4%
Increase fruit set and fruit retention
Mango 263
naphthalene acetic acid (NAA) or deblossoming of terminal panicles either
manually or with chemicals. Both methods delay fl owering and can increase
yield (Table 10.2). The stimulation of auxiliary panicles also occurs if
early panicles are damaged by low temperature or winds in the subtropics.
Sprays of GA can also delay fl owering and fruit maturation by 2 weeks, but
concentration and timing of application are critical if yield is not to be reduced
(Turnball et al., 1996).
Fruit
Fruit morphology
The fruit of the mango is a drupe of variable size and shape, ranging in
weight from a few grams to more than 1 kg. It is fl eshy, fl attened, rounded
or elongated in shape. A number of basic forms of the major morphological
characteristics (Fig. 10.6) are used in describing the fruit (Pope, 1929).
Growth and development
Fruit growth showed a simple sigmoidal growth curve in terms of length,
thickness, mass and volume against days from anthesis (Fig. 10.7). The skin
200
160
120
80
40
Fruit mass (g) and starch (mg/g DM)
Acidity (%) and sugars (%)
0
12
10
Mass
Sugars
Starch
Acidity
8
6
4
2
0
20 40 60 80
Days from fruit set
100 120
Fig. 10.7. Changes in developing ‘Dashehari’ fruit weight, starch and total sugars
(after Tandon and Kalra, 1983) and ‘Nam Dok Mai’ titratable acidity (Kasantikul,
1983, cited by Mendoza and Wills, 1984).

264 Chapter 10
of the young fruit is green, sometimes purplish, and, when ripe, green, yellow,
orange, yellowish-red, purplish or purplish-red. The Indian group tends to
mature with a yellow-and-red colour combination, while the Indochina–
Philippine mangos have yellow- or orange-coloured fruit. Some fruits, such as
‘Nam Dok Mai’, have a slight pink hue on a yellow peel when ripe.
The peel (exocarp) is thick and the fl esh (mesocarp) of ripe fruit is yellow
or orange-yellow and juicy. The pericarp can be separated into exocarp,
mesocarp and endocarp at about 14 days after anthesis. There is a period
9–14 weeks after fruit set when growth rate decreases, and this is associated
with hardening of the endocarp and accumulation of starch and sugars (Fig.
10.7). The endocarp is hard, with fi bres that may extend into the fl esh. The
period from fruit set to maturity depends upon cultivar and climate and can
range from 10 to 28 weeks. The cv. ‘Saigon’ grown in a hot climate is ready for
harvest in 12–13 weeks. In cool areas, where mean temperatures fall below
20°C, maturation is delayed by up to 4 weeks.
A network of latiferous canals and secretory ducts anastomoses in all
directions in the exocarp and mesocarp (Joel, 1978). Cultivars with a poorly
developed duct system are more susceptible to the Mediterranean fruit fl y
(C. capitata Wied.). The latiferous canals in mango begin to disintegrate during
ripening and the fruit becomes susceptible to fruit fl ies. The sap from the skin
contains urushiol, a toxin also found in other members of the Anacardiaceae,
which causes an allergic skin rash on contact. Urushiol is catechol substituted
with an alkyl chain with 15–17 carbon atoms. Some individuals are more
sensitive than others.
CULTIVAR DEVELOPMENT
Genetics, cytogenetics and breeding
Cytological studies of seven species, M. indica, M. sylvatica, Mangifera calo-
neura, M. zeylanica, M. caesia, M. foetida and M. odorata, showed all to have
a chromosome number of 2n = 40 (Mukherjee, 1950). M. zeylanica and
M. odorata have been successfully crossed, indicating the possibility of
interspecifi c crosses without the problem of interspecifi c sterility. This is
supported by the uniformity of chromosome morphology. Although mango
hybridization has been carried out, very few cultivars have been developed by
controlled pollination. Hence, little genetic information is available.
Problems in breeding
Breeding is di cult because of the small number of seeds obtained, the
complex nature of the panicle and fl ower (Fig. 10.1a, b), excessive fruit drop,

Mango 265
long life cycle and heterozygosity of the crop (Knight and Schnell, 1993). The
objectives of mango breeding are to develop regular-bearing dwarf trees that
have an extended cropping season, good cropping in the wet tropics, attractive
fruit of good size (300–500 g), free from internal breakdown and good keeping
and eating quality without fi bre. Active breeding programmes are carried out
in many countries in the world, including India, the USA, Israel and Australia
(Galán Saúco, 1996). Rating scales for important characteristics, such as size,
shape, colour, fi rmness, fi bre content, fl avour, disease resistance and yield,
have been developed. Characteristics are rated on 1 to 5 or 1 to 10 scales (least
desirable to most desirable) in merely descriptive terms (Knight, 1985).
More recent objectives of mango improvement include resistance to
diseases, such as anthracnose and powdery mildew, both devastating to fruit
yields, and tolerance to environmental conditions such as soil salinity and
cold temperatures. There is some variation in the degree of fruit susceptibility
to anthracnose disease. Genetic transformation of mango is possible, using
Agrobacterium as the transformant.
Cultivars
There are hundreds of named mango cultivars throughout the tropics and
subtropics (Table 10.3). Each country or region also has their own selected
cultivars (Table 10.4). Preferences for cultivars vary in di erent regions of the
world. The most important commercial cultivars are derived from selections
among open-pollinated seedling populations. Many excellent cultivars have
been developed through introduction and selection by cooperation between
researchers, growers, nurserymen and hobbyists. Seedlings can be produced
by mass cross-pollination of a collection of mixed cultivars selected for known
desirable characteristics among mono- and polyembryonic races (Whiley et al.,
1993). Only seeds from the monoembryonic cultivars are used as the female
parent and planted for later evaluation. Polyembryonic lines are di cult to
use as the female parent, as there is no assurance that a sexual seedling will be
produced.
It is without doubt that India, with more than 1000 named cultivars and
with di erent regions growing specifi c cultivars, has provided most of the
germplasm for cultivar development. Florida has developed a large number of
cultivars, using mainly Indian cultivars, which have shown wide geographic
adaptability. ‘Haden’ was a major cultivar in the early mango industry in
Florida. It may be a parent of other cultivars, including ‘Irwin’ and ‘Lippens’.
Other well-known Florida cultivars include ‘Keitt’, ‘Sensation’ and ‘Tommy
Atkins’ (Campbell and Campbell, 1992). Most mango varieties planted in the
Americas, the Canary Islands and many African countries were developed
in Florida. The Florida varieties are also dominant in international trade and
include ‘Tommy Atkins’, ‘Haden’, ‘Kent’, ‘Keitt’ and ‘Van Dyke’.

266 Chapter 10
Table 10.3. Some common mango cultivars grown commercially in different parts of the world.
Cultivar Origin
Poly/
Mono Fruit shape Skin colour
Fruit
maturation Fibre
Eating
quality Storage
Anthracnose
susceptibility
Alphonso India Mono Oblique Yellow Mid Low Excellent Low
Baptiste Haiti Poly Oval Bright yellow Mid None Fair to good
Carabao Philippines Poly Long and
slender
Greenish to bright
yellow
Early None Good to
excellent
Poor Low
Haden Mulgoba,
India
Mono Oval Red blush or
yellow
Mid Abundant Good to
excellent
Susceptible
Irwin Lippens,
Haden
Mono Ovate Red Mid None Good Poor Low
Keitt Mulgoba Mono Oval Red blush on
green
Late Little Good to
excellent
Good Moderate
Kensington Australia Poly Oblong ovate Yellow with pink
blush
Mid Low Excellent Low
Langna India Mono Oblong Pink blush or
greenish-yellow
Mid Abundant Fair to good
Manila Philippines Poly Long and
slender
Bright yellow Mid Fibrous Good to very
good
Mulgoba India Mono Oval to ovate Yellow with pink
blush
Mid to late Little Good to
excellent
Very
susceptible
Nam Dok
Mai
Thailand Poly Long slender Bright yellow Mid None Excellent Susceptible
Neelum India Mono Ovate Bright yellow Late None Good to
excellent

Mango 267
Pairi India Mono Round to
oblong
Orange-red Mid Little Good Moderate
Saigon Vietnam Poly Oval to ovate Yellow None Good to
excellent
Low
Tommy
Atkins
Haden Mono Oval to
oblong
Dark red or orange-
yellow
Mid Moderate Fair Good Resistant
Tong Dam Thailand Poly Oblong to
long
Greenish-yellow Early None Good to
excellent
Susceptible
Zill Haden Mono Oval to
ovate
Dark red to crimson
or yellow
Mid None Good to
excellent
Poor Susceptible
268 Chapter 10
In Mexico, the ‘Manila’ and ‘Ataulfo’ varieties of Philippine origin are
grown, while in Brazil, in addition to the main export varieties (Table 10.4),
local varieties from material introduced by the Portuguese are also produced.
In most of Latin America, di erent ‘criollo’ mango varieties are grown, which
belong to the Indochina–Philippine group. These criollo types have survived
as such since they are polyembryonic and almost exclusively propagated
by seeds.
In the last few years some new varieties have been demanded by the US
market, the largest import market, and also by the EU; these include ‘Edward’,
a Florida variety, ‘Ataulfo’, a polyembryonic variety from Mexico, and others,
such as ‘Madame Francis’ from Haiti and ‘Julie’ from Jamaica and the
Caribbean. The EU is also starting to import other varieties such as ‘Amelie’
from Africa and ‘Nam Dok Mai’ from Thailand.
Hawaiian cultivars, such as ‘Pope’, ‘Gouveia’, ‘Momi-K’, ‘Ah Ping’,
‘Harders’ and ‘Joe Welch’, are all derived from monoembryonic parents. The
recently released improved cultivars are ‘Rapoza’ and ‘Exel’, the former being
a seedling of ‘Irwin’ and having large fruit with excellent eating quality;
Table 10.4. Producing countries, selected cultivars and main marketing season.
Country Selected cultivar Marketing season
Australia Kensington Pride, Keitt, Kent, Palmer,
Irwin
October to March
Brazil Haden, Tommy Atkins, Kent, Keitt, Palmer,
Bourbon, Espada, Itamarco, Maco, Rosa,
Carlota
October to February
India Alphonso, Banganpalli, Dashehari,
Bangalora, Langra, Mulgoa, Neelum,
Pairi
April to July
Indonesia Arumanis, Dodol, Gedong, Golek,
Cengkir
September to January
Israel Keitt, Tommy Atkins, Kent, Maya, Haden July to August
Malaysia Harumanis, Golek, Maha 65, MA 200
(Malgoa)
June to August
Mexico Haden, Manila, Esmeralda, Kent, Keitt,
Tommy Atkins, Jan Dyke, Palmer
April to October
Peru Kent, Tommy Atkins, Haden November to February
Philippines Carabao, Pico, Julie June to September
South Africa Peach, Zill, Fascell, Sensation, Tommy
Atkins, Keitt
November to January
Spain Tommy Atkins, Keitt, Lippens, Osteen July to August
Taiwan Irwin, Yellow #1, Haden July to October
Thailand Nam Dok Mai, Rad, Tongdum, Okrong March to May
USA – Florida Keitt, Irwin, Tommy Atkins, Kent, Van
Dyke, Palmer
July to August

Mango 269
it is a late bearer, with fruit maturing from August through October, greatly
extending the season (Hamilton et al., 1992). ‘Exel’ was also selected from a
population of ‘Irwin’ seedlings for its high quality, attractive fruit and regular-
bearing habits, with fruit weight ranging from 400 to 500 g and with 18%
total soluble solids. Another desirable feature of the fruit is the thin, fl at seed,
which results in more than 90% edible fl esh (Ito et al., 1992).
Isozyme analysis has been used to verify or refute parentage of mango
cultivars (Degani et al., 1990). Isozymic banding has shown that ‘Haden’
appears to be a seedling of ‘Mulgoba’, and ‘Zill’ a seedling of ‘Haden’.
‘Mulgoba’ has the ab phenotype and ‘Keitt’ the cc phenotype, while ‘Haden’
shows an aa phenotype, ‘Carabao’ bb and ‘Edward’ ac. Newer molecular-
biology techniques are being used to unscramble the parentage of mangoes
and to determine the extent of genetic diversity (Krishna and Singh, 2007).
Rootstocks
Most mango plants are grafted on to polyembryonic rootstocks in order
to obtain plants that have a uniform root system, since they come from
an asexual process and so are clones. There are several rootstocks in each
country. In Latin America, some of the ‘criollo’ types of the Indochina–
Philippine group are used; however, not all are good rootstock. In Mexico,
the cultivar ‘Manila’ (‘Carabao’), which is exported and consumed locally,
is used. Israel has developed ‘13-1’ for saline and alkaline conditions. South
Africa uses ‘Sabre’ for sandy soils and ‘4/9’ for heavy soils. In the Canary
Islands ‘Gomera-1’ and in mainland Spain ‘Gomera-3’ are used (Galán Saúco,
2009). Australia uses the polyembryonic ‘Kensington Pride’, which is also a
commercial fruit cultivar. Very little has been reported about the infl uence of
rootstock on the cultivar growth.
The Indian cultivars ‘Olour’, ‘Vellai Colamban’ and ‘Saber’ are used as
rootstocks to reduce tree vigour. Researchers have sought rootstocks with
special attributes, such as dwarfi ng habit and tolerance to high pH and saline
conditions. Two Indian hybrid cultivars, ‘Mallika’ (‘Neelum’ × ‘Dashehari’)
and ‘Amrapali’ (‘Dashehari’ × ‘Neelum’), have been reported to exhibit distinctly
dwarfi sh characteristics in terms of trunk circumference, tree height and canopy
diameter in Brazil (Pinto and Sharma, 1984). Tolerance to saline soils has been
identifi ed, and cultivar ‘13-1’ is being used commercially as a rootstock in Israel,
where calcareous soil and saline irrigation water pose serious problems (Gazit
and Kadman, 1980).

270 Chapter 10
CULTURAL PRACTICES
Propagation
Sexual
Mango seeds should be planted while still fresh, as they lose their viability
within a few weeks. The usual procedure is to eliminate the pulp from around
the seed by fermentation and washing, and superfi cially dry the seed in the
shade for a couple of days. Uniform germination can be achieved if the kernel
is removed from the hard endocarp or seed coat; this can be achieved by
using pruning shears to cut the edges and remove the seed. During sowing,
the concave part of the seed should face down and be buried about 4–5 cm
into sand or a sandy mix. This sandy medium allows easy extraction of the
seedlings with little damage to the roots when repotted. The germinated
seedlings are separated and transplanted singly into polyethylene bags (18 cm
(w) × 13 cm (d) × 30 cm (h)), and bags up to 45 cm in height help to avoid
early root deformation. The seedlings are grown under ~30% shade. The seeds
of polyembryonic cultivar produce several seedlings, some of which become
twisted together and have curved stems and roots, and should be discarded
and not transplanted into nursery bags. Monoembryonic seeds from breeding
programmes can be planted directly into the polyethylene bags. Polyembryonic
cultivars, though they can be propagated by seed as they retain the parental
characteristics, are grafted to take advantage of the earlier production and
shorter, stockier trees, avoiding the juvenile characteristics of seedling trees.
Asexual
Cultivars are propagated vegetatively by such methods as cuttings, grafting,
budding or air-layering. Cutting propagation has been successfully achieved
using hardwood or semi-hardwood cuttings, or subterminal cuttings with
adult leaves being put under mist and bottom heat, and the cut surface treated
with auxin (Reuveni et al., 1991). This method is justifi ed in cases of a scarcity
of monoembryonic rootstock material.
Seedlings can be grafted in 6–8 months if fertilized and irrigated regularly.
Grafting is usually some form of side or cleft graft, with the side-wedge method
also being frequently used. Splice grafting is occasionally used. Only a well-
matured terminal and the section below it should be used as scion wood,
immature wood often fails to graft. Terminal scions are preferred because
they will have no wound at the apex end of the scion piece. Budding methods
permit the use of much younger rootstocks. Buds may be prepared in advance
by removing the leaves of mature terminal wood. Removal of the leaves and
the apical bud destroys apical dominance and allows axillary buds to begin
to swell in 1–2 weeks. Air-layering and inarching are used in some areas to
generate experimental plants rapidly. However, these time-consuming methods
are appropriate when only a few plants are needed. Cultivars propagated by

Mango 271
grafting and inarching grow faster than those propagated by stooling and air-
layering. Grafting at a low height produces a spreading tree, while grafting
high up on the stock produces a non-spreading type.
Field preparation
Land preparation is similar to that with other tree crops. Deep ripping may be
necessary to break up any hard subsoil layer. In some cases sulfur has to be
added if the soil is too alkaline. This is also the time when organic matter can
be added without interference from the tree canopy.
Transplanting and spacing
Transplanting should be done just before or early in the wet season if no
irrigation is available. Often, organic matter and P fertilizer are added to the
planting hole (0.5 m × 0.5 m × 0.5 m) before planting. No shade is required
after transplanting. Spacing is largely dependent upon environment, soil
characteristics, vigour of the cultivar and the planned orchard management.
Various patterns, with spacing from 6 m × 3 m to 7 m × 15 m, are recom-
mended. High-density planting (3 m × 2.5 m) has been tested with grafted
trees, and, while individual tree yields are low, total yield is higher, although
extensive pruning is essential (Ram and Sirohi, 1991). In the subtropics, closer
spacing (6 m × 4 m or 6 m × 3 m) is being used, with more detailed formation
pruning and annual maintenance pruning to prevent the trees from becoming
too large.
Irrigation
Young transplants require about 20–30 l of water every 4–5 days for about
2–3 months during establishment. For the remainder of the fi rst year, rates
may be increased to 40–50 l at 7–10-day intervals. During the second year,
rates are increased to about 100–150 l per 10 days. More may be necessary
during particularly dry periods. In the third year, rates of 200–300 l/tree at
15-day intervals may be adequate. Rates may be decreased or increased and
the intervals shortened or lengthened, in accordance with soil type and the
amounts and periods of rainfall.
Four- to 5-year-old grafted trees can begin to bear fruit, and cultural
practices should be adjusted to refl ect this change. For bearing trees,
especially in the tropics, it is desirable to have a 3–4-month dry period prior
to fl owering, to reduce vegetative fl ush growth. This is easily accomplished in
areas where this period coincides with the natural dry period. In dry areas,

272 Chapter 10
irrigation is desirable after the infl orescence appears and as fl owers begin to
set fruit. During the fi rst 4–6 weeks of fruit development, cell division is most
rapid and moisture stress should be avoided. Four-year-old trees may require
around 400–500 l water/tree at 2-week intervals. Irrigation is completely cut
o as the fruits approach maturity, since dry conditions favour higher sugar
content. Heavy irrigation is resumed immediately after harvest to encourage
new vegetative growth (Fig. 10.4). In the monsoon tropics, termination of
harvest coincides with the rainy season, so irrigation is usually unnecessary.
As soon as a major vegetative fl ush occurs, reduction in soil moisture content
is desirable to mature the new fl ush. The choice of irrigation equipment and its
management is based on capacity and e ciency of water delivery, and cost of
the system plus installation. Irrigation should be programmed in accordance
with the phenological growth cycle (Fig. 10.4) to achieve maximum yield.
Pruning
In most subtropical areas, the tendency is to use narrower plant spacings
and keep the trees small by pruning. In the hot tropics, the trees are also kept
smaller than before by pruning, but they are normally larger than in the
subtropics.
Formation pruning can start in the nursery, at transplanting time or
shortly after transplanting. All lateral shoots from the main stem are removed
until the plants reach a height of 70–100 cm, to train the tree to a single
trunk. For varieties with long hanging branches, at this height the top 10–20
cm is cut o below a node. In the subtropics this should not be done in autumn
or winter, otherwise fl oral shoots might develop (Galán Saúco, 2009). Later
lateral buds will sprout and three well-oriented and well-distanced shoots are
left to become the primary tree branches. These shoots should be oriented in
di erent directions, hopefully separated by a 120° angle; at the same time
there should be a distance of 20–30 cm between them along the main stem, so
that the weight of the adult tree is not concentrated at one point. For varieties
that tend to produce very long shoots at a narrow angle, the selected primary
branches can be forced to grow at a 45° angle. This forcing will slow their
growth and induce more laterals to arise and reduce excessive tree height.
To obtain compact trees for narrow plant spacing, once the main lateral
branches have stopped growing and mature, the terminal buds are pinched
o , with as few leaves as possible being removed. From these pinched main
lateral branches, three shoots are left to grow, and they will be pinched the
same way, so that nine secondary branches are formed, and fi nally these can
also be pinched, leaving three equally spaced new shoots on them, completing
a total of 27 tertiary branches. This process is repeated during the fi rst 2–3
years until a well-balanced plant is obtained.

Mango 273
When less-compact canopies are desired, pinching can be done every
second or third fl ush, and later the canopies are opened at the centre by
removing interior branches, so that the plant and fruit receive more light.
In some tropical areas topping of the vegetative shoots at the second or
fi rst internode is done to obtain more-compact trees (Galán Saúco, 2009).
Pruning can also be done to accommodate the trees for espaliers and trellises,
where pinching is done at the height of the fi rst wire so that two new shoots
are directed along the wire and the third shoot grows up to the second wire,
and repeated.
After the trees have been adequately trained, annual pruning is not
usually practised. Mango trees normally make very dense growth, and
occasionally light thinning of branches will become necessary to facilitate
light penetration, air movement, pesticide penetration, removal of dead and
diseased branches and some control over tree height. The tallest branches
are cut back at the fork (point of origin) of the branch. ‘Water sprouts’ and
overlapping branches may be removed annually. In Florida, pruning is done
with machines called ‘ledgers’ and ‘toppers’ by cutting back the height and
width of the trees to about 4 m. This is done annually immediately after
harvest (Campbell, 1988). These trees have two or three vegetative fl ushes
before becoming dormant at the onset of winter and then produce fl owers in
the following spring.
Pruning becomes a major endeavour when trees are allowed to outgrow
the space provided. Yields are reduced and harvesting becomes di cult
and uneconomical. Removal of alternate trees appears only to aggravate
management problems later, as remaining trees will grow even larger. Drastic
pruning of large trees to about 2 m to develop new tops on old trunks may
cause approximately 3 years’ loss of income. Yearly pruning to less than ~3 m
to control tree height is practised in Taiwan, without signifi cant impact on
year-to-year production. The fruits are bagged to reduce disease and insect
damage, and fruits thinned to match fruit load to tree size (Fig. 10.8).
In cultivars with biennial (irregular) fl owering, shoots that have fl owered
are removed after harvest, leaving only shoots that may fl ower next time.
Flowering shoots that do not set fruit should also be removed soon after
fl owering. Removal of apical buds after each fl ushing cycle to increase the
number of terminal shoots that could fl ower can lead to better fruiting and
limits tree size (Oosthuyse and Jacobs, 1995). The number of terminal shoots,
however, is not always related to the number of fruit set (Fig. 10.9). There is
a strong correlation between number of terminal shoots and fruit number
for the cv. ‘Sensation’, which retains a higher number of fruit per shoot than
‘Kent’, indicating that other factors are involved. Deblossoming of the terminal
infl orescences can lead to infl orescence development from axillary buds, a
20–30-day-later harvest and higher yields (Chang and Lion, 1987).
In the lower tropics, where fl owering time is modifi ed, synchronization
pruning is done every year to force the trees to produce new shoots at the

274 Chapter 10
same time. For this, peripheral pruning is done all over the canopy, removing
the tips of the last growth, including leftover fl oral peduncles, which have
an inhibitory e ect on future fl owering. This pruning will force the tree to
produce new growth, simultaneously resulting in more uniform fl owering,
since all new shoots are of the same age and in a similar physiological stage.
This pruning should be rather light to avoid a strong tendency to get a second
vegetative fl ush too soon, since this would not allow enough time for the shoot
to rest and become reproductive. It is done soon after harvest, and it is the fi rst
step in the process of fl ower induction.
Fertilization
One of the basic considerations for fertilization amount and time of
application is the growth and fl owering cycle of the tree (Fig. 10.4). During the
fi rst 3 years, approximately 113–227 g per tree of a complete (NPK) fertilizer
is applied three times a year. From the fourth year, trees are considered to
be mature, as they will begin to produce commercial yields, and fertilizer is
applied twice a year. One application is made when the fi rst infl orescence
begins to appear and the second immediately after harvest, to promote a new
vegetative fl ush. Proper placement is important in ground application, as the
highest feeder-root density is approximately 90–175 cm away from the trunk
to a depth of 20 cm. Irrigation is necessary whenever fertilizer is applied.
The major mango-growing countries have usually developed their own
fertilizer ratios and amount to be applied. Complete fertilizers, with an oxide
ratio of approximately 15:15:15, are usually recommended in Hawaii (Yee,
1958). In Florida, a good crop can be obtained by providing 1.4–1.8 kg of N
and K/tree/year to mature trees (Malo, 1976). Using the triple-15 fertilizer,
(a) (b)
Fig. 10.8. Mango tree showing profuse and uniform fl owering (a). Trees are
managed to less than 2 m. When the fruits are about 7 cm across, the tree is sprayed
with fungicide to control fruit diseases, the fruits are thinned and the selected fruits
are bagged (b). One fruit has 6–8 leaves subtending it on the branch.

Mango 275
an application of 4 kg/tree in February–March provides 0.6 kg each of N
and K. A second application immediately after harvest of 6 kg/tree provides
0.9 kg each of N and K, for a total of 1.5 kg each of N and K for the year. N
and K at these levels are reported to enhance total yields, external colour and
sugar content, while N in excess of 1.8 kg can produce unfavourable e ects
on external colour and fl esh fi rmness (Young and Koo, 1974). When leaf N
levels are greater than 1.5%, reduced fruit size is expected, with an increased
incidence of ‘soft nose’ and soft brown rot and an increase in the number of
days to ripen. Critical levels for various nutrients in mango leaves have been
developed (see Table 4.3).
Flowering synchronization and induction
Ethephon, an ethylene-releasing compound, increases fl owering and fruiting
in ‘o ’ years in biennial-bearing trees. In the past, to produce fl oral induction,
Philippine farmers used smudging by burning rice straw close to the trees, with
the smoke having a stimulating e ect on mango fl owering. This was possibly
due to the ethylene gas in the smoke. In the last two decades more sophisticated
approaches have been developed and are being used commercially.
In the subtropics, fl owering depends on the cool winter temperatures (Fig.
10.4a) and fl owering is enhanced by water stress. In cool winters, the plants
will fl ower profusely (Fig. 10.8a). For young plants, this profuse fl owering
can limit plant growth and the ability to achieve the desired tree size, so the
Fig. 10.9. Relationship between the number of terminal shoots on 2-year-old
‘Sensation’ and ‘Kent’ trees and number of fruit retained. ‘Sensation’ y = 0.98x +
12.8, r
2
= 0.86***, ‘Kent’ y = 0.18x + 11.8, r
2
= 0.31* (after Oosthuyse and Jacobs,
1995).

276 Chapter 10
fl owers and small fruit are removed during the fi rst few years to allow the
plants to become larger. In the tropics, fl ower induction is not dependent on
a cool winter and is regulated by the time since the last fl ush matured after
the sprouting induced by the synchronized peripheral pruning. If this shoot
sprouts too soon, then the new fl ush will be vegetative; if the apex has rested
for a longer period, a reproductive apex will form. The length of the rest period
varies with variety.
The main aim of mango producers in the northern hemisphere is to
harvest in March to April when prices are highest. In Central America, normal
fl owering occurs in January/February and the harvest then coincides with
that of Mexico. Mexico, as the largest world exporter and main supplier to
the USA, makes it di cult for other supplies to compete, and prices are low
because of oversupply. Other northern-hemisphere suppliers use orchard
management practices to induce earlier fl owering and harvest 1–3 months
earlier to coincide with the better market prices. This is possible as a major
portion of Mexican mango production occurs under subtropical conditions
in northern Mexico and they are unable to signifi cantly modify the fl owering
time. Similar procedures are followed in other parts of the world in order to
achieve better economic returns by harvesting before the bulk of production
arrives to the market. During ‘El Niño’ years, when winter temperatures are
higher, Peruvian and Ecuadorean mango production is a ected, though little
can be done to overcome the poor fl ower induction.
For these orchard management practices to work e ectively to advance the
fl owering date, the following factors should be taken into account: (i) the plant
leaf N concentration should be between 1.1 and 1.3% at the time of induction;
higher concentrations result in vegetative shoots; (ii) the soil moisture
content should not be low, in order to achieve a better reproductive response;
this is di cult to control in the tropics if the induction time occurs during
the rainy season; (iii) the age and maturity of shoots, with older shoots that
have been inactive after vegetative growth and maturation being more likely
to di erentiate into a reproductive shoot. To limit vegetative growth an anti-
gibberellin compound such as paclobutrazol is used. Low soil nitrogen and
moisture also play a role in reducing the occurrence of early vegetative fl ush;
and (iv) the e ectiveness of the application of fl owering stimulants, where the
dosage, the tree coverage, age and state of the leaves and the time the product
stays on the leaves infl uence the outcome (Huete and Arias, 2007).
Steps to induce fl owering
Stimulation of new shoots
The primary goal is to stimulate abundant vegetative shoot production. This
is achieved by: (i) synchronizing peripheral pruning after harvest, which will
result in a uniform synchronous production of new vegetative shoots at the

Mango 277
same physiological age. This pruning should also remove all of the last season’s
panicles, which inhibit future fl owering. This pruning should not be extensive;
(ii) a balanced fertilization programme that ensures good vegetative growth
but does not provide excessive amounts of nitrogen, or lead to a defi ciency;
and (iii) soil moisture management that involves limiting irrigation as the
leaves approach maturity to prevent the potential for premature shooting.
CHECKING VEGETATIVE GROWTH
Limiting vegetative growth can be achieved with paclobutrazol and
uniconazole, the latter being ten times more potent. These products are
expensive, sometimes di cult to fi nd in most countries and if used improperly
can cause permanent tree damage. These anti-gibberellin compounds inhibit
sprouting and reduce the time needed between synchronization pruning
and fl oral stimulation by about 1 month. These inhibitors are applied 1–2
months after harvest, when the shoots from the synchronization pruning
have completed their growth phase. Paclobutrazol can be applied at a rate of
5–8 g/plant
or 1 g/m of canopy diameter, while uniconazole is applied at a
rate of 0.1 g/m
2
of the canopy shade. The application is made in 10–20 l of
water put in a shallow trench at the canopy drip line; the soil should be moist
before application and irrigated after, to carry the chemicals to the root zone.
Another anti-gibberellin product, ‘Pix’ (mepiquat chloride), is sprayed at 1%
active ingredient on to the canopy until run o . A 2–3 cm girdle can induce
fl owering but often leads to the death of ~5% of branches per year and is not
recommended. Controlled scoring is now used on subterminal branches with
little danger of tree damage. Controlling water stress is di cult in the tropics
with frequent rain, except in tropical deserts.
Spraying fl owering stimulants
Flowering can also be infl uenced by nitrates, such as potassium nitrate at
2–4%, calcium nitrate at 1–3% and ammonium nitrate at 1–2% sprayed
on trees in the tropics, but apparently not in the subtropics. One to three
additional sprays of the same chemicals can be done at weekly intervals if
the e ect is not satisfactory. Thiourea can be used instead of nitrates. Young
shoots (1.25 months from bud emergence) of the Philippine cvs ‘Carabao’
and ‘Pahutan’ can be induced to fl ower uniformly by spraying 10 and 40 g/l
of KNO
3
, respectively. ‘Carabao’ fl owered in 11 days, while ‘Pahutan’ required
20 days. Spraying older ‘Carabao’ shoots (8.5 months from bud emergence)
with 10–160 g/l KNO
3
induced 100% fl owering, with unsprayed controls
remaining vegetative (Bondad and Apostol, 1979). Genetic di erences among
seedling trees and between cultivars means that variations in response to the
chemical used occur, with the nitrate ion being the e ective ion (Nagao and
Nishina, 1993).
Eight factors will determine the success of these nitrate sprays: (i) the
leaves have to be mature, dark green and brittle when squeezed; (ii) the

278 Chapter 10
nitrogen concentration of the leaves should be less than 1.3%; (iii) soil
moisture should be low; (iv) application at night when cool and days are warm
or after a cold front; (v) rain should not occur for at least 6 h after spraying
to avoid the product being washed o ; (vi) the whole tree should be sprayed to
runo ; this can take 8–20 l/tree. A spreader sticker improves the e ectiveness
of the sprays; (vii) the best time to apply is after 4 p.m.; and (viii) the variety
should be responsive. The Indochina–Philippine varieties respond readily to
stimulation, while Indian variety ‘Haden’ responds better than ‘Kent’ or ‘Keitt’,
and ‘Tommy Atkins’ is the least responsive. Advancing fl owering date should
be done in several small steps (Duarte, 2002) to avoid losing production. In
the fi rst year, fl owering is brought forward 20 or 30 days, with a similar step
in the following year, until the desired fl owering date is achieved. Following
application, fl owering will occur in about 3 weeks.
Nitrates or other products do not induce or change apex di erentiation:
if it is a vegetative apex, a vegetative shoot will sprout; if it is a reproductive
structure, a fl ower panicle will emerge; nitrates induce the bud to start
growing. The factor that determines whether a shoot apex produces a fl ower
panicle or a vegetative shoot is the age of the shoot from the time it started
to grow after the synchronizing pruning. The older the shoot, the greater the
possibility that a fl ower panicle will emerge. Normally ‘Haden’ shoots can be
sprayed 4 months after synchronization pruning, while ‘Tommy Atkins’ needs
5 months for similar results. Davenport (1993) proposed the following model
for mango fl owering induction in the hot tropics:
With paclobutrazol application:
Harvest − pruning + 1 month, paclobutrazol + 3 months = 4 months to
induce ‘Haden’
Harvest − pruning + 2 months, paclobutrazol + 3 months = 5 months to
induce ‘Tommy Atkins’
Without paclobutrazol application:
Harvest − pruning + 5 months to induce ‘Haden’
Harvest − pruning + 6 months to induce ‘Tommy Atkins’
The paclobutrazol treatment reduces by 1 month the time that the shoot
needs to be in a non-active stage to fl ower after nitrate application in the
tropics. The result is explained by postulating that gibberellins are fl ower-
formation inhibitors. Paclobutrazol and uniconazole have not been labelled for
use on fruit crops in the USA, though they are not translocated into the fruit.
Fruit yields
Fruit yields vary with cultivar, climatic and edaphic conditions of the
production site, cultural practices and other factors, such as diseases and
Mango 279
insect pests. Yields over many years exhibit a sigmoidal curve, initially with
low yields, increasing more rapidly and then dropping o as trees become
crowded. The period of maximum production depends upon tree growth rate;
a rapidly growing cultivar is more likely to show decreasing yields earlier, due
to crowding. Mango yield studies over a su cient number of years involving
replicated plantings are relatively rare, due to time and cost.
In Puerto Rico, researchers determined yield potential, year-to-year
consistency of production, estimates of incremental increase in yields over age
of trees, fruit size and tree growth of 16 cultivars in their fi rst 6 crop years.
The adjusted cultivar mean yield can be separated into three yield groups,
with no signifi cant di erences between cultivars within each group (Pennock
et al., 1972). The high-yielding group order was ‘Ruby’, ‘Sensation’, ‘Eldon’.
‘Lippens’ and ‘Irwin’; an intermediate group ‘Earlygold’, ‘Keitt’, ‘Parvin’,
‘Zill’, ‘Haden’ and ‘Palmer’; and a low-yield group of ‘Pillsbury’, ‘Kent’,
‘Edward’, ‘Santaella’ and ‘Jacquelin’. Cultivar consistency of bearing also
gave three di erent groupings, having di erent cultivar make-up: ‘Edward’,
‘Zill’, ‘Pillsbury’, ‘Ruby’, ‘Lippens’ and ‘Irwin’ as regular bearers; ‘Sensation’,
‘Santaella’, ‘Parvin’, ‘Earlygold’ and ‘Jacquelin’ with intermediate consistency;
and ‘Kent’, ‘Eldon’, ‘Palmer’, ‘Haden’ and ‘Keitt’ being highly inconsistent
in yield (Fig. 10.10). The consistent-bearing cultivars with high yields were
‘Ruby’, ‘Lippens’ and ‘Irwin’. ‘Edward’, ‘Zill’ and ‘Pillsbury’ show regular-
bearing habit but consistently produce low yields.
A mango orchard on the south coast of Puerto Rico under similar
conditions to those of the above experimental site began production about 5
Fig. 10.10. Year-to-year variation in yield of three cultivars having different bearing
habits (Pennock et al., 1972).

280 Chapter 10
years after fi eld planting. The fi rst crop had around 2.3 kg/tree, increasing to
about 12.7 kg/tree/year during the next 5 years. On a per-hectare basis, with
173 trees/ha (57.8 m
2
spacing), the initial yield of 5-year-old trees would be
393 kg/ha, with a yearly increase of 2.2 t/ha
(Pennock et al., 1972). Yields
obtained in Florida, Mexico, and Central and South America have shown
that in the fi fth year after planting, 0.9 t/ha can be expected, increasing to
1.7 t/ha in year 6, 3.5 t/ha in year 7, 5.2 t/ha in year 8 and 8.7 t/ha in year
9. Mature trees can yield 10–30 t/ha, with an average of 22–25 t/ha in the
subtropics. In the tropics, commercial yields of 10 t/ha are expected from
high-quality cultivars.
PEST MANAGEMENT
Diseases
Anthracnose (C. gloeosporioides) is perhaps the most important disease
of the mango in almost all production areas (Table 10.5), as it attacks
leaves, fl owering panicles and fruit. Yields are drastically reduced when
the infl orescence is attacked. This disease is especially serious in areas with
high humidity and frequent light showers during the fl owering period. In
Hawaii, where rainfall coincides with the fl owering season, almost the entire
production of infl orescences can be destroyed. In the Ryukyu Islands of Japan
(26°N), a crop of mango can be produced only by constructing polyethylene
shelters over the trees to protect the infl orescence from the frequent light
showers falling during the fl owering season. For this reason, trees are kept
at 1.8–2.4 m in height. In Australia, the cultivars ‘Carabao’, ‘Keitt’, ‘Tommy
Atkins’ and ‘Zill’ have been identifi ed as possessing tolerance to anthracnose
(Whiley and Saranah, 1981). Control measures include weekly sprayings of
the plants during fl owering, alternating a systemic fungicide with a protectant;
spraying during the coolest hours of the day and applying a protectant as soon
as a noticeable weather change occurs are also recommended. Cleaning the
orchard fl oor by picking old leaves and fallen fruit, as well as weed control, will
also help to reduce the inoculum levels.
Powdery mildew (Oidium mangiferae) attacks leaves, stems and
infl orescences and is a common disease of the dry subtropics, and it can also
become serious, especially under drier conditions (Johnson and Coates, 1993).
Normally, the treatments to control anthracnose also control both fungi, and
treatment is necessary until the fruit reaches the size of a cherry.
A disease that has been reported from a number of areas and is very
serious in India, Pakistan and Egypt is mango infl orescence malformation.
Malformation occurs on vegetative shoots and fl owering panicles. Panicles
become short, branchy and compacted and produce solely male fl owers
(Shawky et al., 1980). Lower temperatures during panicle development are

Mango 281
Table 10.5. Some important diseases, disorders and conditions of mango.
Common name Organism Parts affected, symptoms Region or country
Anthracnose
Colletotrichum
gloeosporioides
Infl orescence; leaf black spots, lesions on
fruit
Universal
Powdery mildew
Oidium mangiferae
Leaves, infl orescence; whitish-grey
powdery spores
Universal
Mango scab
Elsinoe mangiferae
Blossoms, leaves, twigs, fruit; greyish-
brown spore masses, cracked tissues
Widespread
Bacterial black spot
Xanthomonas campestris
Leaves, stem and fruit; small watery lesions
that coalesce, causing necrotic spots 2–3
mm diameter with irregular borders
Subtropical cold areas
Infl orescence malformation
Fusarium moniliforme
Infl orescence compact, sex shift to
maleness; no fruit set; vegetative shoot
compact with small leaves
Egypt, India, Pakistan, Africa,
Americas
Stem-end rot
a
Diplodia natalensis,
Fusarium, Alternaria,
Cladosporium
Fruit; blackening of stem-end of fruit, skin
and pulp, over-mature fruit; rotting
Widespread
Sooty mould (not pathogenic)
Capnodium sp.,
Meliola sp.
Leaves, stem, fruit; sooty black appearance;
symptom of scale insects, mites and
mango hopper damage
Universal
Internal breakdown (jellyseed;
soft-nose)
Unknown Fruit; pulp breakdown Florida
a
Some similarity in symptoms; further studies required for clarifi cation.

282 Chapter 10
associated with a higher incidence. It is caused by Fusarium moniliforme, with
mites playing a minor role. A highly signifi cant reduction in malformation is
achieved, with an increase in yield, by spraying with GA
3
and NAA, singly and
in mixtures, with a second application 2 weeks later. Pruning all malformed
vegetative and fl oral fl ushes, followed by spraying with copper oxychloride
(4 g/l), is more e ective than pruning alone to reduce the percentage
of malformed panicles and increases the yield in the following season
(Azzouz et al., 1989).
Two other diseases with unidentifi ed causes can sometimes cause
serious loss, although little is reported in the literature. The fi rst a ects the
bark at the base of the trunk, with increased gummosis on the upper trunk
and large limbs. Wilting of branches occurs, followed by death, particularly
to trees around 8 years old. It is especially serious in Nayarit and Sinaloa
States in Mexico and has been reported from Colima State in Mexico and
from Guatemala. Both Fusarium and Phytophthora are suspected but have
not been confi rmed. The other condition is a disease of the fruit, with no
puncture wounds or insects being observed. The external symptom is a
black spot 0.5–1.5 cm in diameter, usually located at the nak of the fruit.
When the blackened area is cut, there is a hollow ‘tunnel’ leading to the seed
cavity. The seed and seed cavity are blackened. When the fruit is cut open,
many show the peduncular vascular system leading to the endocarp to be
disintegrated (Nakasone, 1979). It was observed to be extremely serious in
orchards of ‘Tommy Atkins’ in Nayarit and on ‘Kent’ in Campeche, Mexico,
and subsequently reported to occur in the Canary Islands and in Guatemala,
where it is called ‘pepita negra’. Lakshminarayana et al. (1985) reported a
similar preharvest stem-end disease of ‘Tommy Atkins’ in Mexico, except that
no mention was made of the black spot on the nak of the fruit. Preliminary
studies isolated a mixture of six species of Fusarium and produced more
diseased fruit with typical symptoms than any other single isolate. Bacterial
black spot (Xanthomonas campestris) of the mango appears to be a relatively
serious disease in South Africa and Australia. It has also been seen in
Hainan, China.
Insect pests
Mediterranean and Oriental fruit fl ies are spread widely throughout the world.
Other fl ies are confi ned to specifi c regions, but together they constitute a
major problem, particularly to the export trade (Table 10.6). Irradiation, hot
water and vapour heat treatment have been developed to meet disinfestation
requirements in importing countries. This is probably the most important
pest of mango, since the presence of a larval form in the fruit renders it non-
exportable to many countries. Exports are generally allowed to countries
where the winters are very cold and the fl ies do not survive. The female fl ies lay

Mango 283
their eggs in the developing fruit as it starts to ripen. Larvae produced by these
eggs feed on the pulp and move through the fruit, causing premature ripening,
rotting and fruit drop. The larvae eventually leave the fruit and bury into the
ground to become pupae; this phase lasts until the adults emerge, and the cycle
is repeated. Control measures include early harvest to shorten exposure time,
eliminating other host plants, collecting and burying fallen fruit, and the use
of traps to monitor adult populations. The release of sterile males can be very
useful in isolated areas or valleys or islands where in-migration of new fl ies is
di cult. Chemical control based on Malathion and hydrolysed protein bait is
also used. Bagging is used in some countries (Fig. 10.8b); though expensive
it results in fruit without fl y injury, very little or no anthracnose, better colour
and about 95% of the harvested fruit is saleable.
The mango seed weevil has been a major deterrent to mango export
to some countries. The weevil is an Old World pest, but is found now in
some parts of the New World: St Lucia, Guadeloupe and Martinique in the
Caribbean region (Pollard and Alleyne, 1986). Field sanitation, chemical
sprays, host-plant resistance, pest-free zones, fruit culling, using X-ray
technology and irradiation are possible solutions to this problem, unless
markets are found in temperate countries that do not require disinfestation. All
cultivars are susceptible, with some cultivars showing lower seed infestations
of 17% to a high of 86%. The weevil deposits its eggs on the surface in the
sinus region of small green fruit in the lower 2 m of the tree. Upon hatching,
the larva burrows through the soft pulp into the seed, goes through the pupal
stages feeding on the developing seed and then fi nally develops into an adult
weevil. When the fruit ripens and pulp decomposes, the adult beetles bore their
way out of the endocarp and enter diapause in cracks and crevices on the tree
until the next season. Since there is no external evidence of infestation and
the fruit normally remains edible, the consumer is unaware of their presence.
The weevil can a ect the appearance of the fl esh if the mature weevil burrows
out, causing decay, which hastens ripening and may even cause premature
fruit drop.
The mango hopper is a serious pest in India, the Philippines and some
other areas. The hopper sucks the sap from fl owering stems, causing them
to wilt. In serious cases, most of the panicles are damaged. Mango fl owers
are destroyed by four mango blossom midge species and others attack the
leaves (Table 10.6). One of these midges is a serious pest of mango fl owers
throughout the state of Hawaii, with several cultivars having 91% of the buds
infested, leading to perfect-fl ower abortion. Eradication by chemical sprays is
not feasible, due to the wide distribution and the large size of the trees, and
biological control is not an alternative as no predators have been reported in
the native habitats.
In Central America, cutting ants (Atta) can be a serious problem,
especially for young plants because they can completely defoliate the tree.
Control can include surrounding plants with an old tyre split in half and

284 Chapter 10
Table 10.6. Some important insect pests of mango.
Common name Organism Parts affected, symptoms Region or country
Mexican fruit fl y
Anastrepha ludens
Larval damage to fruit Caribbean
South American fruit fl y
Anastrepha fraterculus
Larval damage to fruit Americas
Caribbean fruit fl y
Anastrepha suspensa
Larval damage to fruit Caribbean, Florida
Queensland fruit fl y
Batrocera tryoni
Larval damage to fruit Australia
Mediterranean fruit fl y
Ceratitis capitata
Larval damage to fruit Widespread
Marula fruit fl y
Ceratitis cosyra
Larval damage to fruit Africa
Natal fruit fl y
Ceratitis rosa
Larval damage to fruit Africa
Oriental fruit fl y
Dacus dorsalis
Larval damage to fruit Asia, Hawaii, Philippines
Mango seed weevil
Sternochetus mangiferae
Seed India, Hawaii, Philippines,
S. Africa, South-east Asia,
Oceania, Caribbean
Mango blossom midge
Erosomyia indica
Dasineura mangifera
Sucking sap from fl oral parts India, Hawaii
Mango hopper
Idioscopus sp.
Sucking sap from fl owering shoots Philippines, India, Africa,
Oceania, Americas
Red-banded thrips
Selenothrips rubrocinctus
Sucking on underside of young leaves Widespread
Coconut bug
Pseudotheraptus wayi
Sucking sap from young fruit, watery spot
on fruit, fruit drop
Africa

Mango 285
half-fi lling this with water. Baits of chlorpyrifos insecticide used as pellets put
on the ant trails or at the entrance of their nest are used. The ants take the
pellets down the nest and as they come in contact with the fungus they feed
on they die. Another way is to pump chlorpyrifos powder into the nest with
a special blower; this will kill all ants if the product reaches all corners of the
nest.
Other insect pests, such as scale insects, thrips and the red spider mites are
found almost universally but they are relatively easily controlled by natural
enemies and by chemical sprays and do not usually pose any problems.
Weed management
Weed control is essential during orchard establishment. Young trees can be
grown under clean cultivation or sod. Frequently intercropping is practised
during mango establishment with papaya, pineapple or vegetables. Canopy
closure of maturing trees prevents weed growth.
HARVESTING AND POSTHARVEST HANDLING
Harvesting
Harvest maturity is determined by using criteria such as changes in colour,
fullness of cheeks and hardened endocarp. The most reliable indicator of
maturity is when the endocarp has hardened and there is a yellowing of the
fl esh near the seed; however, this is a destructive test. Immature fruit do not
ripen to full fl avour and should not be harvested, hence the importance of
maturity determination. Fruit-set dates can be established as an index for
harvesting. The fruit-set date for each tree is determined when the panicle
shows a high percentage of initial fruit set. An old recommendation to judge
the date of harvest was when the fi rst fruit dropped, the fruits on the tree were
ready to pick. The mango is harvested by hand from the ground, wherever
reachable, or from ladders and by the use of a long pole with a metal basket
or a cloth bag to hold two or three large fruit. Harvesting is also done using
two people, one on a ladder to cut the fruit and let it drop into the hands of the
other on the ground.
Postharvest treatments
Any form of bruising should be avoided during harvesting and transporting to
the packing house. Normally the fruit will be harvested with a 3–4 cm length
of peduncle; the fruit are put under the shade of the trees either on the ground
in small furrows or on a layer of sawdust or in specially designed boxes with

286 Chapter 10
the cut part of the peduncle pointing down for about 30 min until the sap
fl ow stops. In Australia, ‘Kensington Pride’ fruits are washed in the fi eld. The
use of shallow lug boxes minimizes bruising. At the packing house, fruits are
usually placed in a water bath or hand-washed to remove the stem sap from
the surface, and the peduncle is cut according to specifi cations. Sap removal
is essential to prevent sap burn and should be done within 24 h (Lovey et al.,
1992). Fruit anthracnose can be controlled by dipping into hot water (52°C,
5 min). A combination of hot water and a fungicide or chlorine may also be
used. When the fungicide is added to the hot water, the temperature can be
reduced slightly (Akamine, 1976).
Grade standards are usually based upon size, colour and freedom from
injury and defects. Other requirements include full development, freedom from
stains and fi rmness. Since the fruit is easily bruised, fruits are packed in single-
or double-layer cartons with adequate protective material or use of trays.
The US box contains about 4.5 kg and the EU box 5 kg. In the local markets,
mangoes are frequently packed in bamboo baskets or in wooden crates.
At ambient temperatures, shelf-life of this climacteric fruit is short: 7–14
days to fully ripe. Precooling to 10–13°C is benefi cial during hot weather or
when shipping is delayed. Fully ripe fruit can be stored at 8–10°C. The length
of shelf-life varies markedly with cultivar, maturity at harvest, injury, calcium
(Ca) sprays and exposure to ethylene. A dip in 4–6% calcium chloride can
signifi cantly increase shelf-life of some cultivars, with the response varying
with season, fi eld management practices and soil type. Fruit of ‘Keitt’, ‘Tommy
Atkins’ and ‘Muska’ from successive harvests show an increasing rate of
ripening changes during the 21 days’ storage period at 12°C, suggesting a
decrease in storage potential as the season progressed.
Mango is a climacteric fruit and ethylene can be used to reduce the time till
ripening commences. A treatment of 100 ppm for 24–48 h at 25°C and 90%
relative humidity (RH) is adequate. Acetylene generated from calcium carbide
and ethephon can also be used. Skin colour is also enhanced by ethylene
treatment by increasing degreening. The best ripening temperature range is
from 21 to 24°C. At high temperatures of 32°C, ripening can be retarded.
Controlled atmospheres have been tested on mangoes and indicate some
possibilities; storage in atmospheres of 5% oxygen (O
2
) and 5% carbon dioxide
(CO
2
) is possible for 20 days, while o -fl avours and skin discoloration occur at
1% O
2
or high CO
2
(15%). Cultivar di erences in response have been reported
and the extension in shelf-life may not be commercially viable. Controlled
atmosphere for ripe fruit is ine ective. Containers with controlled atmospheres
are available for transport. Modifi ed atmosphere storage using plastic bags
or wraps and waxing shows some delay in ripening. O -fl avours have been
reported with some wraps and waxes that delay ripening. Waxes are widely
and successfully used commercially on mango to reduce water loss.
Postharvest disorders include chilling injury, sap burn, internal
breakdown and bumpy tissue (Wainwright and Burbage, 1989). Chilling

Mango 287
injury is a storage disorder that occurs at temperatures below 12.5°C, the
extent of the injury being dependent upon the storage temperature and
duration: at 0°C injury occurs in 4 days, at 5°C in 8 days and at 10°C in 12
days. The symptoms include skin scald, failure to ripen and increased disease
susceptibility.
Sap burn caused by fruit skin contact with sap exuded from the cut or
broken pedicel reduces consumer acceptance because of the browning and
blackening of the skin after lenticel penetration. The Australian cultivar
‘Kensington’ is very susceptible (Lovey et al., 1992), while ‘Irwin’ is less
susceptible. The sap component in ‘Kensington’ thought to cause the burn
is the major non-aqueous terpene component, terpinolene; it can also burn
‘Irwin’, but in ‘Irwin’ the predominant terpene (6.8%) is car-3-ene. This sap
is present in the latiferous ducts of the fruit and is not interconnected with the
stem ducts. The latex is under some pressure and when the pedicel is broken
can shoot 300 mm or more. Harvesting with the stem attached, draining with
the pedicel down and washing are e ective.
One fruit disorder, occurring especially in ‘Alphonso’ in India, is referred
to as ‘internal breakdown’, ‘spongy tissue’ or ‘soft tissue’. The lower half of the
fruit is most a ected and it may be related to preharvest heat stress. ‘Soft nose’
in Florida is serious, with high Ca inhibiting the disorder and high N increasing
the disorder (Malo and Campbell, 1978). The ‘jelly-seed’ disorder is more
widespread. The disease usually appears during the initial stages of maturity,
with a loss of fi rmness of the pulp near the endocarp, which becomes jelly-like
and translucent with advancing ripeness. The disorder does not develop after
harvest. An open cavity may develop in the pulp at the stem end prior to pulp
breakdown. ‘Tommy Atkins’ has been reported to be especially susceptible to
this disorder, although ‘Kent’, ‘Irwin’, ‘Sensation’, ‘Carabao’, ‘Alohouron’ and
a few other commercial cultivars of importance are also susceptible (Campbell,
1988). No pathogenic organism has been detected. The only recourse for this
disorder is to harvest the fruit at the mature-green stage, before any colour
break occurs on the skin.
Certain cultivars are particularly susceptible to lumpy tissue, which is not
evident in green fruit but develops during ripening. The mesocarp contains
white starchy lumps and the fruit surface develops indentations. Aetiology is
unknown. It has been reported from Thailand and the Philippines. Internal
fruit necrosis fi rst appears as a brown area in the mesocarp and endocarp
of rapidly growing fruit. This later extends to the skin and a brown-black
gummy exudation occurs. These areas then collapse and are surrounded
by corky tissue. This non-pathological disorder has been associated with
boron defi ciency.

288 Chapter 10
Marketing
Consumer preferences vary, with the US market apparently preferring large-
sized and highly coloured mango without the turpentine taste, colour being
the more important. Mango marketing has taken on international dimensions,
with major marketing centres around the world. Canada and the USA are
the major markets in North America, while the major European Union (EU)
markets are located in the UK, France, Germany and the Netherlands. In the
Far East, Japan, Hong Kong and Singapore are lucrative markets for producing
countries such as the Philippines, Malaysia, Thailand and Pakistan. The
Philippine and Mexican mangoes dominate the Japanese market. The major
supplier for Hong Kong is the Philippines.
Countries supplying the North American and European markets are
Mexico, Ecuador, Brazil, Peru, Venezuela, Haiti, Jamaica and other Caribbean-
island countries in the Americas, and Ivory Coast, South Africa and Mali in
Africa. Egypt and Israel are small producers but are looking at the EU market
windows. Increases in demand will largely depend upon increasing consumer
familiarity with the fruit, quality and price structure. By virtue of geographical
location in the southern hemisphere, South Africa, Brazil, Peru and Australia
are able to supply mangoes to the North American, European and Asian
markets during their winter months, thus making mango a fruit that is
available year-round (Table 10.4).
UTILIZATION
The mango is rapidly becoming one of the leading trade crops in the tropics
and subtropics. As postharvest handling techniques and shipping technology
have improved, consumer demand has increased. The fruit is 60–75% fl esh,
11–18% skin and 14–22% seed, depending upon cultivar, with the fl esh
being ca. 20% dry matter. Most of the mangoes produced are marketed in the
fresh state for consumption as a dessert fruit. Fruit can be eaten green, and
this practice is very popular in Thailand, the Philippines and Central America,
with some starchy and crispy cultivars being preferred, such as ‘Khieo
Sawoey’ in Thailand. Fruit may simply be peeled and sliced. Diced pieces
may be added to salads and fruit cocktails. People consume mango simply
because of its pleasant taste and fl avour without much thought about the
content of minerals, vitamins, lipids and amino acids. However, the mango is
a good to excellent source of provitamin A and is considered a fair source of
vitamin C (Table 10.7), although this varies greatly among cultivars, with a
range between a low of 5 mg and as high as 142 mg/100 g of fresh material
(Wenkam, 1990).

Mango 289
A considerable amount of fruit is processed into various products, such
as jellies, jams, marmalades, pulp, juice and canned slices, throughout the
world. Green mangoes make excellent chutney and are being exported for
raw consumption by Latin American and other ethnic groups in the USA and
Canada. Canned mango slices have been processed in India since before 1925
(Hayes, 1966). Canned mango and dehydrated slices are important export
products in the Philippines and some other countries.
Table 10.7. Nutritive values of mango in a 100g edible portion (Wenkam, 1990).
Nutrient Units Haden Pirie
Proximate
Water g 84.12 79.97
Energy kcal
kJ
56
234
72
301
Protein g 0.39 0.55
Lipids (fat) g 0.02 0.20
Carbohydrate g 15.05 18.91
Fibre g 0.54 0.70
Ash g 0.42 0.37
Minerals
Calcium mg 8 6
Iron mg 0.16 0.16
Magnesium mg 12 12
Phosphorus mg 10 15
Potassium mg 159 126
Sodium mg 0 3
Zinc mg – –
Copper mg – –
Manganese mg – –
Vitamins
Ascorbic acid mg 15.10 15.00
Thiamin mg 0.041 0.081
Ribofl avin mg 0.057 0.061
Niacin mg 0.300 0.460
Pantothenic acid mg – –
Vitamin A RE
IU
381
3813
474
4735
Vitamin B6 mg – –
Vitamin B12 mcg – –

290 Chapter 10
FURTHER READING
Cull, B.W. (1991) Mangos crop management. Acta Horticulturae 291, 154–173.
Johnson, G.I. and Coates, L.M. (1993) Postharvest diseases of mango. Postharvest News
and Information 4, 27N–34N.
Litz, R.E. (ed.) (1997) The Mango: Botany, Production and Uses. CAB International,
Wallingford, UK.
Mendoza, D.B. and Wills, R.B.H. (eds) (1984) Mango: Fruit Development. Postharvest
Physiology and Marketing in ASEAN. ASEAN Food Handling Bureau, Kuala Lumpur,
Malaysia.
Ridgeway, E. (ed.) (1989) Mango Pests and Disorders: Information Series QI890O7.
Queensland Department of Primary Industries, Brisbane, Australia.
Scha er, B., Whiley, A.W. and Crane, J.H. (1994) Mango. In: Scha er, B. and Andersen,
E.C. (eds) Handbook of Environmental Physiology of Fruit Crops. Vol. II. Subtropical and
Tropical Crops. CRC Press, Boca Raton, Florida, pp. 165–197.
Wainwright, H. and Burbage, M.B. (1989) Physiological disorders in mango (Mangifera
indica L.) fruit. Journal of Horticultural Science 64, 125–135.

© CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1 291
(R.E. Paull and O. Duarte)
11
PAPAYA
Papaya (Carica papaya L.) is a popular fruit native to tropical America. It
is usually grown for its small to large melon-like fruit. It is a herbaceous
perennial, bearing fruit continuously at the leaf axils spirally arranged along
the single erect trunk. The papaya is also called papaw, pawpaw, papayer
(French), melonenbaum (German), lechosa (Spanish), mamao, mamoeiro
(Portuguese), mugua (Chinese) and betik (Malaysian, Indonesian).
BOTANY
Taxonomy and nomenclature
The cultivated papaya belongs to the family Caricaceae and is the only member
of the genus Carica. Caricaceae is a small family of dicotyledonous plants
with six genera; four of tropical American origin (Carica, Jarilla, Jacaratia,
Vasconcella) and one, Cylicomorpha, from equatorial Africa. Caricaceae species
have been variously classifi ed in families such as Cucurbitaceae, Passifl oraceae,
Bixaceae and Papayaceae. Approximately 71 species have been described,
though Badillo (1993, 2000) reduced the number to 32 species, with the
following distribution: Carica, 1 species, Cylicomorpha, 2 species, Jacaratia,
5 species, Jarilla, 3 species, Vasconcella, 20 species, and Horovitzia, 1 species.
Papaya (C. papaya L.) is the most important economic species in Caricaceae.
Carica and Vasconcella species are dioecious, except for the monoecious
Vasconcella monoica (Desf.) and some Vasconcella pubescens and the polygamous
C. papaya. Most species are herbaceous, single-stemmed and erect. Other than
C. papaya L., the other edible species are Vasconcella candamarcensis Hook. f.,
V. monoica Desf., Vasconcella erythrocarpa Heilborn, Vasconcella goudotiana Solms-
Laubach and Vasconcella quercifolia Benth. and Hook (Storey, 1969). These fruit
are mostly eaten cooked, being normally dry and lacking the juicy fl esh of C.
papaya. Another edible species, Vasconcella pentagona, is called ‘babaco’.

292 Chapter 11
Origin and distribution
C. papaya has not been found wild in nature and is only distantly related to
the Vasconcella species, based upon isozyme and AFLP analysis. The greatest
diversity in C. papaya exists in the Yucatan–San Ignacio–Peter–Rio Motagua
area of Central America. The volunteer population in this area has greater
diversity than domesticated populations (Morshidi, 1996; Van Droogenbroeck
et al., 2002). Papaya origins are rather uncertain, but there is some agreement
among botanists that it originated in the lowlands of Central America, between
southern Mexico and Nicaragua. Early distribution over a wide geographical
region in Central and South America was aided by the abundance of seeds in
the fruit and the seeds long viability. The accounts of 18th-century travellers
and botanists indicated that seeds of papaya had been taken from the
Caribbean to Malacca and on to India (Storey, 1941a). From Malacca or the
Philippines, distribution continued throughout Asia and to the South Pacifi c
region. Don Francisco Marín, a Spanish explorer and horticulturist, is credited
with the introduction of papaya into Hawaii from the Marquesas Islands
during the early 1800s. Papaya is now grown in all tropical countries and in
many subtropical regions of the world (Anonymous, 2003).
ECOLOGICAL REQUIREMENTS
Major commercial production of papaya is found primarily between 23° N and
S latitudes. Man has extended cultivation into regions as far as 32° N and S.
At these latitudes, papayas may be best grown in well-protected areas at sea
level. In Hawaii, at l9–22° N, papaya is grown at sea level and up to 300 m
elevation.
Soil
Papaya can be grown on a variety of soil types, with the most essential
requirement being drainage. A porous loam or sandy loam soil is preferred.
In Hawaii, the crop is frequently grown on rocky, volcanic soil called a’a,
composed of porous lava with some organic matter and excellent drainage;
the planting holes are fi lled with soil prior to planting.
Soil pH should be between 5.0 and 7.0, with the range between 5.5 and
6.5 being more desirable (Awada et al., 1975). At pH levels below 5.0, seedling
growth is poor and mortality high. In soils with a pH range of 5.0–5.5, lime
applications can increase growth and yield. Papaya can be grown successfully
on problem soils. For peat soils, high rates of lime application (6–8 t/ha) are
essential for cultivation. Micronutrients (boron, zinc and copper) are also
applied to ensure production of high-quality fruit on these soils. Since the

Papaya 293
water table is usually high in peat soils and other alluvial soils along streams
and canals, drainage is required. A major constraint is the tendency for trees
to lodge in these soils, especially for heavy bearers, because of poor anchorage
and limited root growth once fruiting commences. Cultivation of papaya on
sandy soils requires the incorporation of large amounts of organic matter
and heavy fertilization (8 kg/plant/year) coupled with irrigation. Boron
defi ciency and nematode infestations are two major problems encountered
on sandy soils. On acid-sulfate soils, which are compact, acidic, contain toxic
concentrations of certain micronutrients and prone to fl ooding, papayas have
a relatively short economic life. This short lifespan is due to a poorly developed
root system and extreme susceptibility to root and collar rot diseases under
poor drainage conditions. Similarly, heavy clay soils should be avoided due to
the poor drainage.
Climate
Rainfall
Papayas grow well and produce substantial yields without supplementary
irrigation if there is a minimum monthly precipitation of approximately
100 mm. Since most tropical areas have monsoon-type climates with
well-defi ned wet and dry seasons, successful production depends upon the
availability of supplemental irrigation during the dry period.
A minimum relative humidity of 66% has been reported for papaya’s
optimum growth. Stomatal opening is controlled by humidity and, as relative
leaf water content is not a ected by drought stress, stomatal closure maintains
leaf water status, allowing rapid return of gas exchange fl ux and growth upon
rewatering (Marler, 1994; Clemente and Marler, 1996). Drought frequently
leads to the rapid shedding of new fl owers and older leaves, and poor fruit
set. Five days of fl ooding leads to abscission of fully developed leaves which
is preceded by chlorosis. Flooding frequently leads to plant death, due to root
rots, while recovery from non-lethal fl ooding is slow, due possibly to the low
root growth rate of fruiting trees.
Papaya has been ranked from extremely sensitive to moderately tolerant to
salt stress. Germination and early seedling growth are the most sensitive stages
to salt stress. It is probably moderately salt sensitive at other growth stages.
Temperature
Optimum temperature for growth is between 21 and 33°C; if the temperature
falls below 12–14°C for several hours at night, growth and production are
severely a ected (Lange, 1961). Dioecious cultivars are better suited to low
temperatures (<20°C), as female trees do not exhibit stamen carpellody shown
by the more temperature-sensitive bisexual (hermaphroditic) cultivars (Fig.
11.1). Hermaphroditic cultivars (Solo type) grown at minimum temperature
294 Chapter 11
<17°C can have 100% carpellodic fl owers. At higher temperatures (>35°C),
bisexual cultivars often become functionally male, with poorly developed and
non-functional female parts. This tendency varies with cultivars and within a
cultivar. Net photosynthetic rate also rapidly declines above 30°C.
Temperature during the growing season signifi cantly infl uences fruit
growth and development from the normal 120–150 days (Fig. 11.2). The
e ect is most pronounced in subtropical areas. In these areas, fruit set does not
normally occur in winter, and fruit set before the winter can take up to 90 days
longer to reach maturity. Final fruit size is determined in the fi rst 4–6 weeks
of fruit development, and temperature plays a dominant role in the process,
especially in subtropical areas. Fruits that develop in the cooler season have
lower total soluble solids and fi nal fruit size.
Radiation
Papaya in its wild state is found developing as a rapid volunteer in areas
where the tree vegetation has been disturbed. The high saturation point above
1000 μmoles/m
2
/s (photosynthetic photon fl ux) (ca. 800 W m
-2
) supports its
rapid development in areas having direct sunlight in newly disturbed areas.
No photoperiodic e ects on tree growth, production or sex expression have
been reported (Lange, 1961). When exposed to shade, the plant is shorter,
Fig. 11.1. The percentage of true hermaphrodite, hermaphrodite with functional
stamens (male), and non-functional pistil and carpellodic fl owers at different times
of the year, on ‘Solo’-type papaya tree growing in Honolulu with a mean minimum
temperature of 21°C and maximum temperature of 27°C (Awada, 1958).
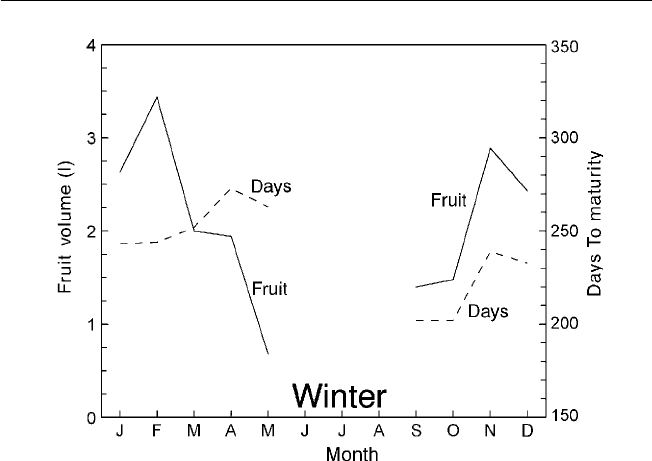
Papaya 295
having smaller leaf area, lower stomatal density, increased internode and
petiole length and increased chlorophyll content, and hence is regarded as a
shade-avoiding species. Partial stomatal closure and opening occur rapidly
with cloud-related changes in irradiance, maximizing water-use e ciency.
Windbreaks
Papaya trees are delicate and require protection from strong winds. When
the root system is well developed, though relatively shallow, the tree can be
uprooted by winds of 64 km/h, especially if the soil is softened by rain. Even
though trees can withstand uprooting, loss of leaf area leads to fl ower and
young fruit abscission, and low total soluble solids in the more mature fruit
on the column. Full recovery from wind damage to the leaves can take from
4 to 8 weeks.
GENERAL CHARACTERISTICS
Stem
The papaya is a large, monoaxial herbaceous plant with an erect stem
terminating with a crown of large leaves and can attain heights of 7–9 m
Fig. 11.2. The effect of date of fruit set on fi nal fruit size and days from anthesis to
the half-ripe stage of plants grown under subtropical conditions (Kuhne and Allan,
1970).

296 Chapter 11
(Fig. 11.3). Although there are occasional lines or cultivars that produce an
abundance of lateral branches, especially during the juvenile period, the main
stem normally grows without branching, unless the growing point is injured.
Natural growth of the axillary branches does occur when the trees become
3–5 years old. The stem is semi-woody and hollow and a major site of starch
storage. The bark is smooth, greyish in colour, with large, prominent leaf
scars. When the stem is wounded, a thin milky sap oozes from the wound.
After transplanting, shoot growth is initially slow, though considerable
root growth is taking place, extending out well beyond the canopy drip line.
Stem growth is then rapid up to fl owering, increasing in circumference up
to 2 mm per day. Growth rate peaks at fl owering then declines as the tree
starts bearing (Fig. 11.4). The rate of growth is infl uenced by nitrogen and
phosphorus supply, irrigation and temperature.
Leaves
A cluster of leaves occurs at the apex of the plant and along the upper part
of the stem and makes up the foliage of the tree. New leaves are constantly
formed at the apex and old leaves senesce and fall. Leaves are palmately lobed
with prominent veination and can measure 40–50 cm or more in diameter
and have an individual leaf area of 1625 cm
2
, with ca 15 mature leaves per
plant (Fig. 11.5). In the tropics, new leaves appear at a rate of two to three
a week (Chan and Toh, 1984); in Hawaii the rate is 2.4 per week during the
cool season and up to 3.0 in the warm season. Petioles are cylindrical, hollow
and 60–90 cm long, depending upon the cultivar. The most recently matured
leaf’s fresh weight (~10th leaf from 2.4 cm juvenile leaf) can vary from ca 50
to 170 g. The leaf petiole dry mass increases at a rapid rate until fl owering
then increases more slowly, peaking after fruit bearing starts (Fig. 11.5).
Infl orescence and fl owers
There are three primary groups of fl owers, i.e. pistillate, staminate and
hermaphrodite, with many variants, especially in the hermaphrodite group
(Fig. 11.3). Flowers are borne on modifi ed cymose infl orescences that appear
in the axils of the leaves. The type of infl orescence depends upon the sex of
the tree. The pistillate tree produces only pistillate fl owers on short, 4–6 cm
peduncles, with a functional pistil devoid of stamens (Fig. 11.3a). The fi ve
petals are separate but are inconspicuously fused together at the very base of
the ovary. In staminate trees, fl owers are sessile and are produced in clusters
on long, pendulant racemes 60–90 cm long. The individual fl ower is tubular
with ten stamens in two series of fi ve, attached to the throat of the corolla
tube, and lacks an ovary. The hermaphroditic form (Fig. 11.3b) is between the
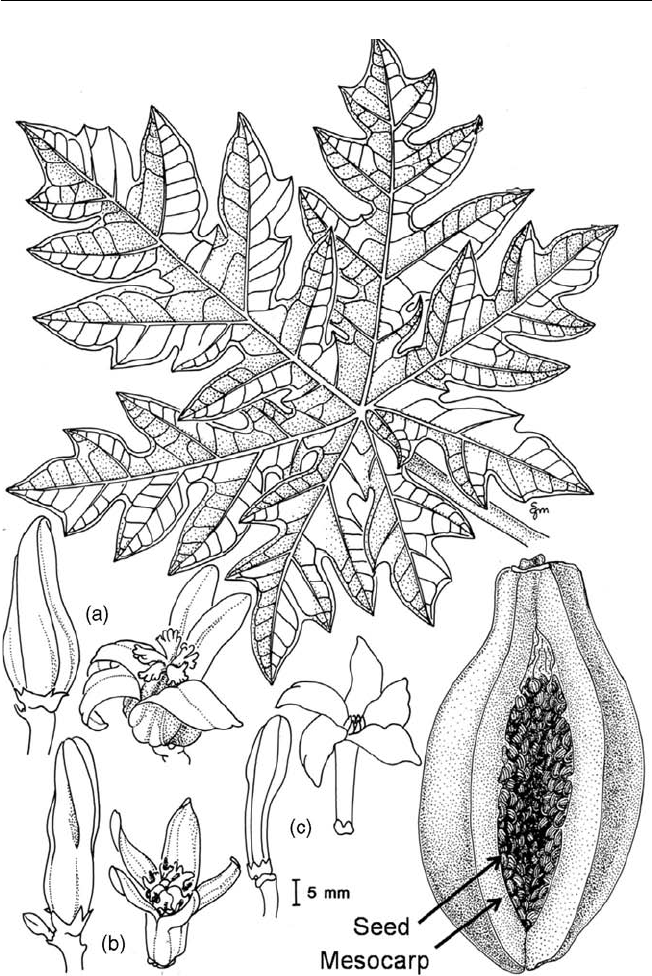
Papaya 297
Fig. 11.3. Papaya leaf, female (a), hermaphrodite (b) and male fl ower (c) types, and
fruit.
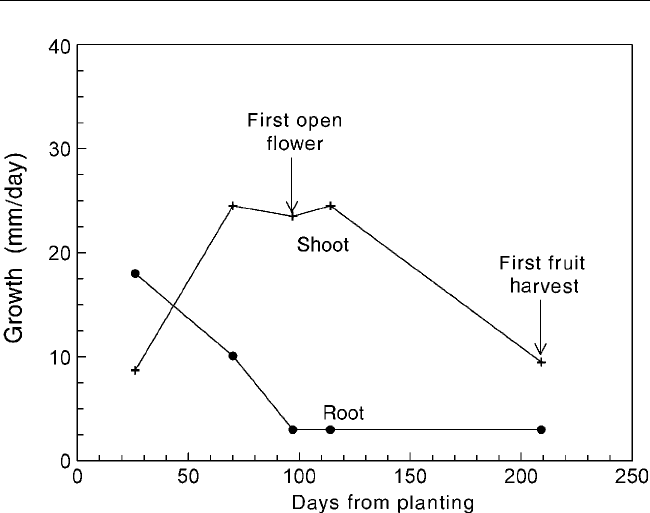
298 Chapter 11
two unisexual fl ower types and exhibits numerous deviations. The basic fl ower
on a short peduncle is characterized by an elongated pistil with fi ve stigmatic
rays and fi ve petals that are fused to about two-thirds of their length, forming
a corolla tube. There are ten stamens in two series of fi ve attached to the
throat of the corolla tube. The pistil is normally fi ve-carpellate and elongated,
oval or pyriform.
Taking into account the numerous deviations, Storey (1941b) classifi ed
papaya fl owers into fi ve basic types (Table 11.1). Type I: pistillate or female
fl ower devoid of stamens, with a distinct ovoid ovary terminating in a fi ve-
lobed stigma (Fig. 11.3a). Type II: hermaphrodite (pentandria) fl ower having
fi ve functional stamens and a globose fi ve-furrowed ovary (Fig. 11.3b).
Type III: hermaphrodite (carpelloid) fl ower having six to nine functional
stamens and an irregularly ridged ovary. Type IV: hermaphrodite (elongata)
fl ower having ten functional stamens and an elongate, smooth ovary. Type
IV+: hermaphrodite (barren) fl ower having ten functional stamens but
the pistil aborts, becomes vestigial and lacks a stigma (Fig. 11.3c). Type V:
staminate fl ower having ten functional stamens only. The ovary is completely
absent and fl owers are bunched in an infl orescence. Although fi ve basic
fl oral types are listed, certain male and hermaphrodite trees undergo sex
Fig. 11.4. The growth rate of papaya root and stem in the fi eld, showing root growth
rate reduction at the start of fl owering and shoot growth as fruits start to develop
(after Marler and Discekici, 1997).
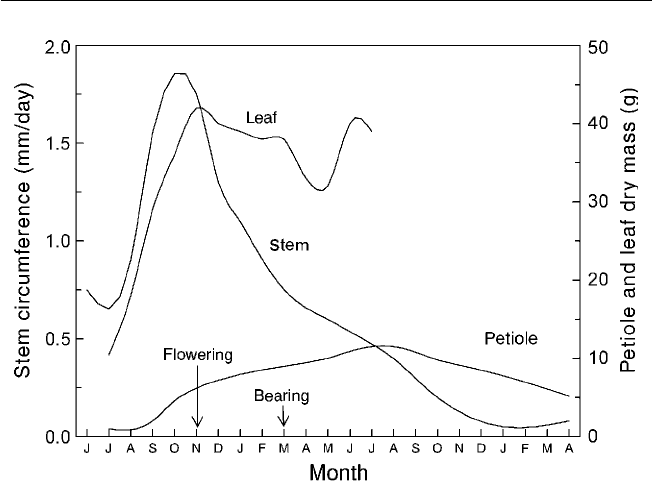
Papaya 299
reversal and morphological changes in various degrees under the infl uence
of environmental changes (Storey, 1958). Type V staminate fl owers may
form functional ovaries and bear fruit during cool months. Type III fl owers
can form carpelloid fruit when exposed to low temperatures about 40 days
before anthesis.
The appearance of a large number of modifi ed fl ower forms occurs in
progenies from appropriate hybridization when grown under a temperature
regime that may range from around 13 to 32°C. Therefore, recovery of
extreme sex modifi cations may not be seen in a breeding programme unless
conducted in an area with wide fl uctuations in seasonal temperatures in
highly heterozygous parental types. Stamen carpellody is expressed under cool
temperatures, with increasing severity as temperatures lower in the ca 40 days
before anthesis (Fig. 11.1). Instead of ten stamens in a double whorl, there are
only fi ve stamens, with the other fi ve fi xed to the normal carpels. The fruits
that develop from this carpellody are severely misshaped and unmarketable
(Fig. 11.6). Female sterility occurs under warm temperatures, again with
increasing severity at higher temperatures in the ca. 40 days before anthesis.
Excessive nitrogen and moisture also favour stamen carpellody, while plant
stress such as N defi ciency and moisture stress infl uences female sterility
(Awada and Ikeda, 1957).
Fig. 11.5. Growth rate of papaya stem circumference, dry weight of the most
recently matured leaf petiole and the 17th leaf from the 2.4 cm juvenile leaf under
dry land conditions of Puna, Hawaii. The dry weight reduction in April is attributed
to low rainfall in the previous 2 months (Awada, 1977).

300 Chapter 11
Table 11.1. Types of papaya fl owers (Storey, 1958).
Types Tree
a
Flower
a
Description
Staminate M M Typical unisexual fl ower on long peduncles.
Teratological
staminate
M M Found in sex-reversing male tree, with some
degree of carpel initiation and development.
A number of hair-like processes – vestigial
carpels – at base.
Reduced
elongata
MF M Modifi ed normal elongata fl ower differs from
staminate fl owers in having a thicker and
stiffer corolla tube, abortion of pistils, and
reduced ovary size and number of carpels.
Frequent warm periods and late summer; last
1–2 weeks up to 6 months, depending upon
cultivar and temperature.
Elongata
(normal type)
MF MF Elongata refers to the shape of the pistil,
terminating in fi ne stigmata; lobes develop
into pyriform or cylindrical fruit, fi ve laterally
fused carpels. Petals fused two-thirds of
length.
Carpelloid
elongata
MF F Transformation of the inner series of stamens
into carpel life structure. Numerous types,
different number of stamens, becoming
carpelloid and degree of carpellody from
slight to developing locules with functional
stigma. Fruit to varying degrees misshapen.
Pentandria MF F Normal hermaphrodite-type, modifi ed,
unisexual, pistillate fl ower through stepwise
stamen transformation to carpels, with loss
of the original carpels. Short corolla tube,
only fi ve stamens of the outer whorl on
long fi laments, globose and furrowed pistil.
Carpels from fi ve to ten.
Carpelloid
pentandria
MF F The stamens of the outer whorl become
carpelloid. Carpellodic forms in carious
stage, especially under cool conditions.
All fi ve stamens fully carpelloid and fuse
laterally, with abortion of original carpels;
fl owers resemble pistillate fl owers – pseudo-
pistillate.
Pistillate F F Unisexual fl owers larger than MF fl ower, lack
stamens. Form stable and unchanged by
environment.
a
M – male; F – female.
Papaya 301
The fl oral primordia of ‘Solo’ is laid down 50–70 days prior to anthesis,
at a rate of about one new fl ower in each leaf every 2–3 days along with
new leaves. Ovaries di erentiation begins 42–50 days prior to anthesis and
is completed within 28 days before anthesis. Stamen di erentiation begins
50–56 days before anthesis and is completed by 35 days prior to anthesis.
Female fl ower bud emergence to anthesis ranges from ~46 days in Hawaii to
80 days in India, with the wide discrepancy being due to temperature.
Pollination and fruit set
In hermaphrodite populations, wind pollination is minimal as the stamens
are packed inside the corolla tube and seldom protrude prominently out of
the fl ower (Fig. 11.3b). The hermaphrodite fl owers are cleistogamous, i.e. the
anthers dehisce and release the pollen prior to anthesis of the fl ower, leading
to self-pollination. Varieties such as ‘Sunrise’, ‘Kapoho’ and ‘Eksotika’ are
enforced self-pollinators, and seeds gathered from hermaphrodite fruit will
usually breed true to type. Self-incompatibility in cultivars is relatively rare,
though there are isolated cases when controlled self-pollinations are made.
Self-pollination in papaya also does not appear to result in any loss of vigour.
In mixed planting of pistillate and hermaphroditic trees or in purely
hermaphroditic stands, no pollination problems are experienced. Problem
occurs when dioecious cultivars are planted with an inadequate number
of male trees. In Australia, the recommended ratio of female to male is
Fig. 11.6. Stamen carpellody induced by environmental conditions (temperature,
water stress, fertilization) on young hermaphrodite trees signifi cantly alters fruit
shape. The mild forms are sometimes referred to as ‘cat face’.

302 Chapter 11
8:1. However, one male tree per 15–20 female trees provides adequate
wind pollination if male trees are located with respect to prevailing winds.
Parthenocarpy in papaya is rare. Seedless fruit or fruit with very low seeds can
be produced on female trees. These fruits are generally smaller in size. Auxins
have also been reported to induce parthenocarpic fruit.
Fruit set is no problem under open pollination in an orchard. On
hermaphrodite trees, it is most common for the terminal fl ower to set while the
laterals abscise. Under favourable conditions one or two laterals may be set and
only persist for 2–3 weeks or remain to produce undersized fruit that crowd
the fruit column. This crowding leads to fruit compaction and misshaped
fruit. Fruit thinning may be practised. The degree of compaction is due to the
peduncle length. Fruit set is variable; if lateral fl owers are not desired and only
one fruit per leaf axil is desired, set is usually 100%; if three fl owers are formed
and only one fruit is desired then the set is 33%. Annual fruit set depends upon
the length of the female sterility period in hot weather, and with one fruit per
peduncle the range is 85–95%.
Fruit
The papaya fruit is a fl eshy berry, from 50 g to well over 10 kg, and superfi cially
resembles a melon, being spherical, pyriform, oval or elongated in shape
(Fig. 11.3). Fruit shape is a sex-linked character and ranges from spherical
to ovoid in female fl owers to long, cylindrical or pyriform (pear-shaped) in
hermaphrodite fl owers. The skin of the fruit is thin and usually green when
immature, turning to yellow or orange when ripe. The fruit is normally
composed of fi ve carpels united to form a central ovarian cavity, which is lined
with the placenta, carrying numerous black seeds. Placentation is parietal,
with the seeds attached by 0.5–1 mm stalks. The seeds are dark grey to black
when mature and enclosed in a sacrotesta. The ovarian cavity is larger in
female fruit than hermaphrodite. The shape of the cavity at the transverse cut
ranges from star-shape with 5–7 furrows, to smooth and circular in shape.
In fruit development, all tissues in gynecia less than 1 mm are meristem-
atic. Later the outer layer of the epidermis increases in size while the
subepidermal layer continues to divide both anticlinally and periclinally. The
central parenchyma of the pericarp increases in size and divides, with the
placenta forming opposite the marginal vascular bundles. This meristematic
lasts 28–42 days and determines fi nal fruit size (Fig. 11.7). Fruit growth shows
two major phases. The fi rst lasts about 80 days post-anthesis, with a large
increase in dry weight occurring just before fruit maturity. Fruit development
takes 150–164 days, which is extended another 14–21 days in Hawaii in the
colder months. In subtropical areas, such as South Africa, development can
vary from 190 to 270 days (Fig. 11.2). The growth of the fl eshy mesocarp
parallels seed development and total fruit growth (Fig. 11.7).
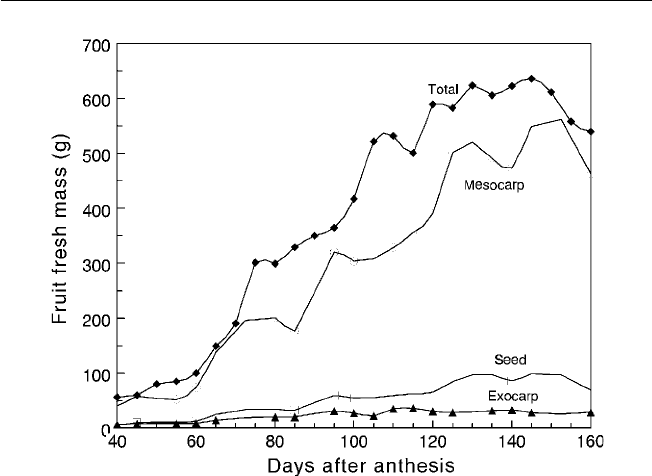
Papaya 303
Flesh colour is white in immature fruit to a pale orange-yellow, salmon
pink or red, depending upon cultivar, when ripe. Total fruit starch declines
from 0.4% to less than 0.1% during the fi rst 80 days of fruit development
(Fig. 11.8). Sugars, however, do not begin to accumulate until 110 days from
anthesis, during the last 28–42 days of fruit development. Flesh total soluble
solids can be as low as 5% up to 19%.
Green fruit contains an abundance of milky latex that contains the
protease papain. The pericarp consists of a network of lacticifers that develop
close to the vascular bundles and anastomose profusely throughout the fruit.
This latex is under pressure and spurts out when the skin is pricked. Laticifers
collapse as the fruit ripens and there is little or no latex at the fully ripe stage.
Commercially, the skin is scarifi ed to induce latex fl ow, which is allowed to dry
then collected to be later processed into papain.
CULTIVAR DEVELOPMENT
Cytogenetics and genetics
The species in the genus Carica possess nine pairs of chromosomes and a small
genome (375 Mb). Meiosis is normal in the three sex types and there are no
Fig. 11.7. The increase in papaya fruit total fresh weight and for skin, seeds and fl esh
(after Qiu et al., 1995).
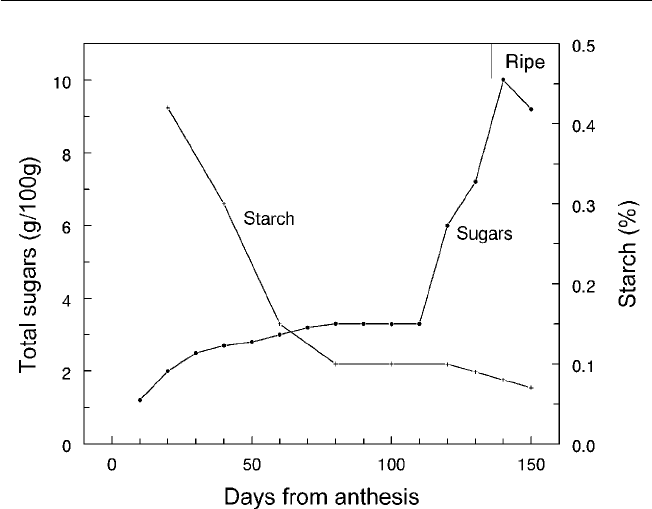
304 Chapter 11
known polyploid cultivars. Seeds of self-pollinated ‘Solo’ and ‘Eksotika’ and
the cross-pollinated varieties ‘Sekaki’ and ‘Khaek Dum’ can be reproduced
readily with good genetic purity, if care is taken in seed production. For the F
1
hybrids, such as ‘Rainbow’ and ‘Eksotika II’, seed is di cult to produce as two
inbred parents have to be crossed for production of the hybrid seeds.
Some Vasconcella species have resistance to diseases to which C. papaya
L. is susceptible, e.g. Vasconcella caulifl ora’s resistance to distortion ringspot
virus. This resistance has lead to attempts to cross these species. Successful
interspecifi c hybrids have been reported between other Vasconcella species but
not with C. papaya L. However, hybrids of C. papaya L. with V. caulifl ora and
with V. pubescens, V. quercifolia and Vasconcella stipulata were obtained using
embryo rescue techniques to overcome post-zygotic barriers to hybridization.
In vitro propagation coupled to rapid disease resistance screening, anther
culture to generate haploid papaya lines and Agrobacterium-mediated gene
transfer has been used to develop disease-resistant papaya lines.
Sex of papaya is determined by monogenic inheritance involving three
alleles (Hofmeyr, 1938). The alleles are M for male, M
H
for hermaphrodite and
m for female. All homozygous dominants, i.e. MM, MM
H
and M
H
M
H
, are lethal
to the zygotes. Therefore male genotypes (Mm) and hermaphrodite (M
H
m) are
Fig. 11.8. Changes in fruit starch and total sugar of developing ‘Solo’ papaya fruit.
Note the dramatic increase in sugars during the last phase of fruit development
(Chan et al., 1979; Chittiraichelvan and Shanmugavelu, 1979).

Papaya 305
enforced heterozygotes, while the female genotype (mm) is a double recessive.
Seeds derived from these cross-combinations will have twice the number of
hermaphrodites compared with females (Storey, 1938, 1941a). It is possible to
self and cross male trees when there is reversal of sex from a staminate fl ower
to a form that has a functional ovary, and these are used to develop inbred lines
of dioecious papayas. Sex is determined at fl owering time, usually 4–6 months
after planting. Molecular probes can be used to predict the sex of papaya at
seedling stage.
Fruit size shows wide variation, ranging from about 5 cm in diameter
and 50 g in weight to over 50 cm in length and 10 kg or more in weight.
Fruit weight is a quantitative character determined by multiple alleles (Chan,
2001). Fruit shape in papaya is a sex-linked character. The female fl ower
has a globose ovary, which develops into round or ovoid fruits. In contrast,
the elongata or hermaphrodite fl ower has a slender, tapering ovary and this
subsequently develops into a fruit that is elongated and cylindrical or pyriform
in shape, depending on the variety of papaya.
Precocity or earliness to fruiting is a factor of the number of nodes
produced to fi rst fl owering node, while the number of nodes to fl owering
and the internode length determine height to fi rst fruit. These characters are
governed by additive gene e ects, with the hybrid having the arithmetric mean
between the two parents (Nakasone and Storey, 1955). Subhadrabandhu and
Nontaswatsri (1997) reported that height of fi rst fl ower and number of nodes
to fruiting were controlled by both additive and non-additive genes.
Carpellody of the stamen is the development of misshapen or ‘cat-faced’
fruits due to fusion of the stamens to the ovary tissues in hermaphrodite
fl owers (Fig. 11.6). Carpellody and female sterility may involve a number of
gene pairs involving three loci, two for carpellody (c/c, c/c) and one for sterility
(s/s). Their normal alleles (+) are partially or completely dominant. The s/+
combination is normal. In c/+, the + is partially dominant over ‘c’, so there
will be some carpellody. The ‘s’ factor, whether homozygous or not (s/-), is
epistatic over the ‘c’ allele when the carpellody factors are heterozygous. The
phenotype would be normal but unstable, depending upon environmental
conditions. If either of the ‘c’ factors is homozygous (c/c), the combined
strength of the two alleles at one locus can overcome epistasis, thus exhibiting
carpellody. High heritability (h
2
= 82%) is obtained for carpellody, but e ective
phenotypic selection may be interfered with by the ‘change-in-rate’ type
of interaction between genotype and plant age (Chan, 1984). Increased
expression of carpellody may also be brought about by cool temperature, high
soil moisture and high nitrogen (Awada, 1961).
Flavour and odour can range from pleasantly aromatic, as in the ‘Solo’
and ‘Eksotika’ varieties, to musky, as in the ‘Maradol’ variety. Muskiness is
due to the homozygous recessive allele of a single gene (Storey, 1969). Total
soluble solids content is usually associated with sweetness and may range from
5% to about 19% total soluble solids (
0
Brix) in the ‘Solo’ varieties. This trait is

306 Chapter 11
governed by quantitative genes with additive e ects, and hybrids are expected
to have intermediate values between the parents.
Papaya fl esh colour ranges from intense gold to deep red, with varying
intermediate shades (Table 11.2). In Malaysia and many South-east Asian
countries, the preference is the red-fl eshed varieties, while Hawaii and
Australia prefer the yellow-fl eshed types. Flesh colour is governed by a single
gene, with yellow (R) being dominant over red (r). Varying intermediate
shades of pink may be attributed to modifi er gene e ects. All red-fl eshed (rr)
varieties, such as ‘Sunrise Solo’ and ‘Eksotika’, will breed true for fl esh colour,
but progenies of heterozygous (Rr) yellow F
1
hybrids, such as ‘Rainbow’, will
segregate in this trait.
The skin colour of papaya fruit is usually green when immature, changing
to yellow or reddish-orange when fully ripe. The ‘Morib’ is an interesting local
selection that has attractive yellow fruit skin even at an immature stage. The
tree is dwarfed and has yellowish-green foliage. Fruit skin colour is governed
by a single gene, with green (G) being dominant and yellow is expressed in a
double recessive (gg). It is di cult to identify the appropriate harvest index of
this fruit.
The old dioecious cultivars, such as ‘Hortus Gold’ (South Africa),
‘Sunnybank’ (Australia), ‘Betty’ and ‘Carifl ora’ (Florida) are cross-pollinated,
yellow-fl eshed and typically round fruit. ‘Carifl ora’ fruits are smaller and
tolerant to the ringspot virus disease. There are also gynodioecious varieties
that are cross-pollinated, such as ‘Sekaki’, ‘Khaek Dum’, ‘Maradol’ and
‘Cibinong’. ‘Sekaki’, also known as ‘Hong Kong’, is the second most popularly
cultivated cultivar in Malaysia after ‘Eksotika’. ‘Khaek Dum’ is Thailand’s best-
known variety and is a vigorous plant that bears red-fl eshed fruit weighing
about 1.2 kg with 10.6% total soluble solids content. ‘Maradol’ originates
from Cuba, is a short-stature variety that bears fruit very close to the ground.
The fruit weighs from 1 to 2 kg, attractive with fi rm red fl esh and 10–11%
Table 11.2. Preference of fruit characteristics in some countries.
Country Fruit size (g) Shape Flesh colour Race
Australia 800–1000 Cylindrical,
spherical
Yellow Dioecious
Cook Islands 500–1134 Pyriform Yellow Hermaphrodite
Fiji 397–510 Pyriform Red, yellow Hermaphrodite
Hawaii 397–510 Pyriform, oval Yellow, red Hermaphrodite
Mexico 1360–5443 Spherical,
elongate
Yellow, red Dioecious,
hermaphrodite
South Africa 1000–1500 Oval Yellow Dioecious
Caribbean
Islands
500–4000 Round, oval,
pyriform,
elongate
Red, yellow Dioecious,
hermaphrodite

Papaya 307
total soluble solids content. It has a characteristic musky fl avour and the fruit
is quite susceptible to anthracnose. ‘Cibinong’ is an Indonesian variety with
large red-fl eshed fruit (2–3 kg) and grown mainly for its high papain yield.
Commercial F
1
hybrids of papaya are rare, although there have been
reports of heterosis and improved yields in cross-combinations between
di erent cultivars of papaya. Agnew (1968) described a vigorous F
1
hybrid
derived from ‘Bettina 100A’ and ‘Petersen 170’ that was, at one time,
important in Queensland. An important hybrid developed in Taiwan that has
resistance to papaya ringspot virus disease is ‘Tainung No. 5’. This was derived
from a cross between ‘Florida’ (FL-77-5) and the ‘Costa Rica Red’. In Malaysia,
an F
1
hybrid called ‘Eksotika II’ was developed from the hybridization of ‘Line
19’ with its sib, ‘Eksotika’ (formerly ‘Line 20’) (Chan, 1993). The new hybrid
has similar features to ‘Eksotika’, but the yield is 14–33% higher due to the
larger fruit, weighing between 600 and 800 g. The appearance of the fruit is
more attractive, with smooth skin and high tolerance to freckles. The fl esh is
fi rmer and the fruit stores longer, making ‘Eksotika II’ more preferred than its
predecessor for export.
The world’s fi rst transgenic papaya was ‘SunUp’, which was transformed
with coat-protein-mediated resistance to papaya ringspot virus disease.
‘Rainbow’ is the fi rst transgenic commercial variety, developed from a cross
between ‘SunUp’ and the conventional cultivar ‘Kapoho’. Transgenic varieties
of ‘Kapoho’ and ‘Kamiya’ have also been developed by introduction of the
coat-protein transgene from ‘Rainbow’ through conventional hybridization
and backcrossing.
Breeding
Some papaya breeding objectives are common to all regions, with objectives
specifi c to di erent localities due to climatic di erences, consumer preferences,
desired sex types and export markets (Table 11.3). These objectives have
changed little in the last 100 years. Desirable tree characteristics are tree
vigour, low and precocious fruiting, minimum expression of stamen carpellody
and female sterility if hermaphrodites are preferred, resistance to diseases
and insect pests, and yielding ability. Universally desired fruit characteristics
are smooth skin, free from blemishes, fi rm fruit with thick fl esh, round seed
cavity, absence of internal lumps and long shelf-life. The desired fruit size,
shape and fl esh colour may di er in di erent regions (Table 11.2). An extreme
example would be a preference for papaya with the heavy musky aroma in
South-east Asia, which would not be suitable in western markets. Preference
for the small (450–650 g) ‘Solo’ fruit is increasing in tropical countries, where
export is considered. Requirements for processing cultivars are the same,
though larger fruit may be more desirable, with size and shape being uniform
if mechanization is to be considered. Flesh colour preference is usually based

308 Chapter 11
upon traditional experience and familiarity with cultivars. Total tree height
is not a good criterion to assess tree vigour, while stem diameter or girth is a
more reliable measure (Fig. 11.5). Stamen carpellody and female sterility do
not occur in dioecious cultivars.
In breeding for hermaphrodite types, selections should be evaluated
during the cool period for occurrence of stamen carpellody and again during
the warm period for female sterility. Trees that show no carpellody in winter
may show a high degree of female sterility in summer, or the opposite. Within
a specifi c locality, if temperature fl uctuations are not too wide, continual
selection and inbreeding can produce lines with minimum carpellody and
female sterility (Chan, 1984). In subtropical climates, where the range of
temperatures between summer and winter is large, dioecious cultivars are
more suitable.
Increased yields may be accomplished by increasing yields per tree, by
increasing density per unit area, or both (Hamilton, 1954). Usually only the
terminal fl ower sets fruit and others abscise or produce small, unmarketable fruit
when they set. However, lines have been observed to produce two, three or even
four normal-sized fruit on each peduncle. Multiple fruiting is strongly infl uenced
by soil fertility. Peduncles must be long enough to prevent overcrowding, which
can produce misshapen, ‘pancake-like’, fl at fruit. Another method to increase
production is by increasing trees per unit area. Petioles of papaya trees are
approximately 75–100 cm, or even longer, and are more or less horizontal. This
requires at least 2 m between plants in a row. A mutant ‘Solo’ line with very poor
yields and short petioles (45–60 cm) that are positioned obliquely upright is
available, and trees can be planted at 0.9–1.2 m apart in the row. Short, upright
Table 11.3. Ideals in papaya breeding have not changed much since the following
were proposed. Additional factors have related to disease resistance (Higgins and
Holt, 1914).
Character
Tree Vigour
Early and low fruiting – wide variation exists
Freedom from branching habits
Fruit Productive but not compact fruiting
Small size for table use
Large size for animal feed and papain production
Uniformity in shape, symmetry and smoothness
Uniformity in ripening
Colouring before softening
Colour of fl esh – yellow, pink or red
Easily separable without scraping fl esh
Flavour – not easily described but easily recognizable
Keeping quality
Other Papain yield

Papaya 309
petiole hybrids have been produced but the yield of the commercial cultivars has
not been achieved.
A common papaya disease is the root, stem and fruit rot caused by
Phytophthora palmivora Butl., which is especially severe during wet seasons.
Early studies in Hawaii involved repeated selection for seedling mortality and
vigour, and replanting each advance generation in the same fi eld resulted in a
few highly tolerant lines (Mosqueda-Vazquez et al., 1981). Cultivars showing
high tolerance to the Phytophthora root rot have also exhibited tolerance
to stem canker and fruit rot caused by the same organism. Other fruit
diseases that appear to be important enough to warrant breeding e orts are
anthracnose and chocolate spot, both caused by strains of C. gloeosporioides,
and stem-end rot caused by Phoma caricae-papayae.
The aphid-transmitted papaya ringspot virus (PRV) (papaya mosaic
virus or distortion ringspot virus) of di erent strains often limits commercial
production in most papaya-growing areas. Tolerant lines have been created,
and a dioecious cultivar named ‘Carifl ora’ with strong tolerance to PRV
has been released (Conover et al., 1986). Using this material, several highly
tolerant hermaphroditic ‘Solo’ selections have been made in Hawaii. More
rapid tolerance is achieved using molecular biology and the transformation of
papaya with the virus coat protein that confers resistance (Fitch et al., 1992).
Two resistant ‘Solo’ lines, one with yellow fl esh (‘Rainbow’) and the other with
red fl esh (‘SunUp’), have been released in Hawaii.
The hermaphroditic papaya is naturally self-pollinating, and continuous
inbreeding has not shown inbreeding depression. Also, F
1
hybrids between
‘Solo’ lines have not shown hybrid vigour, probably due to a close genetic
relationship, with many genes in common, or to the fact that the vigour is
only expressed under poor growing conditions. This narrowness in germplasm
has been confi rmed for ten Hawaii cultivars and three non-Hawaii cultivars
by DNA analysis at 80% similarity. Heterosis has been observed in F
1
s of
crosses between ‘Solo’ and widely di erent papaya accessions and between
interspecifi c crosses involving V. caulifl ora × V. monoica and V. goudotiana × V.
monoica (Mekako and Nakasone, 1975; Manshardt and Wensla , 1989a,b).
Cultivars
Wide variability is shown by the papayas grown in various countries; with
few exceptions, most cannot be classifi ed as horticultural ‘cultivars’ (Table
11.4). Plantings are usually heterogeneous and seeds are obtained from open-
pollinated fruit. A number of horticultural cultivars have been reported to
produce relatively uniform progenies (Table 11.5). Stabilizing characteristics
in dioecious cultivars is more di cult than in hermaphroditic ones, as the
genotype of the male with respect to the fruit characteristics is unknown. In
hermaphroditic cultivars, proper selection and self-pollination can stabilize

310 Chapter 11
characteristics at a more rapid rate. The Hawaiian ‘Solo’ type is perhaps one
of the few cultivars that has been continuously inbred since its introduction to
Hawaii in 1911 from Barbados (Yee, 1970). Solo cultivars such as ‘Kapoho’,
‘Sunrise’, ‘Sunset’ and ‘Waimanalo’ have been inbred for many generations.
Table 11.4. Papaya ‘cultivars’ reported in the literature; many are variable and not
true cultivars.
Country Cultivar Sex type Flesh colour
Australia Improved Petersen Dioecious Yellow
Guinea Gold Hermaphrodite Yellow
Sunnybank/S7 Dioecious Yellow
Richter/Arline Dioecious Yellow
Americas
Mexico Verde
Gialla
Cera
Chincona
USA – Florida Carifl ora Dioecious Yellow
Betty Dioecious Yellow
Homestead Dioecious Yellow
USA – Hawaii Kapoho Solo Hermaphrodite Yellow
Sunrise Hermaphrodite Red
Waimanalo Hermaphrodite Yellow
Rainbow Hermaphrodite Yellow
Venezuela Paraguanera
Roja Red
Caribbean
Barbados Wakefi eld
Graeme 5, and 7
Cuba Maradol Hermaphrodite Red
Trinidad Santa Cruz Giant
Cedro
Dominican Republic Cartagena Hermaphrodite Yellow
Asia Yellow
India Coorg Honey Dew Hermaphrodite
Coimbitor 2 Dioecious Yellow
Indonesia Semangka Hermaphrodite Red
Dampit Hermaphrodite Red
Malaysia Eksotika Hermaphrodite Red
Sekaki Hermaphrodite Red
Philippines Sinta Hermaphrodite Red
Taiwan Tainung No. 5 Hermaphrodite Red
Thailand Sai-nampueng Hermaphrodite Red
Khaek Dam Hermaphrodite Red
South Africa Hortus Gold Dioecious Yellow
Kaapmuiden – Yellow
Honey Gold Dioecious Yellow

Papaya 311
CULTURAL PRACTICES
Propagation
Papaya is almost entirely propagated from seeds in commercial cultivation.
Sound seeds usually germinate after 2 weeks in the polybags and are ready
to transplant at the 8–12-leaf stage, after about 6 weeks. The seeding rate for
papaya is very low as dry papaya seeds are light, weighing about 14.5 g per
1000 seeds. To establish a hectare of papaya, about 100 g of seed is required.
Approximately 100–150 g of seeds with 80% germination are needed to
produce the 2000 plants needed for one hectare.
Papaya seed can be harvested when the fruits reach ‘colour break’ stage.
Growers select trees with desirable characteristics, from which seeds, generally
from open-pollinated fruit, are saved. This seed produces uniform progenies
if from a single cultivar. The seeds are non-recalcitrant and can be dried to
moisture levels of 9–12% for long-term storage. Seeds harvested fresh from
fruit have very low and variable germination. Removal of the sarcotesta and
soaking in gibberellic acid promotes germination; uniformity of germination
is further enhanced by seed drying and cool-temperature storage at 15°C
for 30–50 days. Papaya seeds without the sarcotesta that are well-dried and
stored at 5°C retain 60–70% germination for 5 years (Chan and Tan, 1990).
Seeds may be sown in trays fi lled with a suitable medium, peat pots,
polyethylene bags or directly in the fi eld. Germination occurs in 12–20 days.
Seedlings are transplanted into 7.6 cm peat pots or into 10 cm plastic bags
Table 11.5. Fruit and tree characteristics of fi ve Hawaiian cultivars. Data are
averages from tests conducted at three locations (winter harvest only).
Characteristics Cultivar
Kapoho Higgins Line 8 Waimanalo Sunrise
Height to fi rst fl ower
(cm)
147 97 169 76 91
Fruit weight (g) 343 367 516 695 425
Soluble solids (%) 15.7 15.9 15.0 15.2 14.5
Flesh colour Yellow Yellow Yellow Yellow Red
Carpellodic fruit (%) 0 1.0 1.0 0.8 –
Other culls (%) 16 12 5 10 –
Marketable (kg/tree) 17 31 26 39 –
Phytophthora
resistance
a
ISRRI
Virus resistance
b
IT HT S S S
a
R = resistant; I = intermediate; S = susceptible.
b
HT = high tolerance; IT = intermediate tolerance; S = susceptible.

312 Chapter 11
at the two-leaf (cotyledonary leaves) stage. Seedlings grown in containers
should be hardened gradually in sunlight and fi eld-transplanted around 1.5–2
months after germination, at about 20 cm high. In fi eld planting up to 15–20
seeds are sown in each hole. Upon germination seedlings are thinned out to
leave fi ve or six seedlings to grow to fl owering. So many seeds are planted in
each hole to allow for birds and fi eld mice loss. At fi rst fl owering, a vigorous
plant of the desired sex is kept and the others removed.
Allan (1964) was the fi rst to report the success of propagating papaya by
cuttings. Large, leafy, lateral shoots that developed after winter were initially
used as cuttings for rooting under intermittent mist. In subtropical countries,
the cool winter checks growth and temporarily overcomes apical dominance,
resulting in the proliferation of lateral shoots. Availability of cuttings became
less season-dependent when they were induced from vigorous 1–2-year-
old trees by topping o the shoot terminus to remove the apical dominance.
The methods for induction and proliferation of suitable-sized lateral shoots
for cuttings were improved further with the application of cytokinin and
gibberellic acid mixtures. Vegetatively propagated papaya fl ower 1–3 months
earlier and are 30 cm lower bearing.
Scion shoots from cultivars ‘Co-1’ and ‘Honey Dew’ can be successfully
cleft-grafted on to uniformly established seedlings (Airi et al., 1986). Patch and
T-budding can also be used, but the success rate is poorer than cleft grafting.
Field grafting can be used to replace female trees. The union is established
in about 2–3 weeks and the female tree is cut back to about 60 cm from the
ground.
The early success of in vitro propagation of papaya was limited because
the sex of the seedling plants used could not be ascertained. Later, Litz and
Conover (1978) reported successful regeneration of papaya plantlets by
culturing apices of mature, fi eld-grown papaya plants in modifi ed Murashige
and Skoog media. A fi eld trial of in vitro plantlets indicated that they
propagated true to sex without somaclonal reversion (Drew, 1988). Besides
greater uniformity, the other benefi ts were earlier bearing, lower bearing
height and improved yield.
Field preparation
Field preparation in many areas is poorly done due to lack of appropriate
equipment or rough terrain. Subsoiling or ripping down to 50 cm or more
is desirable on heavy or compacted soils so roots can penetrate deeply (see
Fig. 3.2). Subsoiling provides better drainage if done parallel to the contour
lines. Discing, levelling and furrowing follow standard practice. Planting holes
30–45 cm in diameter are best dug with a soil auger attached to tractors.
Raised beds are used if there is a chance of fl ooding (see Fig. 3.3).

Papaya 313
Transplanting and plant spacing
After about 6–8 weeks in the nursery, the seedlings should reach the eighth
to twelfth-leaf stage and are most suitable for fi eld planting. In rainfed areas,
fi eld planting is done at the beginning of the rainy season. Seedlings grown
in pots and bags are planted directly, with the removal of the pot or bags.
Transplanted plants must be watered soon after planting to settle the soil
around the root system.
Spacing between plants and between rows varies widely. Universally
practised is the single-row system, with between plant spacing ranging from
1.8 to 3 m and between row spacing varying from 1.8 m to as much as 3.6 m
(see Fig. 3.7b). The between-row spacing largely depends upon degree of
mechanization; a standard tractor requires ca 3 m. The most frequently used
spacing is 2.0–2.5 m within row × 2.5 m, giving a density of 1600–2000
plants/ha. The double-row system, with 2 m between a set of rows and 3.5 m
between double rows, is used. Normally, two or three seedlings are planted
in each hole, spaced about 30 cm apart. When the plants start fl owering,
the female plants are removed, leaving only one hermaphrodite plant per
point. Polyethylene mulch over the beds prevents moisture losses and greatly
minimizes weed growth within the beds (Fig. 3.7b). Organic mulches of
grasses, wood shavings, rice hulls or other kinds of material are also benefi cial.
Irrigation
Non-irrigated fi elds in Hawaii are located in a region with 2500–3125 mm
of rain, fairly well-distributed throughout the year. However, occasional
droughts of 2 or 3 months occur. Low soil moisture tends to shift the sex type
to male and results in lower fruit yields, while high moisture levels can lead to
excessive production of misshaped carpellodic fruit, with rapid tree growth.
Dioecious cultivars fare better unless moisture stress is severe. A monthly
minimum rainfall of 100 mm is needed without supplementary irrigation
for some production. Irrigation should replace at least the moisture lost by
pan evaporation, and 1.25 pan evaporation is required for maximum yield of
mature trees (Fig. 11.9). Young trees only need about 0.3–0.5 pan evaporation.
Good production occurs with 60–90 l/tree/week immediately after planting or
during the wet season and 120–240 l/tree/week during the dry period.
Irrigation may be by fl ooding between the row space by furrows running
along both sides of the rows of trees or via microsprinklers or jets or drip.
Irrigation intervals of around 10–15 days may be necessary to sustain
production, unless this interval is broken by rainfall. Papayas are wind-
pollinated, and overhead sprinkling reduces pollen dissemination and
therefore is not recommended. A drip system must be capable of providing
the maximum amount rather than average demand. If a tree requires
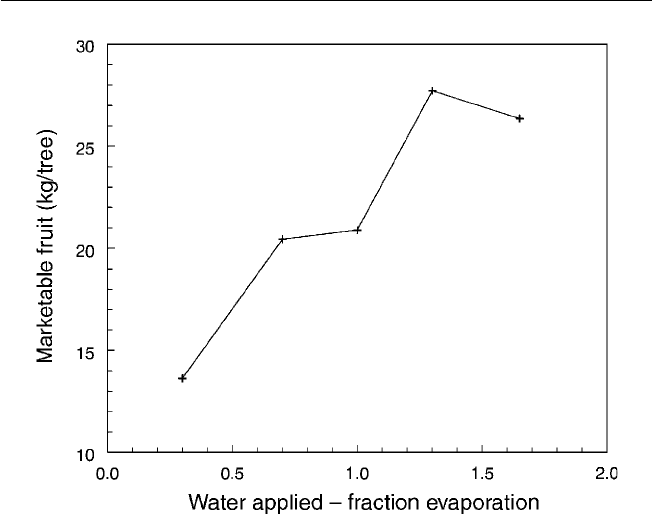
314 Chapter 11
56 l water/day but 114 l maximum during the dry season, the delivery system
must have the capacity to deliver the maximum requirement. One emitter per
tree has been su cient for the fi rst 3–4 months, but thereafter two emitters,
one on each side of the tree, have been found necessary. A single micro-
sprinkler (30 l/h) can also meet requirements.
Pruning
Normal tree pruning is not practised. Side shoots produced by some cultivars
during the juvenile stages or enforced when the plant apex is damaged are
removed as early as possible to maintain a single-stemmed tree. If the apex
is damaged or destroyed, one shoot nearest the terminal is maintained to re-
establish the tree. Some lower leaves are removed to facilitate harvesting,
improve penetration of spray materials and generally to increase marketable
yields (Ito, 1976). No statistical di erences are found in total soluble solids,
fruit size, number of fruits and marketable yield after a year of leaf pruning
when only 15 fully expanded leaves are retained. One fully expanded
mature leaf can provide photosynthetates for about 1500 g of fruit on the
column on ‘Solo’ varieties. Trees that have very large fruits (4–5 kg each)
Fig. 11.9. Relation between yield of marketable fruit and the water application as a
fraction of pan evaporation (Awada et al., 1979).

Papaya 315
may have fewer than ten fruits developing at a time on the fruit column, with
new fl owers abscising.
Fertilization
Papaya is a fast and continuously growing tree that provides fruits all the
year round. An abundant supply of nutrients at regular intervals is needed
to sustain good growth and production. In general, an adequate supply of
nitrogen and phosphorus should be provided during the early stages to ensure
optimum foliage, trunk and root development. However, at the fruiting stage,
the level of potassium should be raised, while the level of phosphorus should
be reduced, as high levels reduce fruit size. The level of nitrogen remains
somewhat unchanged through the juvenile and fruiting stages because foliage
is continuously produced. Tentative ranges of critical levels for bearing plants
of ‘Kapoho’ Solo are: N = 1.10–1.40%, P = 0.15–0.18% and K = 2.5–3.5%
(Awada and Long, 1980).
Papaya is sensitive to boron defi ciency, especially when cultivated
on sandy soil. Plants su ering from this defi ciency exhibit characteristic
symptoms: slight yellowing and downward curling of the leaf tips, with very
slight necrosis of the leaf tips and margins. The leaves are brittle in texture
and claw-like. During fruiting, secretion of milky latex often occurs on the
fruit surface, which subsequently turns brown in colour. At the later stage, the
fruit surface becomes rough or ‘bumpy’, giving the fruit an overall distorted
or malformed appearance. Treatment of boron defi ciency is by soil or foliar
application of borate.
Diversity of soil types, climatic conditions and practices make it necessary
to develop recommendations for specifi c areas based upon soil analysis and
preferably foliar analysis. In the sandy loam soils of North Moreton, Australia,
a pre-plant application of 0.5 kg of 5:7:4 complete fertilizer, 0.25 kg of single
superphosphate and 1.0 kg of lime per individual plant (over 1 m
2
area) is
recommended. For post-plant application, 100 g/plant of 10:2:16 complete
fertilizer is given at about 2-month intervals, with monthly application of
150–200 g per plant site during summer and autumn. In the second year,
250 g per plant is given at 2–3-month intervals. Because of boron defi ciency
problems in this area, 20 g of borax is applied at 3–4-month intervals and
30g/plant is given the second year at about 7–8-month intervals.
Pest management
Diseases
The importance of frequently reported papaya diseases (Table 11.6) varies,
and they may be widespread or localized. Phytophthora commonly causes

316 Chapter 11
collar and root-rot and sometimes stem canker and fruit rot in papaya. Papaya
is predisposed to the disease in areas with a high water table, poor aeration
and persistent high rainfall. Phytophthora with Pythium also cause seedling
‘damping-o ’, a serious disease during the nursery stage. Phytophthora root
rot is a major concern in all areas where the same land is used repeatedly
for production. Depending upon soil type and rainfall, the replant problem
may become very serious within two or three successive plantings, with
seedling mortality up to 45% (Nakasone and Aragaki, 1973). Alternatively,
virgin soil (soil never planted to papaya) is placed into each planting hole in
a replant fi eld; this allows the seedling to become established before the roots
penetrate beyond the virgin soil and they are then less a ected by root rot
fungus. Development of cultivars with strong tolerance through breeding
is slow, with the cv. ‘Waimanalo’ being such a product. Seedling damping o
immediately after germination is caused by a complex of organisms. The use
of sterilized germination medium with good aeration and control of moisture
are preventative measures.
Fruit diseases generally occur postharvest. Incidence of some fruit diseases
can be minimized by fi eld sanitation and fi eld application of appropriate
fungicides, while others can be reduced by careful handling of fruit, as these
organisms infect through wounds. Anthracnose, a preharvest infection fruit
surface rot, and stem-end rot, a harvest wound rot, are the two most common
postharvest rots of papaya. At the early stage of the infection, anthracnose,
C. gloeosporioides, is manifested as tiny dark spots on the skin of fruit, and if
left untreated, they form larger, depressed lesions when the fruits start to ripen.
In more severe cases, especially when the fruits turn full colour, the lesions
may coalesce with each other and the pinkish spores of the fungi are evident
in the depressed lesions. The infected fruit becomes soft, dark coloured and
unattractive.
Bunchy or malformed top is a relatively new disease of papaya. The
disease is a complex involving both thrips and a normally saprophytic fungus
Cladosporium oxysporum. Severely infected plants remain stunted and are
slow to recover. Symptoms are typifi ed by the appearance of new fl ushes
of leaves that are malformed, with leaf spots and ‘shot-holes’ (ranging from
1 to 3 mm wide). Short, light-yellow streaks (due to feeding by thrips) are
usually interspersed with the spots. The ‘Eksotika’ and ‘Solo’ varieties are very
susceptible at the juvenile stage.
The papaya virus disease, papaya ringspot virus (PRSV) has become the
limiting factor for commercial production in many areas. This disease has
been referred to as papaya mosaic virus (PMV) and distortion ringspot virus
(DRV). The fact that PRSV is suspected to be a number of a di erent viruses
is due to the variations in the expressed symptoms associated with di erent
races. Papaya ringspot virus (PRSV) has severely restricted papaya production
in Thailand, the Philippines and Taiwan and has also been recorded as the
major papaya disease in India, Hawaii, the Caribbean, Malaysia and Vietnam

Papaya 317
Table 11.6. Some important diseases and disorders of papaya.
Common name Organism Parts affected Distribution
Phytophthora blight
Phytophthora palmivora
Root, stem, fruit Widespread
Pythium root rot
Pythium aphnidermatum
Root Widespread
Damping off
Pythium aphanidermatum,
P. ultimum, Phytophthora
palmivora, Rhizoctonia sp.
Seedling stem at soil line Widespread
Collar rot
Calonectria crotalariae
Base of trunk, crown roots Hawaii, Thailand
Powdery mildew
Oidium caricae
Underside of leaves, petioles Widespread
Alternaria fruit rot
Alternaria alternata
Fruit body Widespread
Blackspot
Cercospora papayae
Fruit body Widespread
Anthracnose,
chocolate spot
Colletotrichum gloeosporioides
Ripe fruit body Widespread
Soft rot
Rhizopus stoloifer
Injured mature fruit at stem end Widespread
Stem-end rot
Fusarium solani
Wounded mature fruit at stem end Widespread
Phoma rot
Phoma caricae-papayae
Stem end, wounded area Widespread
Phomopsis rot
Phomopsis sp.
Stem end, wounded area Widespread
Stemphylium rot
Stephylium lycopersici
Stem end, wounded area Widespread
Fruit rots
Botryodiplodia sp.
Stem end, wounded area Widespread
Cladosporum sp.
Fruit body
Stem-end rot
Mycosphaerella sp.
Stem end
Continued

318 Chapter 11
Common name Organism Parts affected Distribution
Yellow crinkle Tomato big bud organism Leaves, fl owers Australia
Papaya ringspot
virus
Aphids: Myzus persicae, Aphis
gossypii
Leaves, stems and petioles, fruit ring-spots or
distorted
Widespread
Bunchy top
(mycoplasma-like
bodies)
Empoasca papayae (leafhopper)
Death of growing point Caribbean, tropical
America
Die back Unknown Yellowing and death of crown leaves Australia, Israel
Yellow crinkle Leaf hopper Yellowing of old leaves; crown leaves show
translucent areas
Australia
Table 11.6. Continued.

Papaya 319
(Table 11.6). In Taiwan, papaya is restricted to annual rather than perennial
production and cultivation and is often carried out in large net-houses to keep
out the disease-carrying, non-persistent aphid vectors.
The symptom on the fruit is dark green, concentric rings, hence the name
for the disease. On the younger stem and on the petioles, elongated, green,
water-soaked streaks appear. Infected plants also show chlorosis in the young
leaves, and the upper portion of the crown appears prominently yellowed.
Fruits are also low in sugar content with poor fl avour.
There are no control methods once the plant is infected. Eradication,
sanitation methods and isolating the papaya-growing area by a papaya-free
bu er zone allow papaya to continue to be grown. An orchard or growing
area isolated far enough from disease sources remains uninfected because by
the time aphids can move from the virus source to the healthy orchard, they
are non-infective due to the non-persistent characteristic of the virus in the
aphid. Also, curcurbit plants should not be interplanted in papaya orchards as
they are alternate hosts to the virus. A cross-protection method using a mild
strain confers some immunization, enabling plants to be 82% more productive
than unprotected trees in fi eld trials. Breeding and selection has resulted
in development of tolerant varieties such as ‘Carifl ora’, ‘Tainung No. 5’,
‘Sinta’ and ‘Eksotika’-derived breeding lines. The genetically modifi ed variety
‘Rainbow’ transformed with the virus coat protein gene has shown excellent
resistance to the disease in Hawaii. Transforming of papaya with the viral
coat protein has been successful; however, each virus race has a specifi c coat
protein, so di erent coat protein genes are needed in di erent areas.
INSECTS AND MITES
Many insects have been reported on papaya, but most are unimportant and
the damage is negligible or easily controlled. A few may cause severe problems
in localized areas (Table 11.7). Aphids, such as Aphis gossypii and Myzus
persicae, are important only as vectors of PRSV. Fruit fl ies are troublesome in
the export trade as papayas may need to receive a disinfestation treatment for
fruit fl y eggs and larvae before export. Rarely are fruit at the mature-green to
colour-turning stage infected; egg lay becomes a problem when fruits have
25% or more skin colour (Seo et al., 1982).
Several species of mites are known to infest papaya (Table 11.7), the
commonest being the red spider mite (Tetranychus cinnabarinus) and the broad
mite (Hemitarsonemus latus). The mite population increases dramatically
during the dry and cool spells, and they can be located on the undersurface
of emerging leaves and petioles. In severe infestations, the leaves are shed
prematurely and the shoot terminals su er die back. They also cause extensive
scarring (netted corky appearance) on the surface of the fruits when they feed
on the epidermal tissues.
Thrips (Thrips parvispinus) are common on papaya fl owers during the
blooming season. However, during the early stages of plant growth, they feed

320 Chapter 11
on the supple young leaves and shoot and provide the infection site for the
invasion by the saprophytic fungus C. oxysporum.
There are three species of scale insects known to infest papaya in Malaysia
but only two, i.e. the palm scale, Aspidiotus orientalis, and the oriental scale,
Aonidiella orientalis, are economically important. A. orientalis is considered the
most destructive. Both the nymph and adult feed on the leaf, stem and fruit.
A. orientalis is only found on the fruit and seldom on other parts of the plant.
Table 11.7. Some important insects and pests of papaya.
Common name Scientifi c name Parts affected Distribution
Melon fl y
Dacus cucurbitae
Fruit Widespread
Oriental fruit fl y
Dacus dorsalis
Fruit South-east Asia,
Philippines, Western
Pacifi c, Hawaii
Dacus melanotus
Fruit Cook Islands, South
Pacifi c
Mediterranean fruit
fl y
Ceratitis capitata
Fruit Hawaii, Mexico,
Central, South
America, Middle
East, Africa
Fruit fl y
Toxotrypana
curvicauda
Fruit American tropics,
Florida
American fruit fl y
Anastrepha
fraterculus
Fruit Subtropical, tropical
America
Caribbean fruit fl y
Anastrepha
suspensa
Fruit Florida, Caribbean
Green peach aphid
Myzus persicae
Virus vector Widespread
Red and black fl at
mite
Brevipalus
phoenicis
Fruit Widespread
Broad mite
Hemitarsonemus
latus
Emerging leaves,
leaves of young
seedlings
Widespread
Carmine mite
Tetranychus
cinnabarinus
Lower surface of
mature leaves
Widespread
Texas citrus mite
Eutetranychus
banksi
Mature leaves Widespread
Eutetranychus
orientalis
Mature leaves Thailand
Citrus red mite
Panonychus citri
Mature leaves Widespread
Monkeys Kenya, Barbados
Birds Four species Caribbean, Hawaii
Bats Vanuatu, South Pacifi c

Papaya 321
Nematodes
This pest, common only on sandy soils, damages the root systems and causes
stunting of the plants. Two nematodes, the root-knot nematode (Meloidogyne
incognita) and reniform nematode (Rotylenchulus reniformis), are the major
problems.
Weed management
Hand weeding, especially around young seedlings, mowing and mulching
are often used for weed control. Plastic mulch is very e ective against annual
broadleaved weeds but ine ective against perennial grasses or sedges. Post-
emergence herbicides such as glyphosate are currently widely used. Glyphosate
is e ective for a wide spectrum of weed species, and as a systemic herbicide its
e ects are not visible for a number of days. A common practice in some areas
is to interplant papaya orchards with tree crops that later become the principal
crop. Herbicides selected for the principal crop must also be compatible with
papaya and registered for use for both crops.
Orchard protection
Papayas develop extensive root systems but are vulnerable to strong winds,
especially when accompanied by rain. Windbreaks of appropriate shrubs or
small trees planted close together across prevailing wind directions can protect
the trees from severe damage. Selection of windbreak plants is best left to those
with local experience of the region.
HARVESTING AND POSTHARVEST HANDLING
Harvesting
Harvesting is easy when fruits can be reached by hand; as trees become
taller some form of harvesting aid, such as poles and ladders, must be used.
In Hawaii, most growers use a rubber cup or chisel-shaped metal tool with a
‘V’ attached to a long pole, which is placed against the bottom of the fruit or
against the peduncle and pushed upwards, snapping the fruit at the peduncle.
The falling fruit is caught by the picker with the other hand or removed from
the cup. The harvested fruits are accumulated in a bucket, tray or cloth
picking bag. These methods are possible only with the small ‘Solo’ fruit. When
the container is full, it is emptied into padded or lined bins left on fi eld roads.
An experienced harvester can harvest from 360 to 450 kg per 8-h day. Various
hydraulically operated mechanical harvesting aids, such as mobile platforms,

322 Chapter 11
are sometimes used. The harvesters stand on the platform, which is adjusted
to be at fruit height, as an operator slowly drives it down the rows.
The degree of ripeness for harvesting depends upon distance to markets.
Fruits may be one-quarter to one-half ripe for local markets. Fruits to be
transported long distances or exported are harvested at colour break to one-
quarter ripe, depending upon the cultivar’s ripening characteristics and
season. Colour assessment is based upon the judgement of pickers. The Hawaii
grade standard requires fruits to have 11.5% total soluble solids with the
colour-break stage normally meeting this standard. Green immature fruit do
not ripen well and have a total soluble solids value that is frequently less than
10%, giving a bland taste.
Postharvest treatment
The need for postharvest treatments depends upon the importing countries.
Overseas markets require disinfestation treatments to eradicate fruit fl y larvae
and eggs from the fruit before shipment. The vapour heat method was the
fi rst disinfestation treatment used in the 1950s, and after the banning of the
fumigant ethylene dibromide in 1984, vapour heat and forced heat treatment
again became the industry standard. The fruit core temperature in these
heat treatments reaches at least 47.2°C and the total treatment time is about
7 h (Paull and Chen, 1990). The alternative method is irradiation at a dose
of about 250 Gy. This low irradiation dose sterilized the fruit fl y larvae and
eggs, preventing them from completing their life cycle. Irradiation at 250 Gy
does not provide postharvest disease control and needs to be coupled with a
postharvest disease control programme.
A fungicide–wax combination is recommended prior to packing (Paull and
Chen, 1990). Incidence of storage diseases can be reduced by fi eld spraying
and proper care in harvesting and handling to avoid wounding and bruising.
Skin injury is a major problem and is caused mostly by impact and abrasion
during harvesting (Quintana and Paull, 1993). The latter is mainly caused
when harvested fruits are being dropped into fi eld bins with rough side walls
and bottom. Careful handling is essential to avoid these unsightly blemishes,
which provide an invasion site for postharvest rots.
Following the disinfestation treatment, papayas are packed into cartons in
a screened area to prevent re-infestation by fruit fl ies. Defective fruits are culled
before and/or after disinfestation and fruits are graded for size and colour
and hand-packed into cardboard cartons with a capacity of about 4.5–10
kg. Cartons from areas requiring insect disinfestation are fully sealed to meet
regulatory requirements, while fruits from other areas can be in open-topped
cartons. Count size ranges from 6 to 18, depending upon fruit and carton size.
Fruits are marketed as colour break, one-quarter ripe, half-ripe and three-
quarter ripe and are normally ready to eat when there is three-quarters or

Papaya 323
more colour on the skin. Foam mesh sleeves, foam padding on the bottom
of the carton or paper wrapping prevent abrasion injury, which is a major
problem in fruits still having green areas of skin.
Room cooling and forced air cooling are most commonly used to pre-cool.
Hydro-cooling is possible; however, rapid cooling after insect disinfestation
treatments can lead to skin scalding. Storage recommendations are in the
range of from 7 to 13°C and from 90 to 95% RH. At 7–10°C, storage life is
limited by chilling injury, while at 10–13°C ripening slowly occurs (Chen and
Paull, 1986). Papaya fruit at colour-turning (break) stage can be stored at 7°C
for 14 days and will ripen normally when transferred to room temperature
(Fig. 5.3). Chilling injury symptoms include skin scald, hard lumps in the
pulp around the vascular bundles and water-soaking of fl esh. Fruits become
progressively less susceptible to chilling stress as they ripen, and the symptoms
occur after 14 days at 5°C for mature green fruit and 21 days for 60%
yellow fruit. Skin scald can be induced in colour-break fruit after chilling at
1°C for 24 h.
Shelf-life extension of 1–1.5 days was obtained when papaya were
stored at 12°C in 1–1.5% oxygen for 6 days. Low oxygen (1.0–5%), with or
without high CO
2
(2–10%), reduces decay and delays ripening. High CO
2
(30%) adversely a ected internal colour, aroma and fl avour, while there is
no residual e ect of 10% CO
2
on decay control, though skin degreening is
delayed. At 10°C, fruit could be stored for 36 days in 8% CO
2
, 3% O
2
and still
have 5 days at 25°C for retail. Ethylene removal prior to storage has shown
variable results. Controlled atmosphere has not shown dramatic improvement
in storage life and the economic cost of the treatment needs to be balanced
against the improvement in fruit storage life and quality.
The optimum temperature for fruit ripening is between 22.5 and 27.5°C,
with fruit taking 10–16 days to reach full skin yellowing from the colour-break
stage (An and Paull, 1990). Severe weight loss and external abnormalities
become signifi cant at temperatures higher than 27.5°C. Fully ripe fruit at the
edible stage can be held at 1–3°C. Loss of ca. 8% of initial weight from colour-
break papaya produces ‘rubbery’, low-gloss, unsaleable fruit.
Marketing
Most papayas produced in the tropics are consumed locally, due to long
distances to markets and the di culty in handling the large fruit size.
Marketing standards for trade are normally set by agreement between the
shipper and the wholesale or retail buyer. In Hawaii, standards for Hawaiian-
grown papaya have been developed. Fruit must conform to the ‘Solo’ type,
with similar cultivar characteristics as to size and shape, must be mature
with a defi nite tinge of yellow at the blossom end, must be clean, well-formed
and meet the total soluble solids standards, averaging not less than 11.5%

324 Chapter 11
for any lot of papayas, provided not more than 5% by count of fruit in the
lot have soluble solids less than 10.5%. Fruit is sized into the following size
classifi cations: small 284–369 g; medium 369–454 g; large 454–907 g; and
extra large over 907 g. A major problem with the standard is the di culty in
achieving same size and skin colour that will ripen together in the carton.
Other typical requirements include that the fruit must be free from decay,
breakdown, internal lumps and other undesirable characteristics and free
from various categories of injury, including insect and mechanical injury.
WORLD PRODUCTION AND UTILIZATION
Production
Thirty-eight countries are reported to produce papaya. Six countries produce
a minimum of 100,000 MT/year. Brazil is the largest producer, followed by
Mexico, Indonesia, India, Zaire, Taiwan and the Philippines (Anonymous,
2003). World production is in excess of reported fi gures, as considerable
production occurs in small island countries in the tropics; backyard and
subsistence-farm production and that consumed locally may not enter into
the world production statistics.
Utilization
The fruit is the major product from this species, though young leaves and
male fl owers are used as vegetables and in soap. Nutritionally, the papaya is a
good source (Table 11.8) of calcium (30 mg/100 g), and an excellent source
of provitamin A and ascorbic acid (Wenkam, 1990). Papayas are consumed
fresh as breakfast fruit, dessert or in salads. In Asia, green fruits are cooked
as a vegetable or made into preserves. Papayas are also processed into various
forms, such as dehydrated slices, chunks and slices for tropical fruit salads and
cocktails, or processed into puree for juices and nectar base, usually frozen,
and as canned nectar, mixed drinks and jams. Papaya puree is the basis for
remanufacturing of many products. Papaya puree is processed aseptically
or frozen, with yield of ca. 50%. The fl avour of aseptically processed puree is
stable during processing and after 6 months in ambient storage, with some
colour changes noted.
Papain is a proteolytic enzyme that digests proteins and is used as a
meat tenderizer, as a digestive medicine in the pharmaceutical industry, in
beer brewing, tanning industries and in the manufacture of chewing gum.
Papain production is primarily centred in Tanzania and India, where labour is
abundant and inexpensive. The latex is obtained from green papaya by making
about four surface lancings on the fruit and catching the drippings in cups,

Papaya 325
with yields of 88.5–227 g/tree of dried latex per year. Approximately 2.27 kg
of fresh latex will produce 0.45 kg of dried latex.
In processing papayas 22% of the waste is seed. The seed oil content
of 33% on a dry weight basis is considered high when compared to seeds of
other fruit. Protein content of 29% on a dry weight basis is comparable to that
of soybean, with 35%. Papaya seed meal, with 40% crude protein and 50%
crude fi bre, appears to be a potentially rich animal feed. A salad dressing is
also made from ground papaya seeds.
FURTHER READING
Alvarez, A.M. and Nishijima, W.T. (1987) Postharvest disease of papaya. Plant Disease
71, 681–686.
Gonsalves, D., Vegas, A., Prasartsee, V., Drew, R., Suzuki, J.Y. and Tripathi, S. (2006)
Developing papaya to control papaya ringspot virus by transgenic resistance,
intergeneric hybridization, and tolerance breeding. Plant Breeding Reviews 26,
35–78.
Table 11.8. Nutritive value of hermaphrodite ‘Solo’-type papaya (Wenkam, 1990).
Amount per 100 g
edible portion
Proximate
Water (g) 87
Energy (kJ) 192
Protein (g) 0.39
Lipid (g) 0.06
Carbohydrate (g) 12.2
Fibre (g) 0.58
Ash (g) 0.57
Minerals
Calcium (mg) 30
Iron (mg) 0.2
Magnesium (mg) 21
Phosphorus (mg) 12
Potassium (mg) 183
Sodium (mg) 4
Vitamins
Ascorbic acid (mg) 84
Thiamine (mg) 0.03
Ribofl avin (mg) 0.04
Niacin (mg) 0.33
Vitamin A (IU) 1093

326 Chapter 11
Kim, M.S., Moore, P.H., Zee, F., Fitch, M.M.M., Steiger, D.L., Manshardt, R.M., Paull, R.E.,
Drew, R.A., Sekioka, T. and Ming, R. (2002) Genetic diversity of Carica papaya L. as
revealed by AFLP markers. Genome 45, 503–512.
Marler, T.E. (1994) Papaya. In. Scha er, B. and Andersen, P.C. (eds) Handbook of
Environmental Physiology of Fruit Crops Vol. 2. Subtropical and Tropical Crops. CRC
Press, Boca Raton, Florida, pp. 216–224.
Nakasone, H.Y. (1986) Papaya. In. Morselise, S.P. (ed.) CRC Handbook of Fruit Set and
Development. CRC Press, Boca Raton, Florida, pp. 277–301.
Paull, R.E., Nishijima, W., Reyes, M. and Cavaletto, C. (1997) A review of postharvest
handling and losses during marketing of papaya (Carica papaya L). Postharvest
Biology and Technology 11, 165–179.
Zhou, L., Christopher, D.A. and Paull. R.E. (2000) Defoliation and fruit removal e ects
on papaya fruit production, sugar accumulation, and sucrose metabolism. Journal
of the American Society for Horticultural Science 125, 644–652.

© CAB International 2011. Tropical Fruits, 2nd Edition, Volume 1 327
(R.E. Paull and O. Duarte)
12
PINEAPPLE
Pineapple (Ananas comosus (L) Merr.) is also called piña (Spanish), abacaxi
(Portuguese), annachi pazham (Tamil) or nanas (Malaysian), with many
languages using the South American Tupian language name ananas. This
is the only species in the bromeliad family grown commercially for its fruit.
The fruit was called ‘piña’ by 15th-century Spanish explorers because of its
resemblance to a pine cone. The fruit develops by fusion of fl oral parts (Fig.
12.1). Pineapple is eaten fresh and canned, and the juice is sold singly and in
combination with other fruit juices. Cut pieces are used as a dessert, in salads
and cooked meat dishes, and in fruit cocktail mixes. Today, the pineapple is
found in almost all the tropical and subtropical areas of the world, and it ranks
third in production of tropical fruits, behind bananas and citrus.
BOTANY
Introduction
Pineapple is in the bromeliad family, which has about 45 genera and 2000
species. The Bromeliaceae originated in tropical America except for one species,
Pitcairnia felicana (Aug. Chev.) Harms & Mildbr., a native of tropical West Africa
(Collins, 1960). Plants are herbaceous or shrubby and classifi ed as epiphytic
or terrestrial. Pineapple (A. comosus (L.) Merr.) is by far the most economically
important bromeliad. Other species of Ananas and Bromelia yield edible fruits:
Ananas bracteata (Swartz) Grisebach, Ananas kuntzeana Mez., Ananas longifolia
(Rudge) L.B. Smith & M.A. Spencer, Ananas nudicaulis (L.) Grisebach, Bromelia
antiacantha Bertoloni, Bromelia balansae Mel., Bromelia chrysantha Jacquin,
Bromelia karatas L., Bromelia hemisphaerica Lamarck, Bromelia nidus-puellae
(André) André ex Mez., Bromelia pinguin L., Bromelia plumieri (E. Morren) L.B.
Smith, and Bromelia trianae Mez. The more common are consumed locally,
under names such as cardo or banana-do-mato (bush banana), piñuelas
(small pineapple), or karatas, gravatá and caroata (derived from Amerindian
328 Chapter 12
names given to terrestrial bromeliads). For example, B. balansae Mel. is called
gravata in Brazil and it has 80–120 fruit per plant, each weighing 6–14 g;
the skin is brown and the fl esh is white when ripe. Many other bromeliads
are cultivated as ornamentals, gathered for fi bre extraction or used in
traditional medicine.
Open
Flowers
Floral
Bract
Fig. 12.1. Pineapple infl orescence and fl ower, and fruit. The small purplish fl owers
open from the base of the infl orescence. The fruit is composed of fused fruitlets and
the skin of sepals partially covered by a bract.

Pineapple 329
Origin and distribution
The pineapple was fi rst seen by Europeans when Columbus and his men
landed on the island of Guadaloupe during the second voyage in 1493. Early
exploration by botanists in South America indicated the area of origin to be
south-eastern Brazil, Paraguay and northern Argentina, because of the
abundance of wild species. Based on materials collected in South America,
Leal and Antoni (1980) proposed an area further north, between 10° N and
S latitudes and 55–75° W longitude. This general area includes north-western
and eastern Brazil, all of Colombia and Guyana, and most of Venezuela.
At the time of Columbus’s arrival, the pineapple was already widely
distributed throughout most of tropical America. The antiquity of this fruit
even at that time is evidenced by the presence of distinct types, all of which
were nearly or completely seedless. Its wide uses as food, wine and medicine
at the time of Columbus’s arrival in the Americas and the absence of
recognizable wild progenitors of the cultivated pineapple are further evidence
of pineapple’s antiquity (Collins, 1948). Distribution of pineapple from the
Americas is attributed to Spanish and Portuguese explorers and was aided by
the resistance of crowns and slips to dessication. Pineapple was introduced
into Africa at an early date and reached southern India by l550. Before the
end of the l6th century, it had become established in China, Java and the
Philippines (Collins, 1949).
At least 79 countries in the tropics and subtropics produce measurable
quantities of pineapple (http://www.fao.org, 2008). Yields (kg/ha) are highly
variable and, for those countries producing more than 5000 MT, range
from 8 t/ha in Nigeria to 47 t/ha in Australia. This great variation in yields
results from a number of factors. Small farms in countries that use the best
technology tend to have higher yields than do large plantations utilizing the
same technology, because in-fi eld losses are easier to control on the small farm.
Yields, on average, are much lower in developing countries, where access to
inputs is limited by lack of capital. The exception occurs when a multinational
corporation establishes a plantation in a developing country where labour
costs are low. Cultivar di erences also contribute to variations in yields. Where
the predominant cultivars are ‘Smooth Cayenne’ and the Pineapple Research
Institute of Hawaii hybrid clones 73-50 and 73-114 (also known as MD-2,
‘Gold Extra Sweet’, MG-3 and ‘Mayan Gold’), yields are higher, probably more
than twice as high even with the best technology, than are the spiny-leaved
‘Queen’ and ‘Spanish’ cultivars (Table 12.1). Prevailing climate also has a
moderating e ect on yields, with yields at the same level of technology being,
on average, lower in the warm tropics than at higher latitudes.

330 Chapter 12
ECOLOGY
Major areas of commercial cultivation are found between 30° N and
S latitudes, with some areas considered marginal for various reasons
(Bartholomew and Malézieux, 1994). Minor plantings extend pineapple
Table 12.1. Characteristics of pineapple groups and clones (after Leal and Soule,
1977).
Characteristic
Group
Spanish Queen Abacaxi Cayenne Maipure
Leaves Spiny Spiny Spiny Smooth Smooth
Fruit
Weight (kg) 0.9–1.8 0.5–1.1 1.4 2.3 0.8–2.5
Shape Globose Conical Conical Cylindrical Cylindrical
Colour skin Large, deep
eyes
O, R
Deep eyes
Y
Y Flat eyes
O
Y to OR
Colour fl esh Pale Y to W Deep Y Pale Y to W Pale Y to Y W to deep Y
Core Large Small Small Medium Small–
medium
Taste Spicy acid,
fi brous
Sweeter, less
acid, low
fi bre
Sweet,
tender,
juicy
Sweet,
mildly acid,
low fi bre,
juicy
Sweeter than
‘C’, fi brous,
tender,
very juicy
Market
Canning FFFVGF
Fresh
Local GGGG G
Export VG G F to P F F to P
Disease
problems
Gummosis,
wilt
resistance
More
resistant
than
‘Cayenne’
Resistant Mealy bug
wilt
Unknown
Clones Red Spanish Queen Abacaxi Smooth
Cayenne
Maipure
Singapore
Spanish
MacGregor Abakka Cayenne
Lisse
Perolera
Green
Selangor
Natal Sugar loaf Smooth
Guate-
malan
Lebrija
Castilla Ripley Papelon Typhone Monte Lirio
PRI-67 Alexandria Venezolana St. Michael Abacaxi
Cabezona Amarella Esmeralda Rondon
Abbreviations: C, cayenne; F, fair; G, good; O, orange; OR, orange-red; P, poor; R, red; VG, very
good; W, white; Y, yellow.

Pineapple 331
production to subtropical areas with mild climates beyond 30° N and S
latitudes and even under protective shelters. Pineapple cultivars show
considerable variation in their plant growth and fruit size when grown in
di erent environments (Chan and Lee, 1985). Greater variation in cultivar
yield response occurs in less favourable environment.
Soil
Pineapple can be grown in a wide variety of soil types, with good drainage
and aeration being crucial. It is grown in the peat soils of Malaysia, sandy and
sandy-loam soils of Ivory Coast, Queensland, Australia and Oaxaca, Mexico,
and in the highly weathered red volcanic soils of Hawaii. The ideal soil type for
pineapple is a sandy or well-aggregated clay soil with good drainage to prevent
waterlogging and root diseases (Hepton, 2003).
In Hawaii, pineapple is grown primarily on silt loams, silty clay loams and
silty clays, primarily oxisols and ultisols. These soils are well suited to pineapple
because they occur in areas having high insolation, have good water-holding
capacity but are well aggregated, so drainage is adequate to very good. Most
of these soils are red in colour, are derived from basaltic rocks and volcanic
ash alluvium, and are high in oxides of iron, aluminium and, in some cases,
manganese. Soils with high levels of manganese can result in manganese-
induced iron chlorosis because soil pH is maintained around 4.5, which results
in high levels of soluble manganese in the soil solution. Generally, a pH range
of 4.5–5 is considered best for pineapple (Hepton, 2003), primarily to reduce
the incidence of heart and root rot caused by Phytophthora spp.
Climate
Rainfall
The pineapple is an obligate Crassulacean acid metabolism (CAM) plant with a
xerophytic characteristic that enables it to withstand long periods of drought.
The leaves have a water-storage parenchyma that serves as a reservoir of
moisture during drought. The leaves are covered with trichomes and a highly
cutinized upper epidermis. The stomata are small and are located in furrows
on the underside of the leaf. At the early infl orescence development stage, 38%
of water use occurs at night when the stomata are open. At midday, no water
loss is detected. Water-use e ciency is 3.3 times greater for pineapple than for
wheat (Bartholomew and Malézieux, 1994). Pineapples are produced under
a wide range of rainfall, from 600 mm to more than 3500 mm annually,
with the optimum for good commercial cultivation being from 1000 to 1500
mm. In Hawaii, pineapple is grown in areas where the rainfall ranges from
510 to 2540 mm, with an average of 1190 mm annually. Pan evaporation

332 Chapter 12
in the major growing area of Wahiawa, Hawaii is 1850 mm per year (5 mm
per day). Even if annual rainfall closely approximates the optimal range, poor
distribution can result in periods of serious drought. In Loma Bonita and
Acayucan (Mexico) 89% and 82% of the rainfall, respectively, occurs between
June and November.
Despite the xerophytic characteristics of pineapple, growth is adversely
a ected by prolonged dry periods. Plants may not attain the desirable size
by the scheduled time for chemical fl ower induction. However, most areas of
the world where pineapple is cultivated have fairly high humidity, which can
reduce the impact of drought. At higher elevations, night air is cooled enough
to produce heavy dew, which condenses on the leaves and drains into the
heart of the plant and into the soil, providing supplemental moisture that can
be absorbed by roots in the leaf axils.
Temperature
In the absence of drought, temperature is the most important factor in
pineapple cultivation. The prevailing temperature determines the rate of plant
and fruit growth and, in conjunction with solar radiation, has a dramatic
infl uence on fruit total soluble solids and acidity, which are the primary
determinants of fruit quality. Under controlled conditions, the rate of root
growth at 18°C was less than 15% of the maximum, while the optimum
temperature was 29°C (Fig. 12.2). Leaf growth responds to temperature
in a manner similar to that of the roots, but growth is closer to 20% of the
maximum at 18°C and the optimum temperature is 32°C (Sanford, 1962).
Photosynthesis and plant growth approach a maximum when the day
temperature is near 30°C and there is about a 10°C diurnal range between
day and night temperatures (Malézieux et al., 2003).
Warm days and cool nights provide the optimum environment for net
assimilation and growth. Growth is slow at an average temperature of 20°C
and at night temperatures below 15°C. In the cool season or in the cooler,
higher elevations, growth is delayed; leaves are upright, rigid and shorter;
the number of slips is higher; fruit may be smaller with prominent eyes; the
fl esh is opaque, higher in acidity and sugars are lower (Bartholomew et al.,
2003). However, the e ects of seasonally cool temperature are confounded
with insolation, because irradiance decreases with the temperature during the
winter months.
Pineapple does not tolerate frost, and even night temperatures of 7–l0°C
for a few h for several weeks during winter can result in leaf-tip necrosis. Such
low temperatures can also cause fruit injury. A range of desirable minimum
night temperatures would be 15–20
°
C, while a comparable day temperature
range would be 25–32°C. The interaction of solar radiation and temperature
signifi cantly a ects the days from forcing to harvest. Further away from the
equator the amplitude of the temperature di erence between seasons increases
and number of days from planting to harvest also increases.
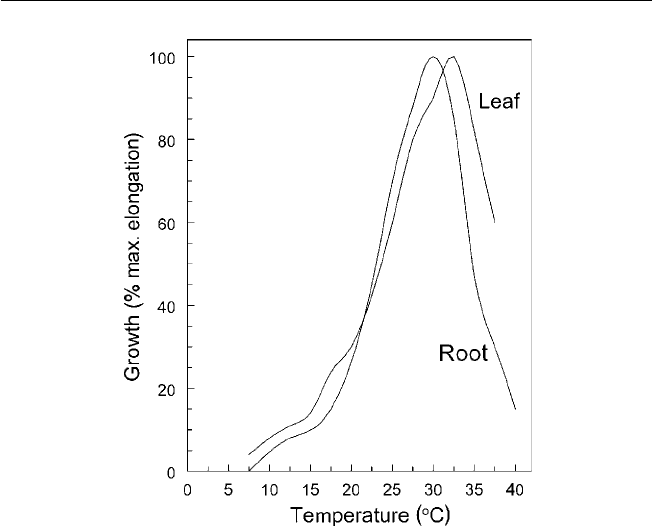
Pineapple 333
Where topographic variation exists, changes in elevation provide a
means of achieving a desirable range of temperature conditions (Fig. 12.3).
Near the equator, the highest yields and fruit of the best quality are generally
obtained at higher elevations. In Kenya, an equatorial country, pineapple
is grown at more than 1400 m, while on Mindinao, the Philippines (8° N),
most pineapple is grown at an elevation of 250 m or higher. While elevation
provides an opportunity to provide an ideal environment for pineapple in
the tropics, economics, particularly labour costs, often determines where the
crop is grown. Thailand (~15° N) is the world’s largest producer of pineapple,
yet most of the crop is grown near sea level in this warm tropical country.
South Africa and southern Queensland, Australia represent the extremes of
the climatic range over which pineapple is grown, and winter temperatures
severely restrict growth rates. In southern Queensland, fruit initiated by forced
fl owering during the 3 coldest months all mature within about 1 month, as
growth resumes as spring temperatures increase (Fig. 12.3). In South Africa
and southern Queensland, minimum temperatures occasionally approach
or reach freezing point for short durations, while in most other areas where
pineapple is grown winter temperatures rarely fall below 10°C.
Cool night temperatures coincident with shortened day length stimulate
fl oral induction if the plant is of su cient size. Cool temperatures also increase
Fig. 12.2. Effect of temperature on leaf and root growth (redrawn from Sanford,
1962).
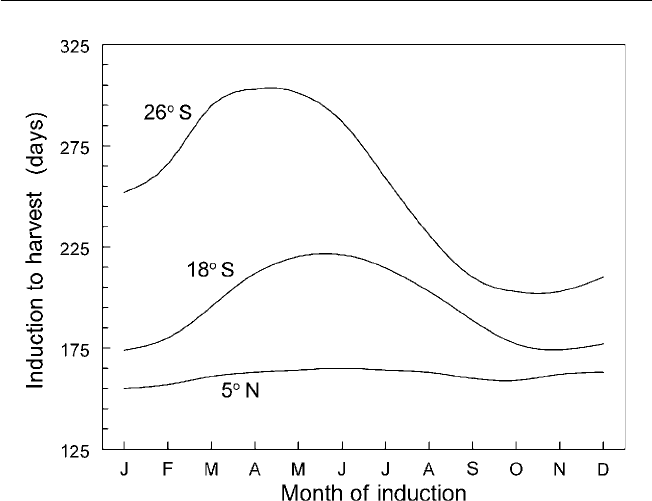
334 Chapter 12
the responsiveness to fl ower-inducing compounds (Bartholomew et al., 2003)
and shorten the time to fl oral induction under a short-day regime. High
temperatures (>28°C) make chemical fl ower induction more di cult, with the
percentage of plants forced decreasing linearly with increasing temperature.
In Mexico between June and August, and in Thailand in most months of the
year, fruit produced for processing generally have low acidity and total soluble
solids (TSS). In Mexico, this lower quality is due to the combined e ect of high
temperature, excessive rain and an increased number of cloudy days, while in
Thailand the response is due primarily to overly warm temperatures.
The ‘Smooth Cayenne’ group is the more productive in tropical conditions,
while the ‘Queen’ group is grown mainly in subtropical areas. ‘Smooth
Cayenne’ is most sensitive to low-temperature-induced internal browning
(chilling injury, blackheart), while ‘Red Spanish’ is less sensitive.
Sunlight
Most pineapple is grown in regions with high insolation, at least in part
because the crop is well adapted to areas with low rainfall. While the light level
required to saturate photosynthesis of a pineapple leaf is believed to be less
than 25% of full sunlight, high irradiance is required to sustain the high levels
of productivity found in commercial plantations, where plant population
Fig. 12.3. Effect of latitude as an indicator of solar radiation and temperature on the
time from forcing to harvest, 26° S is for Nambour, Australia, 18° S is Madagascar
and 5° N is Ivory Coast.

Pineapple 335
densities can reach or exceed 75,000 plants/ha. In Hawaii, the maximum day-
length range between the shortest and longest days is about 2.5 h, but solar
irradiance in December is about 50% of that occurring during midsummer.
Sun injury of leaves is relatively uncommon in most regions where pineapple
is grown, as air temperatures do not exceed about 35°C. When greater than
35°C, the leaf ’s surface temperature on a horizontally displayed leaf is likely
to exceed 50°C. Because of close plant spacing on commercial plantations,
pineapple leaves are erect, as they are supported in an upright position by the
leaves of closely spaced adjacent plants. Erect leaves help to distribute high
midday irradiance uniformly over the leaf surface and help to reduce the heat
load borne by the leaf. Due to relatively erect leaf orientation, about 95% of
light interception occurs in a pineapple canopy at a leaf area index (LAI) of
about 5.0.
Fruit weight is signifi cantly correlated with mean irradiance from planting
to harvest (Fig. 12.4). The lower fruit weight associated with lower irradiance
is due to a lower plant weight at forcing. Fruit acidity in the month before
harvest declines with solar radiation levels and there is no signifi cant e ect on
total soluble solids. Cloudy days reduce pineapple growth and result in smaller
plants and smaller fruit, with higher acid and lower sugar contents. A rule of
thumb is that for each 20% decrease in solar radiation, the yield decreases
~10%. Mutual shading at higher planting densities leads to a linear decrease
in individual fruit weight and a curvilinear increase in total yields as density
increases.
Intense sunlight, particularly during fruit maturation, can cause sun-
scalding of fruit, with the ‘Queen’ group being more susceptible than ‘Smooth
Cayenne’. The damaging e ect can be prevented by shading the fruit or
spraying a refl ective coating, for example lime and a spreader-sticker, as is
done in Queensland, Australia. Fruit covered with newspapers or grass, or the
gathering and tying of the longest leaves over the fruit is observed in Okinawa
and Taiwan and is also a common practice in Mexico and Brazil.
Photoperiod
The normal season for fl owering of ‘Smooth Cayenne’ is during the winter,
in response to cool temperatures and shortened photoperiod. However,
the plant will fl ower naturally at any time of the year, depending upon the
planting material and time of planting (Bartholomew et al., 2003). Because
of this response, Gowing (1961) classifi ed ‘Smooth Cayenne’ as a quantitative
but not an obligate short-day plant. Under controlled conditions, ‘Smooth
Cayenne’ plants grown under 8-h days all fl owered; 69% fl owered under
10-h days, 53% under 12-h and 30% under 16-h days (Friend and Lydon,
1979). Interruption of the dark period by illumination suppresses fl owering.
Before the advent of artifi cial fl oral initiation, plants that were not induced
to fl ower during the winter months generally fl owered in the late summer
and early autumn, producing winter and spring fruit. Some minimum
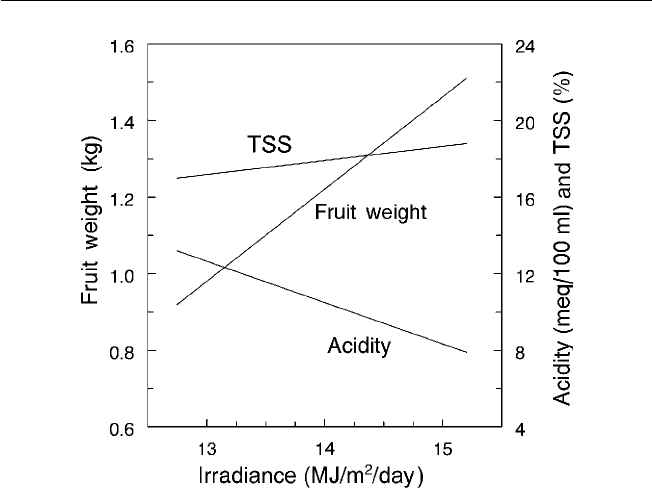
336 Chapter 12
plant size is necessary before natural fl owering will occur, and cool night
temperatures apparently enhance the e ects of short days (Bartholomew et
al., 2003).
GENERAL CHARACTERISTICS
Stem
The stem of a mature ‘Smooth Cayenne’ plant is 30–35 cm long and club-
shaped, with the thickest diameter being 5–8 cm just below the apex.
Internodes are short, ranging from 1 to 10 mm, with the middle region of
the stem having the longer internodes. There is an axillary bud at each node
(Krauss, 1949). Adventitious roots grow through the epidermis and vary in
length from a few millimetres near the top of the stem to several centimetres
at its base. Axillary buds can produce shoots, typically called suckers in the
industry, which are the source of a second or ratoon crop. Suckers may be
retained on the mother plant or they may be harvested for use as planting
material. Such harvesting is a relatively costly operation because the shoots
must be cut from the mother-plant stem. Suckers harvested for use as
Fig. 12.4. Effect of mean irradiance from planting to harvest on average fruit weight,
and mean irradiation 2 weeks prior to harvest on fruit juice titratable acidity and
total soluble solids (TSS) of ‘Smooth Cayenne’ pineapple fruit grown in the Ivory
Coast.
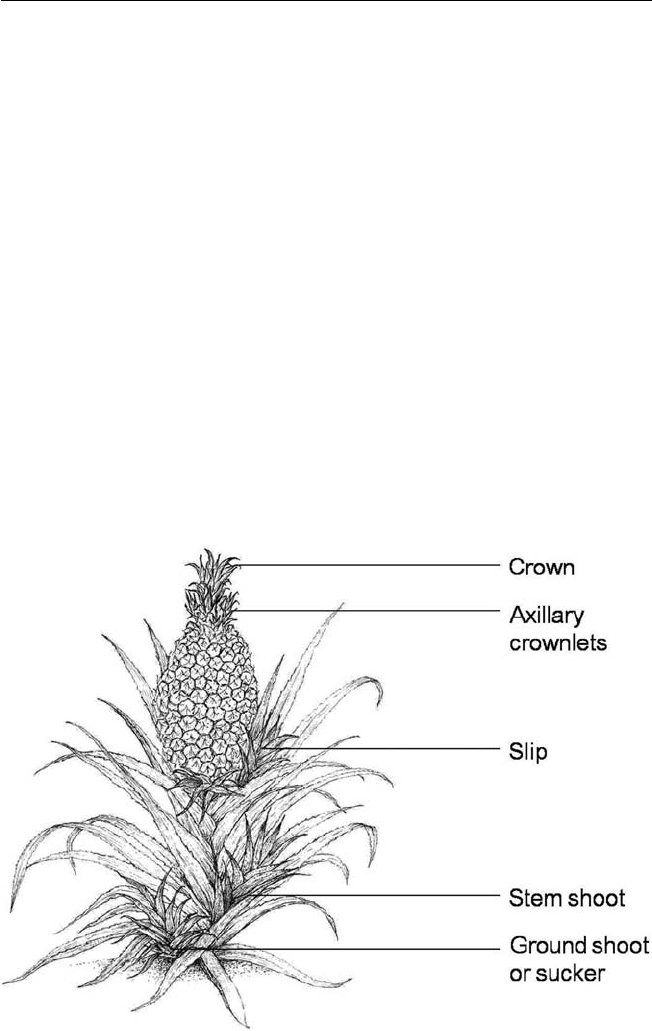
Pineapple 337
planting material can range in weight from a few hundred grams to more
than a kilogram fresh weight (Fig. 12.5). Large suckers are unsuited for use
as planting material because the shock of removal tends to induce precocious
infl orescence development. Shoots, commonly called slips, also are produced
from buds in the axils of the fl oral bracts on the peduncle. The stem of slips is
comma-shaped, in contrast with the straight stem of suckers and crowns. If
slips are allowed to remain on the plant for a few months after fruit harvest,
they can grow to 250–450 g.
Roots
Adventitious roots are produced from preformed root primordia in the stem.
Krauss (1948) separated the roots into ‘soil’ roots, which develop at the base
of the stem and form the underground root system, and ‘axillary’ roots, which
form above the soil surface in the leaf axils. The underground root system is
relatively dense and typically extends to a depth of 15–30 cm. The roots of
pineapple plants grown in deep, loose, fertile soil free from parasitic organisms
and can grow downward more than 50 cm and extend 1.83 m beyond the
plant within a year’s time.
Fig. 12.5. Pineapple fruit showing multiple crowns, slips at the base of the fruit and
suckers at the base of the plants (from Coppens d’Eeckenbrugge and Leal, 2003).

338 Chapter 12
The axillary roots will absorb both water and nutrients, facilitating the
utilization of foliarly applied nutrients. The main absorptive area of the root is
in the unlignifi ed white tissue of the root tip. Roots without white tissues at the
root tips are not actively growing, are ine cient in absorbing water and may
have been lost because of root rot and nematode feeding.
Leaves
Leaves are produced in a spiral around the stem in a tight rosette (Fig. 12.1). A
dormant axillary bud is found at the midpoint of each leaf on the main stem.
By counting from a leaf or bud at a selected node on the stem upwards in a
spiral, the 13th leaf or bud will be directly above the initial leaf or bud and fi ve
turns around the stem will have been made, resulting in a 5/13 phyllotaxy
(Bartholomew and Kadzimin, 1977).
The number of leaves increases regularly, with an average of fi ve or
six leaves per month. Old leaves do not abscise, and a mature plant may
have 70–80 active leaves. The most frequently mentioned developmental
index for carbohydrate and nutrient status is the whorl of leaves designated
and described as the D-leaves by Sideris and Krauss (1936) and fi rst used by
Nightingale (1942). Usually, the D-leaves are in the fourth whorl from the
base of the plant, and not more than three leaves would qualify as D-leaves
at any one time. These are the longest (80–100cm) and the youngest nearly
physiologically mature set of leaves. In non-fruiting plants, the leaf base of
these leaves is only slightly broader than the green blade. In ‘Smooth Cayenne’
a small group of marginal spines is found near the leaf base and near the
tip. Also, a short area of spines is formed on new growth of the leaves after
temporary cessation of leaf growth due to adverse conditions. There is always
a slight constriction at the point where leaf expansion was interrupted.
There are a number of structures in pineapple plants and other
bromeliads that contribute to their strong resistance to moisture stress.
The position and trough shape of the leaves, the presence of trichomes, and
stomata located in furrows beneath trichomes on the underside of the leaf
are features that enhance drought resistance. Long, multicellular trichomes
are found in abundance on the lower side (abaxial) of the leaf and less on the
upper (adaxial) side. It is generally believed that the trichomes absorb moisture
and nutrient solution and reduce water loss through the stomata by forming a
dense covering over the stomata.
A unique internal feature of bromeliads, including the pineapple leaf,
is a water-storage tissue that is colourless and translucent. This tissue can
be identifi ed with the naked eye in the adaxial part of transverse sections of
the leaf and contrasts with the chlorophyllous mesophyll tissue beneath. The
width of both tissues in leaf cross section varies with age and environmental
factors. In the pineapple-growing district of Wahiawa, Hawaii, the water-

Pineapple 339
storage tissue of a turgid, fully developed leaf from a fi eld-grown plant
occupies about half the leaf cross section at the middle of the blade. This tissue
becomes narrower as moisture stress increases. This tissue is absent towards
the tip and margins.
Carbon is assimilated in pineapple leaves by the Crassulacean acid
metabolism (CAM) pathway. The principal features of this pathway are large
diurnal fl uctuations in organic acids, mainly malic, and an inverted pattern
of gas exchange due to the fi xation of CO
2
into malate at night and its release
and reduction to carbohydrate during the day (Malezieux et al., 2003). The
inverted pattern of gas exchange is the result of changes in the intercellular
CO
2
concentration. At night the CO
2
acceptor molecule phosphoenolpyruvic
acid (PEP) is produced using stored carbohydrate. The PEP is carboxylated
to form oxaloacetic acid, which is then reduced to form malate. The malate
is transported to the cell vacuole, where it is stored until the following day.
Shortly after sunrise, malic acid moves from the vacuole to the cytoplasm, is
decarboxylated to produce a molecule of CO
2
and a molecule of pyruvic acid;
the low intercellular CO
2
level begins to increase and the stomata soon close.
In the morning to early afternoon hours, the released CO
2
is fi xed and reduced
to carbohydrate by normal Calvin cycle or C-3 photosynthesis. As the malic
acid level declines, the intercellular CO
2
level also decreases and the stomata
open. During the remainder of the afternoon hours, CO
2
is assimilated from
the atmosphere by conventional C-3 photosynthesis. Normal night opening of
pineapple stomata requires sunlight on the previous day.
Infl orescence and fl ower
The infl orescence is terminal (Fig.12.1), developing from the apical meristem
of the plant. Peduncle elongation begins at the time reproductive development
is initiated and continues after fl ower formation. The fi rst sign of fl oral
initiation, whether natural or induced, is a rapid increase in diameter of the
apical meristem, and 5–6 days after this change takes place, the peduncle
begins to elongate. It continues to elongate as the infl orescence develops.
The infl orescence can consist of up to 200 individual trimerous fl owers with
a phyllotaxy of 21/55 for large fruits of ‘Smooth Cayenne’ and 13/34 for
smaller ratoon crop fruit (Ekern, 1968). Individual fl owers are composed of
three sepals, three petals, six stamens and a tricarpellate ovary. One to several
fl owers open each day over a period of 3–4 weeks, starting from the base of
the infl orescence (Okimoto, 1948). The fruitlets develop from fl owers that do
not abscise and each fl ower is subtended by a fl eshy bract (Fig. 12.1). The style,
stamens and petals wither and the remaining fl oral parts develop into the
fruitlet (Okimoto, 1948). The ovules and pollen grains are functional but seeds
are not normally formed as ‘Smooth Cayenne’ is strongly self-incompatible.
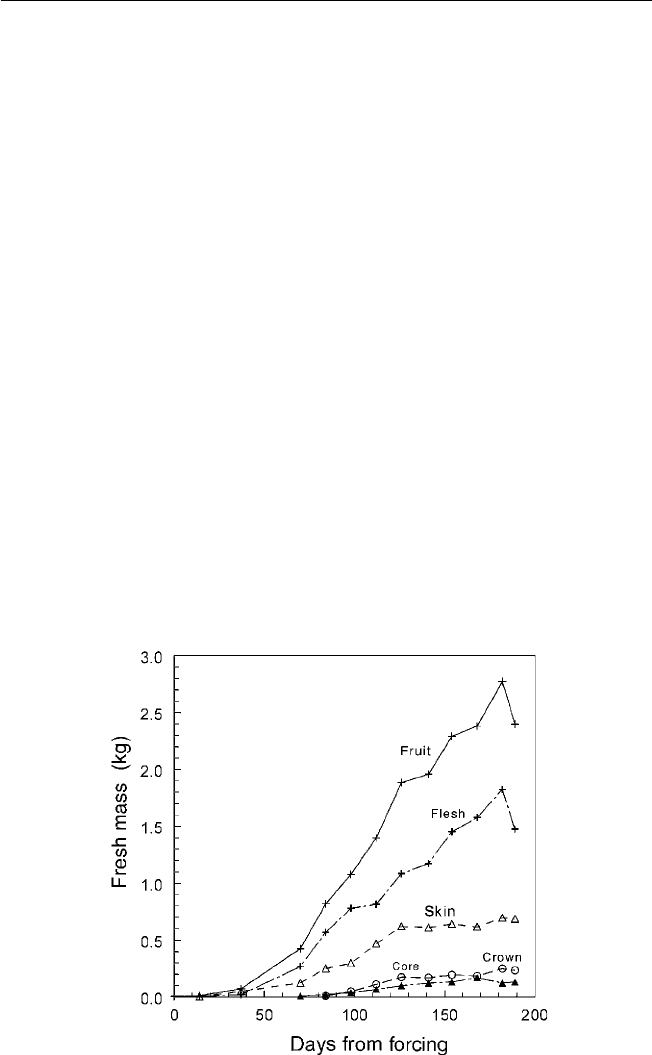
340 Chapter 12
When fl ower production ceases, the apical meristem again reverts to the 5/l3
phyllotaxy, with a transition area of short leafy bracts, followed by the growth
and development of the crown (Fig. 12.1).
Fruit
The fruit, more precisely defi ned as a coenocarpium (a multiple fruit derived from
ovaries, fl oral parts and receptacles of many coalesced fl owers), also commonly
referred to as a sorosis (a syncarp of fused fruitlets from inferior ovaries), is
topped by a leafy stem referred to as the crown (Fig. 12.5). The fruit ‘shell’ is
composed mainly of sepal and bract tissues and the apices of the ovaries, while
the edible fl esh is primarily composed of ovaries, the bases of sepals and bracts,
and the cortex of the axis, which is an extension of the peduncle.
Fruitlets develop from fl owers that do not abscise (Fig. 12.1). The style,
stamens and petals wither, with remaining fl oral parts developing into the
fruitlet (Okimoto, 1948). Yeast and bacteria can enter through the nectary
gland, and mature fruit is not sterile (Rohrbach and Apt, 1986). In the
normal fruit there are 8 gently sloping rows and 13 shorter, steeper rows of
fl owers. The number of fruitlets can be estimated by counting the fruitlets on
the long, gently sloping spiral and multiplying by eight, the number of spirals
(Bartholomew and Paull, 1986). It takes approximately 4 months from the
end of the last open fl ower to fruit maturity, and the total time from fl oral
initiation to harvest takes between 6 and 7 months (Figs 12.6 and 12.7).
Temperature signifi cantly accelerates or delays development.
Fig. 12.6. Pineapple fruit growth and development in Hawaii.
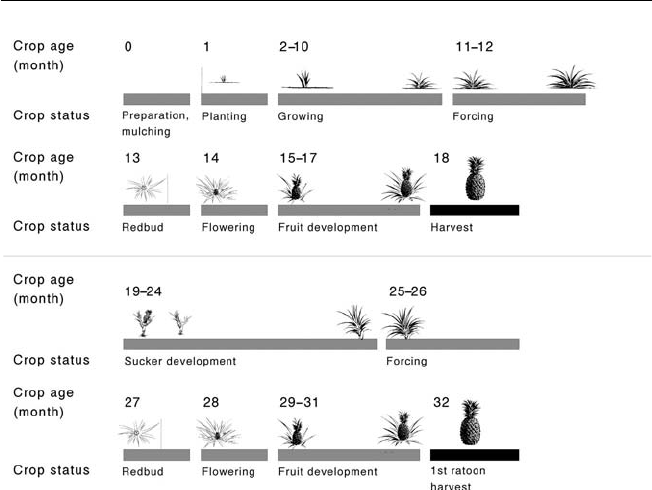
Pineapple 341
Cell division is completed prior to anthesis, with all further development
being the result of cell enlargement (Okimoto, 1948). Fruit weight increases
about 20-fold from the time of fl owering until maturation (Fig. 12.6). Fruit
development studies have shown that fruit weight and its components (core,
fruitlets, the collective fl esh, fruit shell) increase in a sigmoid fashion after the
infl orescence is initiated (Fig. 12.6). Crown growth increases about 30–45
days after fruit growth has commenced. The crown has been reported to have
no direct e ect on the development of the fruit, though crown removal early
in fruiting leads to greater fruit weight. The most marked changes in fl esh
composition occur in the 3–7 weeks prior to, and at, the half-yellow-shell
colour stage. Just prior to this stage, fruit translucence can start to develop,
and translucent development continues after harvest. Titratable acidity
declines and the total soluble solids gradually increases, being more rapid
in the last 6 weeks, as the fruit approaches the fully ripe stage. Fruit sugars
continue to increase through to senescence, unless the fruit is harvested.
Fig. 12.7. The growth cycle of a pineapple crop to harvesting the fi rst ratoon crop in
relation to the various stages of development and crop age (Rohrbach, no date).

342 Chapter 12
CULTIVAR DEVELOPMENT
Cytogenetics and genetics
Information on cytogenetics and genetics comes from early studies conducted
at the former Pineapple Research Institute of Hawaii. Chromosome counts of
‘Cayenne’, ‘Queen’, ‘Spiny Samoa’, ‘Ruby’, ‘Pernambuco’, ‘Spiny Guatemala’,
an F
1
hybrid between ‘Cayenne’ and an unknown wild-type from Brazil and
B. pinquin L. showed the pineapple cultivars to possess n = 25 chromosomes
and B. pinquin has n = 48, with no irregularities in meiosis (Collins, 1960).
Several triploid plants with 75 chromosomes are found among the F
1
hybrids.
The triploids appear to be products of the conjugation between an unreduced
‘Cayenne’ egg cell with 50 chromosomes and normal haploid pollen of a
Brazilian wild type. The commercial cultivar ‘Cabezona’ is a natural triploid
with 75 chromosomes (Collins, 1933).
Collins and Kerns (1938) described about 30 heritable mutant forms in
‘Smooth Cayenne’ fi elds, most being undesirable. One desirable processing
mutant form is for elongated fruit. Collar-of-slips, a condition in which
excessive numbers of slips are either attached directly to fruitlets at the base of
the fruit or massed around its base, occurs in successive vegetative generations
(Fig. 12.5). These plants are continuously rogued out. This character is
dominant and occurs in a heterozygous state in ‘Smooth Cayenne’. Spiny
leaf is due to a homozygous recessive gene, and the smooth leaf condition
of ‘Smooth Cayenne’ is carried as a heterozygous dominant. However, it is
unstable and mutates frequently to the spiny leaf type; other spiny conditions
also occur. Self-incompatibility in pineapple is an advantage in commercial
production of seedless fruit and in cross-pollination. Pollen shows good
viability for several cultivars, except that of the triploid ‘Cabezona’. A single S
locus with multiple alleles and gametophytic control of pollen phenotype are
involved in self-incompatibility (Brewbaker and Gorrez, 1967), in which the
pollen tube growth beyond the upper third of the stylar canal is inhibited.
Breeding and cultivars
Breeding
Pineapple breeding and selection objectives vary with the locality, but almost
always emphasize disease and insect resistance (Coppens d’Eechkenbrugge et
al., 1997; Chan et al., 2003). More recently, development of cultivars for fresh
fruit consumption has been a major focus. Populations have been produced
from crosses to allow selection of improved types. This selection involves
constant roguing of undesirable mutations and selection of superior types.
Mutation continues to occur within selected clones, so the e ects of selection
are not permanent, and without continued rouging and selection, commercial

Pineapple 343
fi elds can revert to conditions of an unselected fi eld population. In Hawaii,
advanced hybrid clones were derived from many years of breeding (Chan
et al., 2003). In Australia, sources of germplasm consist of selections from
Queensland ‘Cayenne’ clones, hybrid populations, meristem-cultured plants
from known clones and introductions from other countries. Hybrid selections
were derived from ‘Cayenne’ crosses with rough leaf types such as ‘Queen’,
‘Ripley Queen’, ‘MacGregor’, ‘Alexandra’ and ‘Collard’. ‘Singapore Spanish’, a
spineless plant with good fruit quality, has been widely used in their breeding
programme. Malaysia has similarly used ‘Smooth Cayenne’ and ‘Singapore
Spanish’ in their hybrid breeding programme. Breeding programmes in Puerto
Rico, Brazil, Taiwan, the Philippines and South Africa maintain the major
cultivars in their germplasm collections.
‘Smooth Cayenne’ is highly susceptible to mealy bug wilt (Table 12.1),
though a few clones have shown variable resistance, which is transmitted to
seedling progenies. Other cultivars and species have shown some resistance:
‘Red Spanish’, ‘Pernambuco’, ‘Queen’, Ananas ananasoides, Ananas bracteatus,
Pseudananas sagenarius and some hybrids involving ‘Cayenne’ and resistant
cultivars (Collins, 1960). In Hawaii, hybrid No. 59-656 is resistant to
P. cinnamoni and Phytophthora parastica root and heartrot diseases.
Concomitant with breeding for disease and insect resistance has been the
attempt to develop cultivars suitable for fresh fruit export. A suitable clone
should have high yield, high sugar, a good balance of sugars to acids, high
ascorbic acid and appealing fl avour. These must be incorporated in a clone
having the desired disease resistance, fruit shape and weight.
Cultivars
Collins (1960) developed a botanical key to the genera and species and the
major characteristics of fi ve ‘Cayenne’ clones for selection of appropriate
parents. A later taxonomic key identifi ed 18 commercial clones with
vernacular names and descriptions (Antoni and Leal, 1980); this included a
new group, ‘Maipure’, composed of smooth-leaved clones (Table 12.1). Clones
in this group are primarily grown in South America. The major fruit and leaf
characteristics of the principal clones can be placed in fi ve phenotypic groups
(Table 12.1). ‘Monte Lirio’ and ‘Perolera’, formerly unclassifi ed, are placed
in the ‘Maipure’ group. ‘Cayenne’ and ‘Maipure’ smooth-leaved groups di er
in that leaves of the former group exhibit a few spines near the leaf tip, while
the latter group shows leaf piping (leaf margins) with a greyish streak, due
to folding over of the lower epidermis on to the upper leaf surface (Collins,
1960). Pineapple isozyme variation indicates fi ve genetically diverse groups
that do not perfectly match these phenotypic groupings (Loison-Cabot, 1992;
Aradhya et al., 1994). A brief discussion of the fi ve horticultural groups
follows.
Cayenne group: ‘Smooth Cayenne’ is the standard for processing and
for the fresh fruit trade because of its cylindrical shape, shallow eyes, yellow

344 Chapter 12
fl esh colour, mild acid taste and high yields. In most areas, ‘Smooth Cayenne’
constitutes a mixture of clones due to new introductions from mutations, lack
of roguing and other various sources. Local selections are mostly known by
their areas of origin, such as ‘Sarawak’ in Malaysia. ‘Champaka’ is a selection
of ‘Smooth Cayenne’ originating in India and widely grown in Hawaii. The
group is susceptible to mealy bug wilt and nematodes.
Queen group: This group generally produces smaller plants and fruit with
spiny, shorter leaves than the ‘Cayenne’ group. ‘Queen’ is grown in South
Africa, Australia and India for the fresh fruit market. ‘Z-Queen’ or ‘James
Queen’ is reported to be a mutant of ‘Natal Queen’ and is a natural tetraploid.
Spanish group: The plants are generally small to medium, spiny-leaved,
vigorous and resistant to mealy bug wilt, but susceptible to gummosis caused
by the larvae of the Batrachedra moth. It is acceptable for the fresh fruit
market but not favoured for canning, due to deep eyes and poor fl esh colour.
‘Red Spanish’ or ‘Espanola roja’ is the major cultivar in the Caribbean region.
‘Singapore Spanish’, or ‘Singapore Canning’ and ‘Nanas Merah’, are the
principal canning pineapple in West Malaysia because of their adaptability to
peat soil. The fl esh has a bright yellow colour. Other Malaysian cultivars are
‘Masmerah’, a spineless type with large fruit, and ‘Nanas Jabor’, a Cayenne–
Spanish hybrid that is susceptible to fruit marbling and cork spot. ‘Cabezona’,
a natural triploid, is an exception, having large plants and fruit weighing
4.5–6.5 kg. It is grown primarily in the Tabasco State of Mexico and a small
area of Puerto Rico where local consumers prefer the larger fruit. The Puerto
Rico clone PR 1-67 is suspected to be a hybrid between ‘Red Spanish’ and
‘Smooth Cayenne’, as these were the only clones grown in adjacent fi elds. The
fruit has light yellow fl esh with adequate sugar and resistance to gummosis,
is fairly tolerant to mealy bug wilt, and has good slip production and good
shipping qualities.
Abacaxi group: This group is grown mostly in Latin America and in
the Caribbean region. Py et al. (1987) called this the Pernambuco group.
The fruit is not considered suitable for canning or for fresh fruit export, but
the juicy, sweet fl avour of the fruit is favoured in the local markets. ‘Perola’,
‘Pernambuco’, ‘Eleuthera’ and ‘Abacaxi’ are the principal clones in Brazil,
along the eastern Espirito Santo in the south through Bahia and Pernambuco
to Paraibo.
Maipure group: This group is cultivated in Central and South America as
fresh fruit for the local markets. These clones may be of interest to breeders in
the western hemisphere as they constitute a gene pool of adapted forms almost
unused in breeding programmes.
The ‘Smooth Cayenne’ cultivar dominates commercial production for
canning and is also one of the major fresh fruit varieties. However, ‘Smooth
Cayenne’ has objectionably high acidity during the winter months, so newer
hybrids such as 73-114 (MD-2, MG-3), which have comparable yield and a
better sugar to acid balance during the winter months, have rapidly expanded

Pineapple 345
in importance as fresh fruit varieties and now dominate international trade.
Other varieties of some importance commercially include ‘Queen’ and
‘Spanish’, both of which are primarily consumed fresh.
CULTURAL PRACTICES
Planting material and propagation
Propagules used in commercial pineapple production include fruit tops
(crowns), shoots borne on vestigial fruits at the base of the fruit (slips) and
shoots borne at any position on the stem (suckers) (Fig. 12.5). Genetic
variation in numbers of slips produced per plant exists between clones
and cultivars. Two or three crowns can be produced as a result of high-
temperature injury during early infl orescence development, but large numbers
of crowns (multiple) or fasciation of crowns is considered a genetic defect and
such plants typically are rogued. Types and sources of vegetative propagules
are kept separate, because weight, nutritional history and development
environment are believed to a ect time to establishment and optimum plant
size for fruiting, as well as having the potential to introduce variability into
the fi eld (Hepton, 2003). Time from planting to harvest is dependent primarily
on the weight of the propagule, and crowns produce fruit in 18–24 months,
slips in 15–20 months and suckers in 12–17 months. Planting materials are
treated with a fungicide or are ‘air-cured’ by drying the butt end, or both,
before planting to prevent rots.
A shortage of planting material can occur when fruits are sold fresh,
because the fruit is usually marketed with the crown. Most commercial clones
of ‘Smooth Cayenne’ produce only one to two suckers per plant and seldom
more than three slips. Several techniques are available to speed up propagule
production required for variety and clone development or to replace planting
material sold with the fruit. Cost of labour often determines which techniques
are used.
Stem sectioning utilizes the axillary buds, usually on stems of mature
plants. Leaves are stripped o the stem; the stem is quartered longitudinally
and each quarter is divided into sections approximately 5 cm in length. The
sections are immersed in a fungicide solution or air-cured for several days, or
both, before planting in well-prepared nursery beds with good drainage and
aeration. Shoots that grow from axillary buds can usually be transplanted in
4–6 months. A normal-sized ‘Smooth Cayenne’ plant stem can produce an
average of 25 sectioned plants (Collins, 1960). Established crowns and shoots
with adequate root systems can be split longitudinally into quarters, which
forces one to several axillary buds to develop. These small plants (plantlets)
can be transplanted when large enough and the process repeated. A similar
technique is to gouge out the plant’s apical meristem, thereby breaking apical

346 Chapter 12
dominance over axillary buds. One to a few suckers are produced, which,
when harvested, result in the production of additional suckers. Once plant
reserves are exhausted, a sucker can be allowed to grow and gouged when of
reasonable size, resulting in the production of additional suckers. In Okinawa,
crown leaves planted in sand produce plantlets at the leaf base. Only one
plantlet is produced per leaf at a low rate. As no axillary buds are present on
the leaf, shoots must be generated from meristematic callus formed at the leaf
base tissue or from stem tissues adhering to the leaf base. From 40 to 70 leaf-
bud cuttings can be produced from a single crown.
Plantlet production is induced by treating plants with the morphactin
chlorofl urenol (Maintain CF-125
®
; Multiprop R
®
) after reproductive develop-
ment has been forced with ethephon. Chlorofl urenol is applied 1 or 2 days after
forcing and before signifi cant fl ower di erentiation has occurred. If timing and
concentration of chlorofl urenol are appropriate, up to ten good-sized plantlets
are produced (Hepton, 2003). Plantlets have also been produced by meristem
culture, but care must be taken to prevent excessive somaclonal variation. It
is reported that up to one million plants can be produced from a single bud in
2 years through tissue culture, but this technique is generally more expensive
than other propagation techniques.
Field preparation and layout
The fi rst step in fi eld preparation on an established pineapple farm is the
destruction of existing plants, since pineapple can be a serious weed if live
plants are plowed under. The fresh plant mass in a ratooned pineapple fi eld is
huge, and residue incorporation or disposal is a costly step in fi eld preparation.
Plants usually are disced multiple times or chopped to hasten desiccation,
and when thoroughly dried are plowed under or burned. The decision as to
whether to plow or burn is often determined by how soon the fi eld is scheduled
for replanting. Conventional tillage implements are used for primary land
preparation on most pineapple farms and plantations, though in Hawaii, a
tefl on-coated moldboard plow was developed that would scour in clay soils and
that permitted fi elds to be plowed to a depth of more than 80 cm. Deep plowing
aids in the incorporation of the large amounts of plant residues produced by a
pineapple crop. Except where deep plowing is done, the size of equipment used
is determined mostly by farm size. Where the soil is compacted, subsoiling is
desirable to break the hard pan to improve drainage and soil aeration. Discing
to break up soil clumps and improve soil texture is important to improve
dispersion of fumigants and to provide good plant-to-soil contact.
Black polyethylene mulch (∼50 microns thick and 81 cm wide) helps to
prevent rapid escape of fumigants, maintains warmer soil temperatures during
the cool season, retains moisture at the soil surface, reduces fertilizer leaching
during rainy periods, controls weed growth in the beds and increases yield. In

Pineapple 347
many pineapple-growing areas where plastic mulching is too costly, mulching
with straw, grass, sugar cane baggasse or other available materials is done.
Crop rotation for nematode control is a possible practice when economical.
Fumigation
‘Smooth Cayenne’ is susceptible to several plant parasitic nematodes, which
if left unchecked can devastate the plant. Pre-plant fumigation of the soil
with a volatile nematicide to control root-knot and reniform nematodes is an
established practice in Hawaii. In recent years, loss of some fumigants has
occurred due to health and environmental concerns. Non-volatile nematicides
are applied post-planting through drip irrigation systems, to provide nematode
control later in the plant crop and during ratoon crop development. Even
with signifi cant nematode feeding pressure, it is often possible to produce an
acceptable mother-plant crop. However, under such pressure ratoon crop
growth and yield will probably be severely reduced, possibly to the point of
complete ratoon crop failure, because ratoon yields are highly dependent upon
the health of the plant crop root system. In South Africa, where Meloidogyne
and Helicotylenchus nematodes are serious problems in pineapples, pre-plant
dipping in a systemic nematicide followed by post-plant spraying at monthly
intervals for 12 months increases a plant crop yield by 11 t/ha. In the absence
of any history of nematode problems, soils should be assayed for nematodes
before embarking on costly nematode control programmes.
Planting
Planting is commonly done manually with a 25 cm-long, trowel-shaped
planting tool. Good soil preparation makes it easy to place the propagule deep
enough into the soil to assure good plant-to-soil contact. Marked cords may
be stretched from one end of the fi eld to the other to keep rows aligned and to
indicate plant spacing. In Hawaii, spacing is established by factory marking on
polyethylene mulch. In Queensland, Australia, South Africa and Mexico, some
growers use a planting machine similar to a vegetable transplanter.
Crowns, slips and suckers are planted separately in di erent fi elds.
Crowns are smaller than and somewhat more uniform in size than slips and
suckers. Slips and suckers can be large, with size mostly determined by the
length of time they remain on the plant. Sizing prior to planting is crucial
in order to obtain uniform-sized plants at the time of fl oral forcing, to assure
a uniform-ripening fruit crop (Py et al., 1987). Large suckers have a high
tendency for precocious fruiting, being physiologically mature, especially
when sucker weight exceeds 600 g. The following weight classes are

348 Chapter 12
suggested (Py et al., 1987): small crowns 100–200 g and medium crowns
200–300 g, and slips or suckers small 200–300 g, medium 300–400 g and
large 400–600 g.
Spacing
Plant spacing or density a ects pineapple average fruit weight and yield per
unit area (Fig. 12.8). Plant density and other cultural practices for ‘Smooth
Cayenne’ are directed towards the production of a fruit size appropriate to the
principal use: the fresh fruit markets or processing. Spacing is also dependent
upon cultivar. Cultivars of the Spanish group produce smaller plants but have
spiny leaves, so wider spacing is used than for smooth-leaved cultivars (Fig.
12.8). On small farms, growers may use a single-row system with relatively
wide spacing between plants and between rows, giving plant densities of
15,000–25,000 plants/ha. In commercial plantings, a double-row system
has been widely adopted, although three- and four-row systems also are fairly
common. Multiple-row systems allow for increased planting density but may
make harvesting operations more di cult and require good management to
assure plant requirements are met. In the conventional two-row bed system,
various spacing regimes are employed to achieve desired plant densities. In
areas where plastic mulch is used, changes in bed width are limited by the
width of mulch used. The spacing between plants in a row should not be less
than ~20 cm, and spacing between rows in a two-row system would be about
35 cm, with between 90 and 120 cm from bed centre to bed centre. Planting
densities typically range from 60,000 to 80,000 for ‘Smooth Cayenne’ and
hybrids with similar growth habit (Fig. 12.8). When plants of the same size
are forced, average fruit size decreases linearly as planting density increases,
with the extent of the decrease being determined by cultivar and environment
(Hepton, 2003). Planting densities as high as 75,000 plants/ha are used
where smaller fruits are desired. There is a fruit size decrease of about 45 g for
each population increase of about 2500 plants/ha.
Irrigation
The pineapple has a low water requirement and can survive long periods
of water stress under natural conditions. However, under non-irrigated
conditions yields are low, with poor-quality fruit of unacceptable size.
Irrigation of pineapple in the Ivory Coast increased yields by 14–22 t/ha,
with a cost equivalent to a yield of 5 t/h. The potential evapotranspiration
of pineapple can reach 4.5 mm per day and a soil’s water-holding capacity
rarely exceeds 100 mm, so without rains, the water supply will be exhausted
within 3 or 4 weeks. Water defi cits can be indexed by the relative thickness
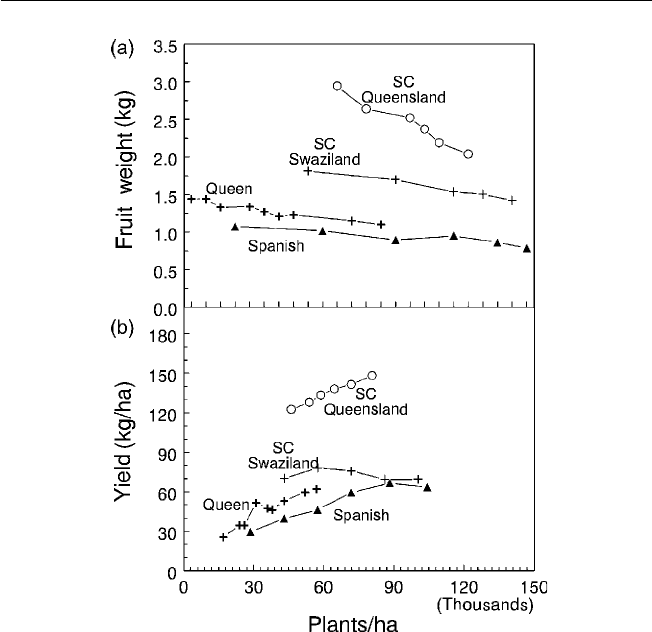
Pineapple 349
of the water-storage tissue of the youngest, physiologically mature leaf
(D-leaf) and by the percentage of white root tips visible on the roots in the soil
(Sanford, 1962).
Irrigation allows year-round planting, allows plants to attain a desirable
plant size for forced fl ower initiation at scheduled times of the year and for
harvesting on a fi rm schedule, allows the use of soils with poor water retention
and allows fertilizing even during the dry season (Fig. 12.7). Weed problems
are, however, increased by overhead irrigation. Sprinkler delivery systems,
including a self-propelled boom sprayer, have been used. Drip irrigation has
replaced other methods of irrigation in most fi elds in Hawaii, with one tubing
orifi ce for every two plants. The quantity of water applied is low compared
with most other crops, typically being in the range of 1.5 cm/ha/week. Drip
irrigation allows fertilizers and nematicides to be applied at the root system
after planting with increased e ciency and safety, and yields are increased.
Fig. 12.8. Effect of planting density on fruit weight (a) and fruit yield (b); data are
for ‘Queen’ grown in Ghana, ‘Singapore Spanish’ grown in Malaysia and ‘Smooth
Cayenne’ grown in Queensland and Swaziland (from Hepton, 2003).

350 Chapter 12
Yield increases have been especially evident in ratoon crops, due to a decrease
in plant mortality and the healthier condition of the plants at plant crop
harvest. The healthy condition of the ratoon plants is due primarily to frequent
delivery of nematicides.
Fertilization
Various published reports on pineapple nutrition indicate that the quantity
of N required ranges from 225 to 350 kg/ha. The requirements for the other
major fertilizer elements are best determined by soil analysis (Malezieux and
Bartholomew, 2003). If K is required, the amount applied usually ranges
from 225 to 450 kg/ha. The crop has a low requirement for P, and 20 ppm of
P in soil is considered adequate. The P requirement can be met by pre-plant
application of this element, and usually from 20 to 80 kg/ha is applied, as
P, P
2
O
5
or, in acid soils, rock phosphate. Some idea of pineapple fertilizer
requirements may be obtained by analyses of elements immobilized in the
various plant parts (Table 12.2). Large amounts of N and K are found in the
plant, fruit and slips. In ratoon fi elds, which develop on suckers on the mother
plant, nutrients removed by the fi rst fruit crop must be replenished. This
amounts to approximately 175 kg N, 27 kg P, 336 kg K, 47 kg Ca and 27 kg
Mg per hectare.
Pineapple responds well to foliar fertilization, and nitrogen, boron,
iron, zinc and sometimes potassium are applied this way (Malezieux and
Bartholomew, 2003). While almost any inexpensive fertilizer can be used,
urea and UAN32 are commonly applied as foliar sprays on a weekly or
biweekly basis, from about 3 months after planting. Urea can be applied as a
foliar spray at up to 20% concentration without damage, if the biuret is less
than 1%. Iron nutrition is a problem in the Hawaiian pineapple soils due to
the high manganese content. The Fe/Mn ratio is more critical than the level
of either nutrient alone (Py et al., 1987). Ferrous sulfate at up to 17 kg/ha per
application is given, as needed, as a foliar spray. Plants with Fe defi ciency show
yellowing of inter-veinal areas. However, Fe defi ciency also a ects absorption
Table 12.2. Minerals immobilized or removed by pineapple plants at a density of
54,340 plants/ha.
Amount immobilized or removed from plant (kg/ha)
NP K CaMg
Plant 437 47 538 134 134
Fruit 135 20 269 33.6 20.2
Slip 40 6.7 67 13.4 6.7
Total 612 73.7 874 181 160.9

Pineapple 351
of N, via its e ects on the root system, leading to a general yellowing of the
entire plant. Zinc defi ciency is usually found in eroded areas, in soils with
high amounts of coral sand and in soils with high pH. Defi ciency causes the
younger leaves to curve and twist; the symptom is unique and diagnostic.
When Zn defi ciency is severe, apical dominance is lost and plants sucker
profusely. Zinc sulfate 0.5% (w/v), applied at 935 l/ha, is sprayed as needed.
Boron defi ciency is characterized by chlorosis of young leaves, development
of red margins and even death of the apical region, resulting in profuse
suckering. Boron defi ciency is a common problem in Australia and 1–4 ppm
boron is applied with ethephon at the time of forcing.
The nutrient requirements of pineapple in Hawaii are based on the
concept of the crop log, which involves the development of laboratory soil and
foliar indices, visual defi ciency symptoms for N, Fe and Zn, and others that
measure growth rate, pathogens, root parasites, moisture stress and weather
conditions (Sanford, 1964; Malezieux and Bartholomew, 2003). A crop log
not only indicates defi ciency symptoms but also attempts to determine causes
of defi ciencies. Soil and foliar analyses are both important, as the former
shows reserve amounts of soil nutrients and the latter indicates e ciency in
absorption. An element may be su cient in the soil but defi cient in the plant,
due to causes such as moisture stress, root disease or loss of the root system to
pests, or an elemental imbalance causing unfavourable interactions a ecting
absorption (Sanford, 1962). Fertilizer is applied on a schedule to meet di erent
requirements at di erent stages of growth (Table 12.3). Foliar applications
should cease after forcing to prevent injury to the infl orescence and reduced
fruit yields.
Chemical fl ower induction
The accidental discovery in the Azores that smoke from burning organic
materials induces premature fl owering in greenhouse-cultured pineapples
led to the wide practice of burning rubbish around the periphery of the
fi elds in Puerto Rico (Rodriguez, 1932). The active ingredient in the smoke
is ethylene gas, with acetylene and calcium carbide also inducing fl owering
in pineapple (Aldrich and Nakasone, 1975). Forcing plants into fl owering
allows synchronization of harvest and makes it possible to control harvest
dates to meet anticipated fresh market and cannery needs (Fig. 12.7). Fruit
harvest date can be predicted with good accuracy with only daily maximum
and minimum air temperature to calculate fruit heat units (Malezieux et al.,
1994). This model can be adjusted using historical data.
The relatively soluble sodium salt of alpha-naphthaleneacetic acid (SNA)
was the fi rst plant growth regulator used commercially to force fl owering
in Hawaii (Bartholomew and Criley, 1983). Ethylene and acetylene gases
dissolved in water have been used in many pineapple-growing regions. The

352 Chapter 12
gases require specialized equipment for e ective application and safe use.
In the warm tropics, SNA has not been e ective, while a water solution of
acetylene produced from calcium carbide or calcium carbide as granules has
been successful.
Ethephon (2-chloroethylphosphonic acid) is probably the most widely
used chemical in commercial pineapple production because of its e ectiveness
and ease of application. This chemical breaks down to produce ethylene
at neutral pHs (Bartholomew and Criley, 1983). The e ective ethephon
concentration ranges from 500 to 1500 μg/l, with greater amounts required
to force fl owering in warm than in cool months. At least 90% fl owering is
obvious 40–60 days after applying about 1000 μg/l solutions (Fig. 12.7) with
50 kg/ha urea (Bartholomew, 1977). In some regions, adjusting the solution
pH above 7 with sodium borate improves forcing success.
Acetylene and ethylene are less e ective when applied during the day
but highly e ective when applied at night or during the early morning hours,
when the stomates are open (Bartholomew and Kadzimin, 1977). In warm
seasons, forcing success may be greater on days when temperatures are less
than 30°C. High N level in the plant at the time of forcing may further reduce
forcing success in warm weather, while withholding nitrogen fertilizer for 4–6
weeks before forcing can improve induction. Plants approaching the natural
Table 12.3. Fertilizer protocol for pineapple planted at 58,710 plants/ha, applied
using a spray valve of 2500 l/ha for different stages of plant development (after Evans
et al., 1988).
Stage of development (month)
0 0–3 4–8 9–10 11–12
Stage Prior to
planting
Growing Growing Growing Forcing
Method Into soil Foliar Foliar Foliar Foliar
Number of
applications
1352 2
Desired crop
colour
Pale yellow
green
Darker
yellow
green
Dark
green
All green
Fertilizer Rate (kg/ha/application)
Total
(kg/ha/
year)
Urea 22 22 33 45 55 450
Potassium nitrate 22 22 33 45 55 450
Iron sulfate 1.5 1.5 2.25 3.0 3.70 30
Zinc sulfate 0.5 0.5 0.75 1.0 1.25 10
Magnesium sulfate 2.75 2.75 3.75 5.5 6.75 55

Pineapple 353
period of fl owering show greater susceptibility to induction (Fig. 12.9a), as
smaller plants are not as susceptible to natural fl owering and are not as easily
forced to fl ower. The minimum size for forcing is much larger under optimum
growing conditions than when growth is restricted by nutrient, water or low-
temperature stress. Plant weights are used to estimate suitability for forcing,
as the larger the plant weights at forcing the bigger the fruit harvested (Fig.
12.9b). For ‘Smooth Cayenne’, when the plant fresh weight is between 2 and
4 kg the fi eld will be forced to fl ower, depending on plant growth status and
weather. Ratoon suckers are more easily induced to fl ower than the plant crop.
The Spanish group is also easier to force in conditions impossible for Cayenne.
Pest management
Except for small plantings in subsistence-farming systems, pineapple is
largely a monocultured crop, involving relatively large fi elds under more or
less standardized cultural practices. Monoculture, as well as the perennial
nature of the crop, is conducive to accumulation of diseases and pests, some
confi ned to certain regions, while most have become established worldwide
(Lim, 1985; Rohrbach and Schmitt, 1994) as vegetative germplasm has been
transported around the world since the 1600s. The ‘Smooth Cayenne’ cultivar
is relatively resistant to most pineapple diseases (Rohrbach and Johnson,
2003). Occasionally, damage associated with specifi c pests or diseases can
cause serious economic losses.
Diseases and nematodes
One of the most important diseases worldwide is mealybug wilt (Table 12.4).
This wilt is caused by a closterovirus and associated feeding by the mealybugs
Dysmicoccus brevipes and Dysmicoccus neobrevipes (Sether and Hu, 2002), both
of which are able to transmit the virus; the disease does not occur if only the
closterovirus or the mealybugs are present. Ants, which protect the mealy
bug colonies and transport individuals from plant to plant, also are essential
to disease development. The dominant species of ant on pineapple in Hawaii
and in South Africa is the big-headed ant (Pheidole megacephala). Ant control
is essential to the control of mealybugs and the wilt; in the absence of ants,
parasitoids and predators keep mealybugs under control. Mealybugs feed on
the roots, on the stem above the roots, on leaf sheaths or leaves and in fruitlet
fl oral cavities. Wilt-a ected plants are stunted, becoming yellowish fi rst and
reddish later. These symptoms resemble the e ects of severe drought.
Some pineapple cultivars possess partial resistance to the mealybug
wilt. However, the only practical control of mealybugs and the associated
ants is by use of insecticide baits to control the ants. Cultivation eliminates
mealybugs, and in a newly planted fi eld they gradually move into the fi eld
from uncultivated grassy or weedy borders, where mealybugs survive on
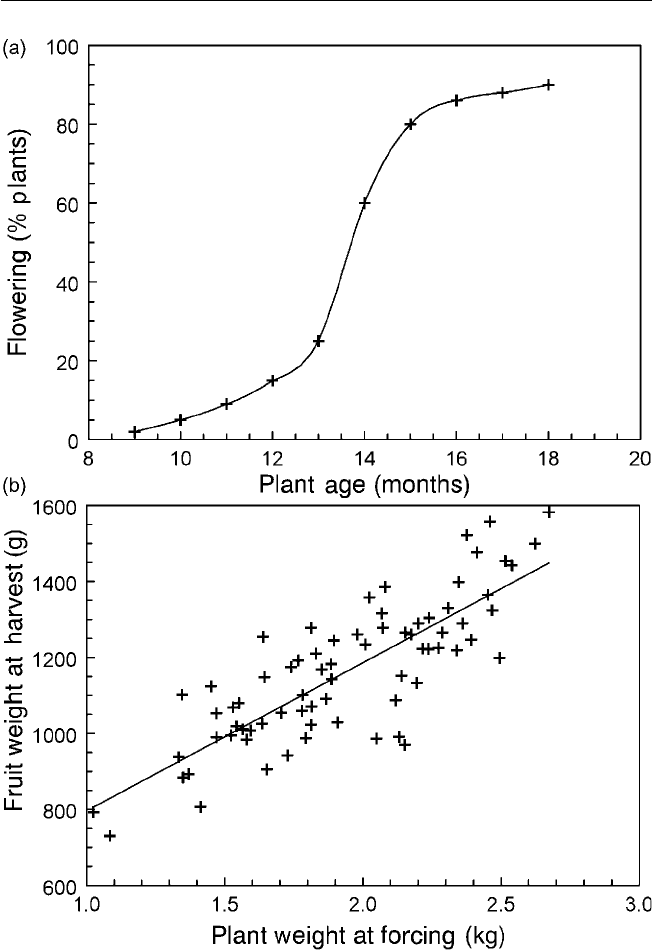
354 Chapter 12
Fig. 12.9. Effect of plant age (a) on susceptibility to natural fl owering (redrawn from
Lacoeuilhe, 1975), and plant weight (b) at forcing and fruit weight at harvest for
‘Smooth Cayenne’ (from Bartholomew et al., 2003).

Pineapple 355
Table 12.4. Some important diseases and nematodes of pineapple.
Common name Organism(s) Parts affected and symptoms
Region or country of
importance
Mealy bug wilt Viral Plant Universal
Pink disease
Gluconobacter oxydens
Acetobacter aceti
Erwinia herbicola
Dark brown discoloration of infected fruit fl esh
upon heating
Hawaii, Australia, Philippines
Interfruitlet corking
Penicillium funculosum
Fruitlets Hawaii
Leathery pocket
Penicillium funculosum
Fruitlets Hawaii, South Africa
Fruitlet core rot
Penicillium funculosum
Fusarium moniliforme
Fruitlets
Pineapple butt rot
Chalara paradoxa
Stems of planting material, and fresh fruit rot Universal
Root/heart rot
Phytophthora cinnamomi
Phytophthora nicotianae
Root, plant, fruit Universal
Root knot nematode
Meloidogyne javanica
Galls, root destruction, stunts plant Universal
Reniform
Rotylenchulus reniformis
No galls; destroys lateral root system, retards
growth
Universal
Root lesion
Pratylenchus brachyurus
Lesion on root and destruction Universal
Root lesion
Rotylenchulus unisexus
Root destruction South Africa
Spiral
Helicotylenchus dihystera
Root destruction Ivory Coast, South Africa,
Puerto Rico, Hawaii

356 Chapter 12
alternate hosts. Movement from fi eld edges led to a practice of planting several
beds of pineapple in a strip parallel to the edge of the fi eld and separated from
the main fi eld by a road as a bu er. These bu ers were sprayed regularly. Ant
control using baits is more common in Hawaii.
Heart and root rot caused by P. cinammomi Rands and P. nicotianae
B. de Haan var. parasitica Dast, Waterh. and P. palmivora (Butler) Butler
does not occur uniformly and levels of infection can vary from year to year.
P. cinammomi is virulent in cooler conditions while P. nicotianae and P. palmivora
are associated with warmer and more tropical environments. Conditions
conducive to Phytophthora rots include poorly drained soils, high rainfall and
a soil pH above about 5.5. Crown plantings, especially during the wet season,
are highly susceptible to Phytophthora rots, with the loss of 30–100% not being
uncommon. The disease is controlled by pre-plant dips of planting materials
and by foliar post-plant spray using fungicides.
Butt or black rot is a universal problem of stored planting material and
of fresh fruit. The disease, caused by Chalara paradoxa (De Seynes) Sacc, is
characterized by a soft rot and blackening of the basal portion of the stem
of vegetative propagules, and similar symptoms are seen on infected fruit.
Infected propagules can completely decay if kept moist, and an infection can
quickly spread through an entire pile. In the absence of fungicide treatment,
planting material can be protected by air-curing for several days. Fruit rot is
managed by careful handling of the fruit to avoid bruising, by refrigeration
and with a fungicide dip. Infection occurs within 8–12 h following wounding.
Susceptibility varies with the cultivar, ‘Red Spanish’ types being more resistant
than ‘Smooth Cayenne’.
Other fruit diseases – fruitlet core rot, interfruitlet corking, pink disease
and marbling disease – are universally distributed and occasionally can be
important. Brown rot or fruitlet core rot occurred in only 7% of inspected
fruit. Other postharvest pineapple diseases that begin prior to harvest may
cause sporadic economic problems.
Nematodes are a serious problem wherever pineapple is grown on the
same land over many years. Yield losses of one-third are common in the plant
crop, with total failure in the fi rst ratoon crop without nematode control.
Injury caused by nematodes feeding on the roots is compounded by the roots
non-regenerative nature. Fumigants do not completely eradicate nematodes.
Eggs and dormant larvae are harder to kill, repopulating the soil with time and
necessitating periodic post-plant application of nematicides. For maximum
e ciency of fumigants, soil must be well prepared, with deep ploughing and
the breaking of all clods. Too much soil moisture inhibits good dispersion of
the fumigant in the soil, while fumigant escape is rapid when the soil is too
dry. Polyethylene mulch over beds helps to retain volatile fumigants in the
soil. Application of nematicides by drip irrigation is e ective in controlling
nematodes, particularly in the ratoon crop.

Pineapple 357
Pests
Insects can cause direct damage to various plant parts or vector diseases
(Table 12.5). Prior to 1953, pineapple fruit were fumigated with methyl
bromide before importation into continental USA. Pineapple fruits that are
more than 50% ‘Smooth Cayenne’ are not now regarded as a host for tephritid
fl ies: Mediterranean fruit fl y, C. capitata (Wiedermann); the Melon fl y, D.
cucurbitae (Coquillet); and the Oriental fruit fl y, D. dorsalis Hendel; hence, insect
disinfestation is no longer required (Armstrong and Vargas, 1982).
The pineapple scale, Diaspis bromeliae (Kerner), occurs wherever pineapple
is grown. In Hawaii, pineapple scale is not normally a major problem in
fi elds, probably because of scale parasites and predators. However, because
of the US quarantine requirement, fruit have to be insect-free, and even low
levels of pineapple scale at harvest present quarantine problems. Scale can
be controlled relatively easily by preharvest insecticide applications, taking
into consideration label requirements relating to last application prior to
harvest time.
The pineapple fruit mite, Steneotarsonemus ananas Tryon, occurs uni-
versally on the growing plant, developing infl orescence, fruit and crown. The
fruit mites feed on developing trichomes on the white basal leaf tissue and
fl ower bracts and sepals, causing light brown necrotic areas. The pineapple red
mite, Dolichotetranychus fl oridanus Banks, feeds on the white basal leaf tissue,
particularly of the crown. Severe damage occurs when the fruits mature under
drought conditions, and it may cause death of the basal crown leaves, thereby
a ecting fruit quality (Rohrbach and Schmitt, 1994).
Other insects are mentioned frequently in the literature. In Latin America
and in some Caribbean islands the larvae of the Thecla butterfl y (Thecla
brasiliodes) cause fruit damage, occasionally in serious proportions. Dusting
or spraying the fruit at fl owering stage with appropriate insecticides has given
satisfactory control. In the Caribbean region, the larvae of the Batrachedra
butterfl y (Batrachedra sp.) damage fruit at the fl owering stage, causing
gummosis. Field observations in Jamaica during 1972 showed high incidence
of damage in ‘Red Spanish’ but not in ‘Smooth Cayenne’.
Weed control
Weeds can cause serious crop losses, and on small farms, neglect of weeding at
monthly intervals can result in 20–40% declines in yields. Average fruit weight
in Guinea can be 0.60 kg from an unweeded fi eld, as compared to 1.55 kg with
good weed control. Weed control is a major cost item in pineapple production
and is essential to assure high yields as well as removal of plants that host or
harbour nematodes and insects such as mealy bugs. The weed problem can be
lessened by complete mulching of the beds and interbeds, hence delaying or

358 Chapter 12
Table 12.5. Some important insect pests of pineapple.
Common name Organism Parts affects and symptoms Region or country
Bigheaded ant
Pheidole megacephala
Associated with mealybugs and wilts Hawaii
Ants
Solenopsis sp.
Anaucomyrmex sp.
Associated with mealybugs and wilts Guyana
Pineapple scale
Diaspis bromeliae
Leaves, fruit Universal
Souring beetle (pineapple
beetle)
Carpophilus humeralis
Plant, ripe fruit Universal
Mealybug
Dysmicoccus brevipes
Roots, stems, leaves Universal
Pineapple fruit mite
Steneotarsonemus ananas
Tryon
Developing infl orescence, fruit and crown Universal
Pineapple red mite
Dolichotetranychus
floridanus Banks
Feeds on the white basal leaf tissue, especially
crown
Universal
Black maize beetle
Heteronychus arator
Lower stems and roots South Africa
White grub
Several beetle species
Root system South Africa
Thrips
Thrips tabaci
Yellow spot disease (virus transmitted by thrips
from Emilia sonchifolia)
Hawaii, other areas
Batrachedra butterfl y
Batrachedra methesoni
Fruit at fl owering stage causes gummosis, larvae
causes damage
Caribbean
Thecla butterfl y
Thecla brasiliodes
Fruit at fl owering stage causes gummosis, larvae
causes damage
Mexico
Central America
South America
Caribbean
Symphylids
Hanseniella unguiculata
Root tips, severe stunting Universal

Pineapple 359
minimizing weed growth. Polyethylene sheet mulching of beds substantially
alleviates weeding within beds.
Weed control in commercial plantations is achieved by chemical means,
unless labour is abundant and inexpensive. Chemical weed control is e cient
and rapid if done correctly at appropriate times, using mechanical applicators.
Pre-emergence application, either before or immediately after planting, is low
in cost and e ective. For herbicides, as with other agricultural biocides, there
usually are restrictions on timing of applications and limits to the quantity
that can be used per hectare, per crop cycle or per year.
HARVESTING AND POSTHARVEST HANDLING
Harvesting
Prior to the use of chemicals to induce fl owering, many passes were required
to complete harvesting of a large fi eld, due to the wide variation in time of
fl owering during the winter season, when most fl ower induction occurred.
Chemical induction concentrated the period of fl owering, therefore
condensing harvesting to two to three passes (Paull and Chen, 2003). Natural
or precocious fl owering during cool weather with short days can signifi cantly
disrupt harvesting and marketing schedules. In Hawaii, fruit developing from
precocious fl owering in some years leads to a second peak, followed by a mid-
year dip in production (Fig. 12.10).
Controlled ripening of fruit with ethephon prior to harvest can further
reduce the number of harvesting passes. Ethephon is applied 48 h or more
before harvest to accelerate shell degreening (Soler, 1992). This accelerated
shell degreening is due to destruction of chlorophyll, giving the shell a
more uniform colour. The application should occur when natural colouring
has started, to assure good fruit quality, and is sometimes less e ective in
hot weather.
Fruits destined for the cannery are usually harvested at the half- to three-
quarter-yellow stage. Fruit to be transported to distant fresh fruit markets is
picked anywhere from mature green (no yellow colour) to quarter-coloured
stage. Fruit maturity is evaluated on the extent of fruit ‘eye’ fl atness, skin
yellowing and acidity to total soluble solids (TSS) measurements. Consumers
similarly judge fruit quality by skin colour and aroma. A minimum reading
of 12% total soluble solids (
o
Brix) is required for fresh fruit in Hawaii, while
others have suggested 14%. A sugar to acid ratio of 0.9–1.3 is recommended
(Soler, 1992).
Pineapple for the fresh market is hand harvested, with pickers being
directed as to stage or stages (shell colour) of ripeness required. Fruit is
packed either in the fi eld or at a central packing shed. In Hawaii, pickers walk
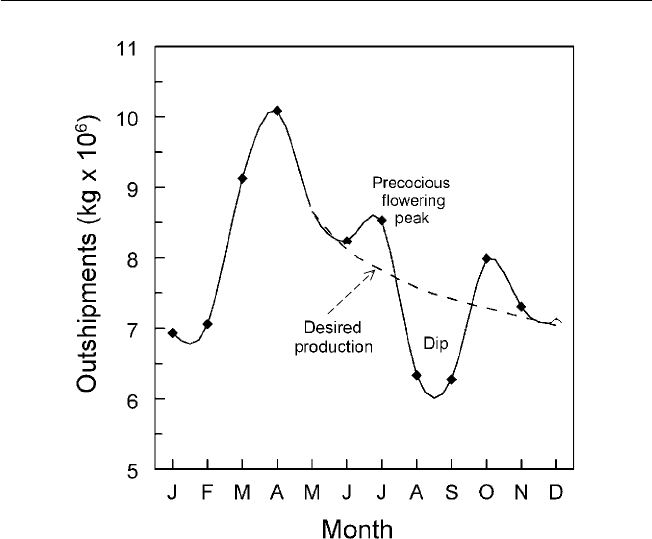
360 Chapter 12
in the inter-rows and place the fruit on a conveyor belt running on a boom,
which transfers the fruit to a truck fi eld bin. Fruit is placed in the bin by hand,
upside-down on the crown to avoid injury. Fruits are also harvested by pickers
carrying large baskets on their backs. When the baskets are full, the fruits are
dumped at the ends of the rows or at the side of fi eld roads, to be later loaded
into trucks or trailers. When the fruit arrives at the packing shed, it is unloaded
by hand, by submerging the fi eld bin in water or by sliding the fruit out of the
fi eld bin into water. Fruit with high translucency (‘sinkers’) are separated at
this step. Fully ripe, translucent fruit are unsuitable for transporting to distant
markets and less-mature fruit are selected. Immature fruits are not shipped,
since they do not develop good fl avour, have low sugars, and are more prone to
chilling injury. Care is taken to avoid mechanical damage to the crown leaves
and mechanical injury to the fruit shell.
Postharvest handling
Fruits are waxed after washing, frequently with polyethylene and para n or
carnauba and para n waxes. The selected wax reduces internal browning
Fig. 12.10. The effect of precocious fl owering in the January to February period on
outshipment of pineapples from Hawaii contrasted with desired production.

Pineapple 361
symptoms of chilling injury, reduces water loss, improves fruit appearance and
assures a more even application of fungicide used to control rots (Paull and
Rohrbach, 1985; Paull and Chen, 2003). There is no worldwide uniformity in
acceptance of wax components, so importing-country restrictions need to be
considered. If the wax injures the crown leaves, only the fruit body is waxed.
Postharvest fungicide is normally included in the wax.
Fresh fruit quality standards are based upon recognized appearance
characteristics (Table 12.6). The fruit needs to be mature, fi rm, well formed,
free of defects, have fl at eyes and a minimum soluble solids of 12% in Hawaii.
Crown size is a crucial grade component, with a minimum size and a set ratio
of crown to fruit length (0.33–1.5) for the higher grades. Crowns that develop
during the summer in Hawaii tend to be larger and may require ‘gouging’
at harvest to meet the standard. This ‘gouging’ leaves a wound for possible
disease entry and detracts from overall appearance. ‘Gouging’ 2 months
before harvest to limit crown growth avoids visible scarring. Fruits are graded
by degree of skin coloration, size (weight), absence of defects and disease, and
other market needs before packing (Soler, 1992).
Temperatures in the range 7.5–12°C are recommended for storage, with
relative humidity of 70–95%; the higher humidity signifi cantly reduces water
loss. At a temperature of 0–4°C, the fruit may be stored for weeks, but upon
removal, the fruit fails to continue ripening and shows severe chilling injury
(Paull, 1993). Half-ripe ‘Smooth Cayenne’ fruit can be held for about 10
days at 7.5–12.5°C and still have about a week of shelf-life, with no chilling-
induced internal browning.
Table 12.6. USA fresh pineapple fruit standards. All grades have 10% limits on
defects, 5% on serious damage and 1% on decay.
Standards
Varietal
character-
istics Maturity Free from Slips knobs
Crown to fruit
length ratio
US Fancy Similar Mature, fi rm,
dry, well
formed, well-
developed
eyes
Decay, sunburn,
injury,
bruising, well-
cured butt
Single
crown,
no slips
Ratio less than
1.5, not less
than 12 cm
in size
US #1 Similar Mature, fi rm,
dry, well
formed, well-
developed
eyes
Decay, sun-
burn, injury,
bruising, fairly
well-cured
butt
<5 Slips Ratio less than
2.0 and
greater than
10 cm in
size
US #2 Similar Mature, fi rm,
dry, fairly
well formed
eyes
Serious decay,
sunburn,
injury, bruising
Slips Any ratio,
double
crown

362 Chapter 12
Physiological disorders
Chilling injury
The maximum storage life of pineapple at 7°C is about 4 weeks; however, when
removed, chilling injury develops within 2–3 days. The symptoms of chilling
injury (CI) include: (i) wilting, drying and discoloration of crown leaves; (ii)
failure of green-shelled fruit to yellow; (iii) browning and dulling of yellow
fruit; and (iv) internal fl esh browning (Paull and Rohrbach, 1985). Preharvest
shading and pre- and postharvest low temperatures are the major factors
increasing CI symptom intensity. CI symptoms have been called endogenous
brown spot, physiological breakdown, blackheart and internal browning.
Postharvest CI symptoms develop after fruits are returned to physiological
temperatures (15–30°C). Susceptible fruits are generally lower in ascorbic acid
and sugars and are opaque (Teisson, 1979a,b). Partial to complete control of
CI symptom development has been achieved by waxing, polyethylene bagging,
heat treatments, controlled atmospheres and ascorbic acid application.
Flesh translucency
Flesh translucency increases fruit sensitivity to mechanical injury. This
condition begins before harvest and continues after harvest. TSS, fl esh
pigments and palatability increase to a maximum at about 60% translucency,
then decrease in fruit with greater translucency. Translucency is more severe
and has a higher incidence when maximum and minimum temperatures
3 months before harvest are both low, less than 23 and 15°C or, to a lesser
extent high, greater than 29 and 20°C, respectively. Fruit with a larger crown
has a lower incidence and severity of translucency (Paull and Reyes, 1996).
Bruising
Fruit bruising is a major problem during harvesting and packing. Bruising can
be caused by impact damage, a 30 cm drop causing signifi cant damage. This
injury is normally confi ned to the impact side of the fruit. The damaged fl esh
appears slightly straw-coloured. Mechanical injury of translucent fruit can
lead to leakage of fruit cell contents and loss of marketable fruit.
Sunburn
Sunburn is common during hotter periods (>35°C) of the year, when the fruit
is not shaded by leaves and especially in ratoon crops. The condition is more
prevalent in the outer rows and when fruit is lodged. Sun-scorched fruit fi rst
show a bleached yellow-white skin, which turns pale grey and brown, with
damage to the fl esh underneath. These damaged areas are more susceptible to
disease organisms, particularly yeasts and bacteria.

Pineapple 363
Malformations
Knobs on the base of fruit occur in o -types. Culling of the crowns of these
fruits as planting material reduces the subsequent fi eld incidence. These fruits
are not marketed, since trimming generally breaks the fruit skin and allows
rots to develop. The other genetic o -type is multiple crowns (fasciation), two
or more on each fruit, with the fruit taking on a fl attened appearance. This
condition is often related to high-temperature injury after forcing. These
fruits should not be marketed and crowns should not be used for planting.
Fruits with pronounced ‘eyes’ or fruitlets normally do not meet most grade
standards, and the thicker skin means lower fl esh recovery. This condition is
common in fruits that fl ower during cool weather. Some ‘Spanish’ varieties
are susceptible to broken core, in which the central fruit core has a transverse
break, leading to the upper part of the fruit ripening ahead of the bottom.
UTILIZATION
Pineapple does not sweeten after harvest, though the acid level may
decline. Canning is more successful with an acid fruit, as a lower processing
temperature can be used (Hepton and Hodgson, 2003). Because canned
product can be stored for long periods without deterioration, fruits destined
for canning tend to be harvested during the summer months, when fruit
quality is highest. The sugar to acid ratio varies widely with cultivar, growing
condition and stage of harvest from 80 to 200. Ascorbic acid also varies widely
with cultivar from 2.5 to 180 g/kg FM of fl esh. Pineapple is a good source
of ascorbic acid (vitamin C), some vitamin A, calcium, phosphorus, iron,
potassium and thiamine. It is low in sodium (Table 12.7).
Most fruit produced goes into the fresh fruit market (Fig. 12.11), mostly
to markets within a country. Much of this fruit is not recorded in world
production fi gures. There is an increasing interest in minimally processed
pineapples, with the shell and core removed just before purchase. The major
processed products are canned slices or solid pack, with a recovery percentage
varying from 20% in ‘Singapore Spanish’ to ca. 60% for ‘Smooth Cayenne’.
Cans of several sizes are fi lled with the best slices. Uncanned and broken slices
are packed as chunks, tidbits or crush (Fig. 12.11). Flesh remaining on the
shell, cut ends, core and trimmings are processed into juice. Much of the juice
is now concentrated, with the volatile fl avour components being recovered
and added back to the concentrate, and frozen to maintain a higher fl avour
quality than the single strength. Shipping costs for the concentrate also are
greatly reduced relative to those for single-strength juice. The residue of the
juice recovery stream is processed into animal feed or other by-products. This
residue from pressed fruit shells and pulp has been sold to dairy farms either
wet or dried (Fig. 12.11).
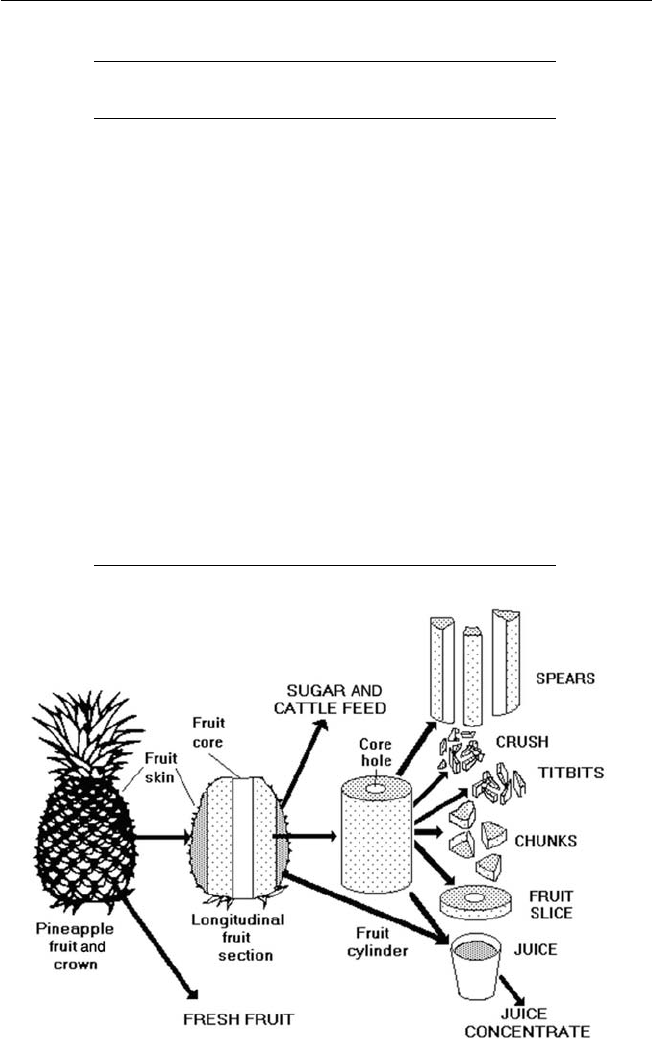
364 Chapter 12
Table 12.7.
Proximate analysis of ‘Smooth Cayenne’ fruit (Wenkam, 1990).
Amount per 100 g
edible portion
Water (g) 86
Energy (kJ) 218
Protein (g) 0.5
Lipid (g) 0.2
Carbohydrate (g) 13.5
Fibre (g) 0.5
Ash (g) 0.3
Minerals
Calcium (mg) 18
Iron (mg) 0.3
Magnesium (mg) 12
Phosphorus (mg) 12
Potassium (mg) 98
Sodium (mg) 1
Vitamins
Ascorbic acid (mg) 10
Thiamin (mg) 0.09
Ribofl avin (mg) 0.04
Niacin (mg) 0.24
Vitamin A (IU) 53
Fig. 12.11. All parts of the pineapple fruit are used in different products (Rohrbach,
2003).

Pineapple 365
Bromelain, the proteolytic enzyme found in pineapple plants, particularly
in the stem, has a number of industrial and medicinal applications. Some
bromeliads, including pineapple, are grown for their leaf fi bres, and many
more are grown as ornamentals.
FURTHER READING
Bartholomew, D.P. and Malézieux, E.P. (1994) Pineapple. In. Scha er, B. and Andersen,
P.C. (eds) Handbook of Environmental Physiology of Fruit Crops Vol. II. Subtropical and
Tropical Crops. CRC Press, Boca Raton, Florida, pp. 243–291.
Bartholomew, D.P., Malézieux, E., Sanewski, G.M. and Sinclair, E. (2003) Infl orescence,
and fruit development and yield. In: Bartholomew, D.P., Paull, R.E. and Rohrbach,
K.G. (eds) The Pineapple: Botany, Production and Uses. CABI Publishing, Wallingford,
UK, pp. 167–202.
Hepton, A. (2003) Culture system. In: Bartholomew, D.P., Paull, R.E. and Rohrbach,
K.G. (eds) The Pineapple: Botany, Production and Uses. CABI Publishing, Wallingford,
UK, pp. 109–142.
Leal, F. and Coppens d’ Eeckenbrugge, G. (1996) Pineapple. In. Janick, J. and Moore,
J.N. (eds) Fruit Breeding, Vol. I: Tree and Tropical Fruits. John Wiley & Son, New York,
pp. 515–557.
Paull, R.E. and Chen, C.-C. (2003) Postharvest physiology, handling, and storage of
pineapple. In: Bartholomew, D.P., Paull, R.E. and Rohrbach, K.G. (eds). The Pineapple:
Botany, Production and Uses. CABI Publishing, Wallingford, UK, pp. 253–279.
Py, C., Lacoeuilhe, J.J. and Teisson, C. (1987) The Pineapple, Cultivation, and Uses. G.P.
Maisoneuve et Larose, Paris, France.
Rohrbach, K.G. and Johnson, M. (2003) Pests, diseases and weeds. In: Bartholomew,
D.P., Paull, R.E. and Rohrbach, K.G. (eds) The Pineapple: Botany, Production and Uses.
CABI Publishing, Wallingford, UK, pp. 203–251.
Soler, A. (1992) Pineapple – Quality Criteria. CIRAD-COLEACP, Montpellier, France.

366
REFERENCES
CHAPTER 1. INTRODUCTION
Codex Alimentarius (2001) Food Labelling Complete Texts. Joint FAO/WHO Food, Standards
Programme Codex Alimentarius Commission. FAO, Rome.
Crane, P.R. and Lidgard, S. (1989) Angiosperm diversifi cation and paleolatitudinal
gradients in Cretaceous fl oristic diversity. Science 246, 675–678.
FAO (2009) FAO Crop Production Statistics. FAO, Rome. http://faostat.fao.org/site/567/
default.aspx#ancor, accessed 17 November 2009.
F
avier, J.C., Ireland-Ripert, J., Laussucq, C., and Feinberg, M., CIQUAL – CNEVA (1993)
Table de composition des fruits exotiques, fruits de ceuillette d’Afrique. ORSTOM editions,
Lavoisier, INRA editions.
Meyer, N., Mittermeier, R.A., Mittermeier, C.G., da Fonseca, G.A.B. and Kent, J. (2000)
Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
Nakasone, H.Y. and Paull, R.E. (1998) Tropical Fruits. CAB International, Wallingford,
UK.
Sabbe, S., Verbeke, W. and van Damme, P. (2009) Confi rmation/disconfi rmation
of consumers’ expectations about fresh and processed tropical fruit products.
International Journal of Food Science & Technology 44, 539–551.
CHAPTER 2. THE TROPICS, ITS SOILS AND HORTICULTURE
Bluthgen, J. (1966) Allgemeine Klimageographie, 2nd edn. Walter de Gruyter, Berlin.
Cerri, C.E.P., Sparovek, G., Bernoux, M., Easterling, W.E., Melillo, J.M. and Cerri, C.C.
(2007) Tropical agriculture and global warming: impacts and mitigation options.
Scientia Agricola. 64, 83–99.
Easterling, W.E. and Apps, M. (2005) Assessing the consequences of climate change for
food and forest resources: a view from the IPCC. Climatic Change 70, 165–189.
Gates, D.M. (1966) Spectral distribution of solar radiation at the earth’s surface. Science
151, 523–529.
Gray, W.M. (1968) A global view of the origin of tropical disturbances and storms.
Monthly Weather Review 96, 669–700.

References 367
Ingram, J.S.I., Gregory, P.J. and Izac, A.M. (2008) The role of agronomic research in
climate change and food security policy. Agriculture, Ecosystems & Environment
126, 4–12.
Kalpage, F.S.C.P. (1976) Tropical Soils: Classifi cation, Fertility and Management. Macmillan,
London.
Oliver, J.E. and Hidore, J.J. (1984) Climatology. Charles E. Merrill Publishing Company,
Columbus, Ohio.
Sanchez, P.A. (1976) Properties and Management of Soils in the Tropics. Wiley-Interscience
Publishers, New York.
Sanchez, P.A. and Buol, S.W. (1975) Soils of the tropics and the world food crisis. Science
188, 598–603.
Sanchez, P.A. and Salinas, J.G. (1981) Low input technology for managing oxisols and
ultisols in tropical America. Advances in Agronomy 34, 279–406.
Schultz, J. (2005) The Ecozones of the World: the Ecological Divisions of the Geosphere, 2nd
ed. Springer, Berlin.
Sutherst, R., Baker, R.H.A., Coakley, S.M., Harrington, R., Kriticos, D.J. and Scherm, H.
(2007) Pests under global change – meeting your future landlords? In: Candell, J.G.
(ed.) Terrestrial Ecosystems in a Changing World. Springer, New York, pp. 211–226.
Thornthwaite, C.W. (1948) An approach toward a rational classifi cation of climate.
Geographic Review 38, 55–94.
Walter, H. (1973) Vegetation of the Earth. In Relation to Climate and Ecophysiological
Conditions, 2nd edn. Translated by Joy Weiser. Springer-Verlag, Berlin.
Watson, B.J. and Moncur, M. (1985) Criteria for Determining Survival: Commercial and
Best Minimum July Temperatures for Various Tropical Fruits in Australia. Wet Tropical
Regional Publication, Queensland, Australia.
CHAPTER 3. CULTIVATION
Avilán, L., Leal, F. and Bautista, D. (1989) Manual de Fruticultura. Editorial América,
Caracas, Venezuela (in Spanish).
Campbell, C.W. and Goldweber, S. (1985) Rare and Exotic Tropical Fruits: Trees and Plants.
Caloosa Rare Fruit Exchange, Ft. Myers, Florida.
Maxwell, L.S. and Maxwell, B.M. (1985) Florida Fruit. B.M. Maxwell Publisher, Florida.
Razeto, B. (1993) Para Entender la Fruticultura, 2nd edn. Vivarium, Santiago de Chile,
Chile (in Spanish).
Rice, E.L. (1974) Allelopathy. Academic Press, New York.
Wilkinson, K.M. and Elevitch, C.R. (2000) Multipurpose Windbreaks, Design and Species
for the Pacifi c Islands. Agroforestry Guides for Pacifi c Islands No. 8. Permanent
Agriculture Resources, Holualoa, Hawaii.
CHAPTER 4. TREE MANAGEMENT
Avilán, L., Leal, F. and Bautista, D. (1989) Manual de Fruticultura. Editorial América,
Caracas, Venezuela (in Spanish).
Baldini, E. (1992) Arboricultura General. Editorial Mundi-Prensa, Madrid (in Spanish).

368 References
Garner, R.J. and Chaudhri, S.A. (1976) The Propagation of Tropical Fruit Trees. Horticultural
Review No. 4. Commonwealth Bureau of Horticulture and Plantation Crops,
Commonwealth Agricultural Bureaux, Farnham Royal, Slough, UK.
Harris, R. (1983) Arboriculture: Care of Trees, Shrubs and Vines in the Landscape. Prentice-
Hall Inc., Englewood Cli s, New Jersey.
Jones, J.B., Wolf, B. and Mills, H.A. (1991) Plant Analysis Handbook. Micro-Macro
Publishing, Athens, Georgia.
McNeil, R.J. (2001) California Avocado Production. Horticulture and Crop Science
Department. Cal Poly State University, San Luis Obispo, California.
Penman, D., Close, R. and Jackson, D.I. (2003) Protección de los cultivos. In: Jackson,
D.I. and Looney, N.E. (eds) Producción de Frutas de Climas Templados y Subtropicales.
Ed. Acribia, S.A., Zaragoza, Spain (in Spanish).
Razeto, B. (1993) Para Entender la Fruticultura, 2nd edn. Vivarium, Santiago de Chile.
Chile (in Spanish).
Reuter, D.J. and Robinson, J.B. (eds) (1986) Plant Analysis: an Interpretative Manual.
Inkata Press, Melbourne, Australia.
Verheij, E.W.M. (1986) Towards a classifi cation of tropical fruit trees. Acta Horticulturae
175, 137–150.
CHAPTER 5. POSTHARVEST TECHNOLOGY
Abbott, J.A. (1999) Quality measurement of fruits and vegetables. Postharvest Biology
and Technology 15, 201–206.
Ben-Yehoshua, S. (1987) Transpiration, water stress, and gas exchange. In: Weichmann,
J. (ed.) Postharvest Physiology of Vegetables. Marcel Dekker Inc. New York, pp.
113–170.
Brackett, R.E. (1999) Incidence, contributing factors, and control of bacterial pathogens
in produce. Postharvest Biology and Technology 15, 305–312.
Chen, N.M. and Paull, R.E. (1986) Development and prevention of chilling injury
in papaya fruit. Journal of the American Society for Horticultural Science 111,
639–643.
Cohen, E., Shalom, Y. and Rosenberger, I. (1990) Postharvest ethanol buildup and o -
fl avour in ‘Murcott’ tangerine fruits. Journal of the American Society for Horticultural
Science 115, 775–778.
Cook, R. (1999) An overview of key food industry drivers: implications for fresh produce
industry. Journal of Food Distribution Research 30, 1–4.
Epperson, J.E. and Estes, E.A. (1999) Fruit and vegetable supply chain management,
innovations and competitiveness: Cooperative Regional Project S-222. Journal of
Food Distribution Research 30, 38–43.
Ga rey, J.J. (1978) Humidity: basic principles and measurement techniques. HortScience
13, 551–555.
Hewett, E.W. (2003) Perceptions of supply-chain management of perishable horticultural
crops: an introduction. Acta Horticulturae 604, 37–46.
Kader, A.A. (1993) Modifi ed and controlled atmosphere storage of tropical fruits. In:
Champ, B.R., Highley, E. and Johnson, G.I. (eds) Postharvest Handling of Tropical
Fruits. ACIAR, Canberra, Australia, pp. 239–249.

References 369
LeBlanc, D.I., Stark, R., MacNeil, B., Goguen, B. and Beraulieu, C. (1996) Perishable food
temperature in retail stores. In: New Development in Refrigeration for Food Safety and
Quality. International Institute for Refrigeration Commission C2, 1996-6, 42–57.
O’Hare, T.J. (1993) Postharvest physiology and storage of carambola (starfruit): a
review. Postharvest Biology and Technology 2, 257–267.
O’Hare, T.J. (1995) Postharvest physiology and storage of rambutan. Postharvest Biology
and Technology 6, 189–199.
Paull, R.E. (1993) Tropical fruit physiology and storage potential. In: Champ, B.R.,
Highley, E. and Johnson, G.I. (eds) Postharvest Handling of Tropical Fruits. ACIAR,
Canberra, Australia, pp. 198–204.
Paull, R.E. (1994) Response of tropical commodities to insect disinfestation treatments.
HortScience 29, 16–24.
Paull, R.E. (1999) E ect of temperature and relative humidity on fresh commodity
quality. Postharvest Biology and Technology 15, 263–278.
Paull, R.E. and Armstrong, J. (1994) Introduction. In: Paull, R.E. and Armstrong, J.W.
(eds) Insect Pests and Fresh Horticultural Products: Treatments and Responses. CAB
International, Wallingford, UK, pp. 1–36.
Paull, R.E. and Chen, N.J. (2000) Heat treatments and fruit ripening. Postharvest Biology
and Technology 21, 21–37.
Paull, R.E., Nishijima, W., Reyes, M. and Cavaletto, C.G. (1997) Postharvest handling
and losses during marketing of papaya (Carica papaya L). Postharvest Biology and
Technology 11, 165–179.
Prussia, S.E. (2000) Soft systems methodologies for modeling postharvest chains. Acta
Horticulturae 536, 653–660.
Prussia, S.E., and Shewfelt, R.L. (1993) Systems approach to postharvest handling.
In: Shewfelt, R.L. and Prussia, S.E. (eds) Postharvest Handling. A Systems Approach.
Academic Press, New York, pp. 44–71.
Qi, S.C. (1982) The application of mechanical refrigeration to cave storage of fruit.
International Journal of Refrigeration 5, 235–237.
Robinson, J.E., Browne, K.M. and Burton, W.G. (1975) Storage characteristics of some
vegetables and soft fruits. Annals of Applied Biology 81, 399–408.
Sastry, S.K., Baird, C.D. and Bu ngton, D.E. (1978) Transpiration rates of certain fruits
and vegetables. ASHRAE Transactions 84, 237–255.
Shewfelt, R.L. (1999) What is quality? Postharvest Biology and Technology 15, 197–200.
Snowdon, A.L. (1990) A Color Atlas of Post-harvest Diseases and Disorders of Fruits and
Vegetables Vol 1: General Introduction and Fruits. CRC Press, Boca Raton, Florida.
Thompson, A.K. (1996) Postharvest Technology of Fruit and Vegetables. Blackwell Science
Ltd, Oxford.
Wills, R.B.H. and McGlasson, W.B. (1970) Loss of volatiles by apples in cool storage:
a di erential response to increased water loss. Journal of Horticultural Science 45,
283–286.
Yahia, E.M. (1998) Modifi ed and controlled atmospheres for tropical crops. Horticultural
Reviews 22, 123–183.
Zagory, D. (1999) E ects of post-processing handling and packaging on microbial
populations. Postharvest Biology and Technology 15, 313–321.

370 References
CHAPTER 6. ANNONAS: CHERIMOYA, ATEMOYA AND
SWEETSOP
Alvarez-Garcia, L.A. (1949) Anthracnose of the Annonaceae in Puerto Rico. University
of Puerto Rico Journal of Agriculture 33, 27–43.
Campbell, C.W. (1985) Cultivation of fruits of the Annonaceae in Florida. Proceedings of
the American Society for Horticultural Science of the Tropical Region 29, 68–70.
Chen, C.C. and Paull, R. (2008) Annona squamosa, sweetsop. In: Janick, J. and Paull, R.
(eds) The Encyclopedia of Fruit and Nuts. CAB International, Wallingford, UK, pp.
48–53.
Cresswell, G. and Sanewski, G. (1991) Diagnosing nutrient disorders. In. Sanewski, G.
(ed.) Custard Apples – Cultivation and Crop Protection. Information Series QI90031,
Queensland Department of Primary Industry, Brisbane, Australia.
Dhingra, J., Mehrotra, R.S. and Aneja, I.R. (1980) A new postharvest disease of Annona
squamosa L. Current Science 49, 477–478.
Duarte, O. and Escobar, O. (1994) Mejora del cuajado de chirimoya (Annona cherimola
Mill.) cv. Cumbe, mediante polinización manual autógama y alógama. Proceedings
Interamerican Society for Tropical Horticulture 41, 162–165.
Duarte, O., Ramírez, A. and Franciosi, R. (1974a) Improving cherimoya fruit set with
plant regulators. Proceedings of the XIXth International Horticultural Congress.
Warsaw. IB, 459.
Duarte, O., Villagarcía, J. and Franciosi, R. (1974b) Efecto de algunos tratamientos en la
propagación de chirimoya (Annona cherimola Mill.) por semillas, estacas e injertos.
Proceedings of the Tropical Region of the American Society for Horticultural Science 18,
41–48 (in Spanish, English summary).
Duarte, O., Pineda, A. and Rodriguez, P.P. (1997) Mejora del cuajado en atemoya ‘Gefner’
(Annona cherimola × Annona squamosa) con diversos tratamientos de polinizacion
manual. Proceedings Interamerican Society for Tropical Horticulture 44, 92–94.
Ellstrand, N.C. and Lee, J.M. (1987) Cultivar identifi cation of cherimoya (Annona
cherimola Mill.) using isozyme markers. Scientia Horticulturae 32, 25–31.
Franciosi, R. (1992) El Cultivo del Chirimoyo en el Perú. Ediciones FUNDEAGRO, Lima,
Peru (in Spanish).
Gardiazabal, F. and Rosenberg, G. (1993) El Cultivo del Chirimoyo. Ediciones Universitarias
de Valparaíso, Valparíso, Chile (in Spanish).
Gazit, S. and Eistenstein, D. (1985) Floral biology of Annona squamosa and Annona
cherimola in relation to spontaneous appearance of atemoya in Israel. Proceedings
of the Tropical Region of the American Society for Horticultural Science 29, 66–67.
George, A.P. and Nissen, R.J. (1986a) E ect of pruning and defoliation on precocity
of bearing of custard apple (Annona atemoya Hort.) var. African Pride. Acta
Horticulturae 175, 237–241.
George, A.P. and Nissen, R.J. (1986b) The e ects of root temperature on growth and dry
matter production of Annona species. Scientia Horticulturae
31, 95–99.
George, A.P. and Nissen, R.J. (1987a) E ects of cincturing, defoliation and summer
pruning of vegetative growth and fl owering of custard apple (Annona cherimola
× Annona squamosa) in subtropical Queensland. Australian Journal of Experimental
Agriculture 27, 915–918.
George, A.P. and Nissen, R.J. (1987b) The e ects of day/night temperatures on growth
and dry matter production of custard apple. Scientia Horticulturae 31, 269–274.

References 371
George, A.P. and Nissen, R.J. (1988) The e ects of temperature, vapor pressure defi cit and
soil moisture stress on growth, fl owering and fruit set of custard apple (atemoya)
‘African Pride’. Scientia Horticulturae 34, 183–191.
George, A.P., Nissen, R.J. and Carseldine, M.L. (1989) E ect of season (vegetative
fl ushing) and leaf position on the leaf nutrient composition of Annona spp. hybrid
cv. Pink’s Mammoth in south-eastern Queensland. Australian Journal of Experimental
Agriculture 29, 587–595.
Kshirsaga, S.V., Shinde, N.N., Rane, D.A. and Borikar, S.T. (1976) Studies on the fl oral
biology in atemoya (Annona atemoya Hort.). South Indian Horticulture 24, 6–10.
Kumar, R., Hoda, M.N. and Singh, D.K. (1977) Studies on the fl oral biology of custard
apple (Annona squamosa Linn). Indian Journal of Horticulture 34, 252–256.
Lo, S.S. (1987) Pruning technique, use of sprouting chemical and fl ower initiation in
sugar apple (Annona squamosa). In: Chang. L.R. (ed.) Forcing Culture of Horticultural
Crops. Special Publication No. 10. Taichung District Agricultural Improvement
Station, Taichung, Taiwan (in Chinese, English summary), pp. 147–150.
Marler, J.E., George, A.P., Nissen, R.J. and Andersen, P.J. (1994) Miscellaneous
tropical fruits – annonas. In: Scha er. B.C. and Andersen, P.C. (eds) Handbook of
Environmental Physiology of Fruit Crops, Vol. II. Subtropical and Tropical Crops. CRC
Press, Boca Raton, Florida, pp. 200–206.
Pascual, L., Perfectti, F., Gutierres, M. and Vargas, A.M. (1993) Characterizing isozymes
of Spanish cherimoya cultivars. HortScience 28, 845–847.
Paull, R. (2008) Annona cherimola, cherimoya. In: Janick, J. and Paull, R. (eds) The
Encyclopedia of Fruit and Nuts. CAB International, Wallingford, UK, pp. 37–42.
Razeto, B. and Diaz de Valdes, E. (2001) E ect of summer pruning and bark girdling
on cherimoya (Annona cherimola Mill.) var. Concha Lisa. Agricultura Tecnica 6,
215–220.
Saavedra, E. (1979) Set and growth of Annona cherimola Mill. fruit obtained by hand-
pollination and chemical treatment. Journal of the American Society for Horticultural
Science 104, 668–673.
Samuel, R., Pineker, W., Balasubramaman, S. and Morawetz, W. (1991) Allozyme
diversity and systematics in Annonaceae – a pilot project. Plant System Evolution
178, 125–134.
Sanewski, G.M. (ed.) (1991) Custard Apples – Cultivation and Crop Protection. Information
Series QI90031, Queensland Department of Primary Industry, Brisbane,
Australia.
Smith, D. (1991) Insect pests. In: Sanewski, G. (ed.) Custard Apples – Cultivation and
Crop Protection
. Information Series QI90031, Queensland Department of Primary
Industry, Brisbane, Australia, pp. 73–79.
Thakur, D.R. and Singh, R.N. (1964) Studies on pollen morphology, pollination and fruit
set in some annonas. Indian Journal of Horticulture 22, 10–17.
Thakur, D.R. and Singh, R.N. (1965) Studies on fl oral biology of Annonas. Indian Journal
of Horticulture 23, 238–252.
Wenkam, N.S. (1990) Foods of Hawaii and the Pacifi c Basin. Fruits and Fruit Products, Raw,
Processed, and Prepared, Vol. 4, Composition. Research Extension Series 110, HITAHR,
College of Tropical Agriculture and Human Resources, Honolulu, Hawaii.
Yang, C.S. (1987) Production of sugar-apple fruits in the winter. In: Chang, L.R. (ed.)
Forcing Culture of Horticultural Crops. Proceedings of a Symposium. Special Publication
No. 10, Taichung District Agricultural Improvement Station, Taichung, Taiwan,
pp. 129–140.

372 References
Yang, C.S. (1988) Application of plant growth regulators on Annona culture. In: Lin,
H.S., Chang, L.R. and Lin, J.H. (eds) The Application of Plant Growth Regulators on
Horticultural Crops. Symposium Proceedings. Special Publication No. 12, Taichung
District Agricultural Improvement Station, Changhua, Taiwan (Chinese, English
summary), pp. 305–320.
CHAPTER 7. AVOCADO
Ahmed, E.M. and Barmore, C.R. (1980) Avocado. In: Nagy, S. and Shaw, P.E. (eds) Tropical
and Subtropical Fruits. AVI Publishing, Westport, Connecticut, pp. 121–156.
Anon. (1995) International Standardisation of Fruits and Vegetables, Avocado. Organization
for Economic Cooperation and Development, Paris.
Arpaia, M.L., Bender, G.S. and Witney, G.W. (1992) Avocado clonal rootstock production
trial. In: Proceedings of the 2nd World Avocado Congress, Vol. I. University of California,
Riverside, California, pp. 305–310.
Ashworth, V.E.T.M. and Clegg, M.T. (2003) Microsatellite markers in avocado (Persea
americana Mill.): genealogical relationships among cultivated avocado genotypes.
Journal of Heredity 94, 407–415.
Barmore, C.R. (1976) Avocado fruit maturity. In: Sauls, J.W., Phillips, R.L. and Jackson,
L.K. (eds) The Avocado: Proceedings of the 1st International Tropical Fruit Short Course.
Fruit Crops Department, University of Florida, Gainesville, Florida, pp. 103–109.
Barrientos-Priego, A.F., Muñoz-Pérez, R., Borys, M.W. and Martinez-Damián, M.T.
(2000) Cultivares y portainjertos del aguacate. In: Téliz, D. (coord.). El aguacate y su
manejo integrado. Ediciones Mundi Prensa, México, Madrid, Barcelona.
Ben-Ya’acov, A. and Michelson, E. (1995) Avocado rootstocks. Horticulture Reviews 17,
381–429.
Ben-Ya’acov, A., Solís, A. and Peri, E. (1995) Progress in the study of avocado genetic
resources. II. The avocado genetic resources in Costa Rica. Programme and book
of abstracts of the 3rd World Avocado Congress. 22–27 October, Tel Aviv, Israel,
p. 109.
Bergh, B.O. (1969) Avocado. In: Ferweda, F.P. and Wit, F. (eds) Outline of Perennial
Crop Breeding in the Tropics. Landbouwhogeschool, Miscellaneous Paper No. 4,
Wageningen, The Netherlands, pp. 23–51.
Bergh, B.O. (1975) Avocados. In: Janick, J. and Moore, J.N. (eds) Advances in Fruit
Breeding. Purdue University Press, West Lafayette, Indiana, pp. 541–567.
Bergh, B.O. (1976) Avocado breeding and selection. In: Sauls, J.W., Phillips, R.L. and
Jackson, L.K. (eds) The Avocado. Proceedings of the 1st International Tropical Fruit
Short Course. Fruit Crops Department, University of Florida, Gainesville, Florida,
pp. 24–33.
Bergh, B. (1990a) The avocado and human nutrition, I. Some human health aspects of
the avocado. In: Proceedings of the 2nd World Avocado Congress, Vol. I. University of
California, Riverside, California, pp. 25–35.
Bergh, B. (1990b) The avocado and human nutrition, II. Avocado and your heart.
In: Proceedings of the 2nd World Avocado Congress, Vol. I. University of California,
Riverside, California, pp. 37–47.
Bergh, B. O. and Ellstrand, N.C. (1986) Taxonomy of the avocado. California Avocado
Society Yearbook 70, 135–145.

References 373
Bower, J.P. (1981a) Climatic Requirements of Avocado. B.l, Farming in South Africa,
Department of Agriculture and Water Supply, Pretoria, South Africa.
Bower, J.P. (1981b) Layout of an Avocado Orchard. D.3, Farming in South Africa,
Department of Agriculture and Water Supply, Pretoria, South Africa.
Bower, J.P. (1981c) Avocado Pollination and Pollinator Interplanting. G.4, Farming in
South Africa, Department of Agriculture and Water Supply, Pretoria, South Africa
(reprinted 1986).
Broadley, R.H. (ed.) (1991) Avocado Pests and Disorders. Information Series QI90013,
Queensland Department of Primary Industry, Brisbane, Australia.
Brokaw, W.H. (1975) Root rot resistant avocado clonal rootstocks. Plant Propagator
21(4), 7–8.
Campbell, C.W. and Malo, S.E. (1976) A survey of avocado cultivars. In: Sauls, J.W.,
Phillips, R.L. and Jackson, L.K. (eds) The Avocado. Proceedings of the 1st International
Tropical Fruit Short Course. Fruit Crops Department, University of Florida,
Gainesville, Florida, pp. 20–24.
Chaikiattiyos, S., Menzel, C.M. and Rasmussen, T.S. (1994) Floral induction in tropical
fruit trees, e ects of temperature and water supply. Journal of Horticultural Science
69, 397–415.
Chen, H., Morrell, P.L., Ashworth, V.E.T.M., de la Cruz, M. and Clegg, M.T. (2009) Tracing
the geographic origins of major avocado cultivars. Journal of Heredity 100, 56–65.
Co ey, M.D. (1987) Phytophthora root rot of avocado – an integrated approach to
control in California. Plant Disease 71, 1046–1052.
Crane, J.H., Scha er, B., Davenport, T.L. and Balerdi, C. (1992) Rejuvenation of a mature,
non-productive ‘Lula’ and ‘Booth 8’ avocado grove by topping and tree removal.
Proceedings of the Florida State Horticultural Society 105, 282–285.
Currier, W. (1992) New variety introduction under US conditions. In: Proceedings of the
2nd World Avocado Congress, Vol. II. University of California, Riverside, California,
pp. 609– 613.
Cutting, J.G.M., Cocker, B. and Wolstenholme, B.N. (1994) Time and type of pruning
cut a ect shoot growth in avocado (Persea americana (Mill.)). Journal of Horticultural
Science 69, 75–80.
Davenport, T.L., Parnitzki, P., Fricke, S. and Hughes, M.S. (1994) Evidence and
signifi cance of self-pollination of avocado in Florida. Journal of the American Society
for Horticultural Science 119, 1200–1207.
de Villiers, A. and Ernst, A. (2007) Practical value of the Allesbeste microcloning
technique. Proceedings of the 6th World Avocado Congress, Viña del Mar, Chile.
Available at http://www.avocadosource.com/WAC6/en/Extenso/1a-5.pdf
(accessed 22 May 2010).
Diaz-Avelar, J. (1979) The Cultivation of Avocado. XXV Anniversary, FIRA, Banco de
Mexico (in Spanish).
Duarte, O., Balvin, A. and Franciosi, R. (1975) Efecto de diversos tratamientos con
ácido giberélico sobre el crecimiento de plántulas de palto (Persea americana Mill)
en vivero. Proceedings of the Tropical Region, American Society for Horticultural Science
19, 45–55.
Finazzo, S.A., Davenport, T.L. and Scha er. B. (1994) Partitioning of photoassimilates
in avocado (Persea americana Mill.) during fl owering and fruit set. Tree Physiology
14, 153–164.
Furnier, G.P., Cummings, P.M. and Clegg, M.T. (1990) Evolution of the avocado as
revealed by DNA restriction fragment variation. Journal of Heredity 81, 183–188.

374 References
Gabor, B.K., Guillemet, F.B. and Co ey, M.D. (1990) Comparison of fi eld resistance to
Phytophthora cinnamoni in twelve avocado rootstocks. HortScience 25, 1655–1656.
Garcia, V.A. (1975) Cytogenetic studies in the genus Persea (Lauraceae). I. Karyology of
seven species. Canadian Journal of Genetic Cytology 17, 170–180.
Garcia, VA. and Tsunewaki, K. (1977) Cytogenetical studies in the genus Persea
(Lauraceae). III. Electrophoretical studies on peroxidase isoenzymes. Japanese Journal
of Genetics 52, 379–386.
Gazit, S. (1976) Pollination and fruit set of avocado. In: Sauls, J.W., Phillips, R.L. and
Jackson, L.K. (eds) The Avocado. Proceedings of the 1st International Tropical Fruit
Short Course. Fruit Crops Department, University of Florida, Gainesville, Florida,
pp. 88–92.
Gomez, R.E., Soule, J. and Malo, S.E. (1973) Anatomical aspects of avocado stems with
reference to rooting. Proceedings of the American Society for Horticultural Science of
Tropical Region 17, 23–28.
Gustafson, D. (1976) Avocado water relations. In: Sauls, J.W., Phillips, R.L. and Jackson,
L.K. (eds) The Avocado. Proceedings of the 1st International Tropical Fruit Short Course.
Fruit Crops Department, University of Florida, Gainesville, Florida, pp. 47–53.
Hatton, T.T. Jr and Campbell, C.W. (1959) Evaluation indices for Florida avocado
maturity. Proceedings of the Florida State Horticultural Society 72, 349–353.
Hatton, T.T. and Reeder, W.F. (1972) Relationship of bloom date to the size and oil
content of ‘Booth 8’ avocados. Citrus Industry 53, 20–21.
Kadman, A. and Ben-Ya’acov, A. (1970a) Avocado, selection of rootstocks and other
work related to salinlty and time. In: 1960–1969 Report. Division of Subtropical
Horticulture, Volcani Institute of Agricultural Research, Bet Dagan, Israel, pp.
23–39.
Kadman, A. and Ben-Ya’acov, A. (1970b) Vegetative propagation, A. Rooting of leaf-
bearing cuttings. In: 1960–1969 Report. Division of Subtropical Horticulture,
Volcani Institute of Agricultural Research, Bet Dagan, Israel, pp. 47–50.
Kaiser, C. and Wolstenholme, B.N. (1994) Aspects of delayed harvest of ‘Hass’
avocado (Persea americana Mill.) fruit in a cool subtropical climate. II. Fruit size,
yield, phenology and whole tree starch cycling. Journal of Horticultural Science 69,
447–457.
Kawano, Y., Hylin, J.W. and Hamilton, R.A. (1976) Plant Products of Economic Potential in
Hawaii. III. Quantity and Quality of Oil Obtained from Hawaii-grown Avocado Varieties.
Research Report 211, Hawaii Agricultural Experimental Station, Honolulu,
Hawaii.
Knight. R.J. Jr (1976) Breeding avocados for cold hardiness. In: Sauls, J.W., Phillips, R.L.
and Jackson, L.K. (eds) The Avocado. Proceedings of the 1st International Tropical Fruit
Short Course. Fruit Crops Department, University of Florida, Gainesville, Florida,
pp. 33–36.
Kremer-Köhne, S. and Köhne, J.S. (2007) 25 years of avocado rootstock development
in South Africa. Proceedings of the 6th World Avocado Congress
, Viña del Mar,
Chile. Available at http://www.avocadosource.com/WAC6/en/Extenso/1a-8.pdf
(accessed 22 May 2010).
Lavi, U., Lahav, E., Cenizi. A., Degani, D., Gazit, S. and Hillel, J. (1991) Quantitative
genetic analysis of traits in avocado cultivars. Plant Breeding 106, 149–160.
Lavi, U., Lahav, E., Degani, C. and Gazit, S. (1992) The genetics of the juvenile phase
in avocado and its application for breeding. Journal of the American Society for
Horticultural Science 117, 981–984.

References 375
Lavi, U., Lahav, E., Degani, C. and Gazit, S. (1993a) Genetics of skin color, fl owering
group and anise scent in avocado. Journal of Heredity 84, 82–84.
Lavi, U., Lahav, E., Degani, C., Gazit, S. and Hillel, J. (1993b) Genetic variance components
and heritabilities of several avocado traits. Journal of the American Society for
Horticultural Science 118, 400–404.
Malo, S.E. (1976) Mineral nutrition of avocados. In: Sauls, J.W., Phillips, R.L. and
Jackson, L.K. (eds) The Avocado. Proceedings of the 1st International Tropical Fruit
Short Course. Fruit Crops Department, University of Florida, Gainesville, Florida,
pp. 42–46.
McKellar, M.A., Buchanan, D.W., Ingram, D.L. and Campbell, C.W. (1992) Freezing
tolerance of avocado leaves. HortScience 27, 341–343.
McMillan, R.T. Jr (1976) Diseases of avocado. In: Sauls, J.W., Phillips, R.L. and Jackson,
L.K. (eds) The Avocado. Proceedings of the 1st International Tropical Fruit Short Course.
Fruit Crops Department, University of Florida, Gainesville, Florida, pp. 66–70.
Miller, C.D., Bazore, K. and Bartow, M. (1965) Fruits of Hawaii. Description. Nutritive
Value, and Recipes. University of Hawaii Press, Honolulu, Hawaii.
Mora, A., Teliz, D., Mora, G. and Etchevers, J. (2000) Enfermedades de la raíz. In: Téliz, D.
(coord). El aguacate y su manejo integrado. Ediciones Mundi Prensa, México, Madrid,
Barcelona.
Nishimoto, R.K. and Yee, W.Y.J. (1980) A Guide to Chemical Weed Control in Tropical and
Subtropical Fruit and Nut Crops in Hawaii. CES Circular 423 (revised), University of
Hawaii, Honolulu, Hawaii.
Ploetz, R.C., Ramos, J.L., Parrado, J.L. and Shepard, E.S. (1991) Shoot and root growth
cycles of avocado in south Florida. Proceedings of the Florida State Horticulture
Society 104, 21–24.
Popenoe, W. (1920) Manual of Tropical and Subtropical Fruits. Macmillan, NewYork.
Rainey, C.A., Gilette, G., Brydon, A., McIntyre, S., Rivers, O., Vasquez, C.A. and Wilson,
E. (1992) Physiological maturity and percent dry matter of California avocado.
In: Proceedings of the 2nd World Avocado Congress, Vol. II. University of California,
Riverside, California, pp. 379–385.
Rainey, C., A ick, M., Bretschger, K. and Alfi n-Slater, R.B. (1994) The California
avocado. Nutrition Today 29(3), 23–27.
Salazar-García, S. (2000) Fisiología reproductiva del aguacate: In Téliz, D. (coord.).
El aguacate y su manejo integrado. Ediciones Mundi Prensa, México, Madrid,
Barcelona.
Scha er, B., Pena, J., Lara, S.P. and Buisson, D. (1986) Net photosynthesis, transpiration,
and stomatal conductance of avocado leaves infested by avocado red mites.
Proceedings of the Interamerican Society for Tropical Horticulture 30, 73–82.
Scora, R.W. and Bergh, B.O. (1992) Origin of and taxonamic relationship within the
genus Persea. In: Proceedings of the 2nd World Avocado Congress, Vol. II. University of
California, Riverside, California, pp. 505–514.
Sedgley, M. and Grant, W.J.R. (1983) E ecct of low temperature during fl owering on
fl oral cycle and pollen tube growth in nine avocado cultivars. Scientia Horticulturae
18, 207–213.
Sedgley, M., Scholefi eld, P.B. and Alexander, McE.D. (1985) Inhibition of fl owering
of Mexican- and Guatemalan-type avocados under tropical conditions. Scientia
Horticulturae 25, 21–30.
Valmayor, R.V. (1967) Cellular development of the avocado from blossom to maturity.
Philippine Agriculturalist 50, 907–976.

376 References
Violi, H.A., Brown, J.S., Tondo C.L., Borrone, J.W. and Schnell, R.J. (2009) Microsatellite
markers reveal low breeding system e cacy and pollen contamination can limit
production of full-sib avocado progeny. Scientia Horticulturae 120, 360–366.
Wenkam, N.S. (1990) Foods of Hawaii and the Pacifi c Basin. Fruits and Fruit Products: Raw,
Processed, and Prepared, Vol. 4, Composition. Research Extension Series 110, College
of Tropical Agriculture and Human Resources, University of Hawaii, Honolulu,
Hawaii.
Whiley, A.W. (1984) Lauraceae. In: Page, P.E. (compiler) Tropical Tree Fruit for Australia.
Information Series Q183018, Queensland Department of Primary Industry,
Brisbane, Australia, pp. 70–77.
Whiley, A.W., Saranah, J.B., Cull, B.W. and Pegg, K.G. (1988a) Manage avocado tree
growth cycles for productivity gains. Queensland Agriculture Journal 114, 29–36.
Whiley, A.W., Chapman, K.R. and Saranah, J.B. (1988b) Water loss by fl oral structures
of avocado (Persea americana cv. Fuerte) during fl owering. Australian Journal of
Agricultural Research 39, 457–467.
Whiley, A.W., Saranah, J.B. and Rasmussen, T.S. (1992) E ect of time of harvest on fruit
size, yield and trunk starch concentrations of ‘Fuerte’ avocado. Proceedings of the
2nd World Avocado Congress, Vol. I. University of California, Riverside, California,
pp. 155–159.
Whiley, A.W., Hargreaves, P.A., Pegg, K.G., Doogan, V.J., Ruddle, L.J., Saranah, J.B.
and Langdon, P.W. (1995) Changing sink strengths infl uence translocation of
phosphorate in avocado (Persea americana Mill.) tree. Australian Journal of Agricultural
Research 46, 1079–1090.
Williams, L.O. (1976) The botany of the avocado and its relatives. In: Sauls, J.W., Phillips,
R.L. and Jackson, L.K. (eds) The Avocado. Proceedings of the 1st International Tropical
Fruit Crop Short Course. Fruit Crops Department, University of Florida, Gainesville,
Florida, pp. 9–15.
Wolstenholme, B.N. and Whiley, A.W. (1992) Requirements for improved fruiting
e ciency in the avocado tree. Proceedings of the 2nd World Avocado Congress, Vol. I.
University of California, Riverside, California, pp. 161–167.
Yee, W. (1978) Producing Avocado in Hawaii. CES Circular 382 (revised), University of
Hawaii, Honolulu, Hawaii.
Zentmyer, G.A. (1976) Soil-borne pathogens of avocado. In: Sauls, J.W, Phillips, R.L. and
Jackson, L.K. (eds) The Avocado. Proceedings of the 1st International Tropical Fruit Crop
Short Course. Fruit Crops Department, University of Florida, Gainesville, Florida,
pp. 75–82.
CHAPTER 8. BANANA AND PLANTAIN
Acedo, A.L. and Bautista, O.K. (1991) Enhancing ripening of ‘Saba’ banana (Musa, BBB
group) fruits with Gliricidia leaves as ethylene source. Philippine Agriculturalist 71,
351–365.
Baldry, J., Coursey, D.G. and Howard, G.E. (1981) The comparative consumer acceptability
of triploid and tetraploid banana fruit. Tropical Science 23, 33–66.
Belalcazar, S.W. (1991) El cultivo del plátano (Musa AAB Simmonds) en el Trópico. Manual
de Asistencia Técnica No. 50. Instituto Colombiano Agropecuario, Centro Nacional

References 377
de Investigaciones para el Desarrollo (Canadá), Comité Departamental de Cafeteros
de Quindío, Red Internacional para el Mejoramiento del Plátano y Banano. Cali,
Colombia.
Daniells, J.W. and O’Farrell, P.J. (1987) E ect of cutting height of the parent pseudostem
on yield and time of production of the following sucker in banana. Scientia
Horticulturae 31, 89–94.
Duarte, O. (1991) Manual del Cultivo del Banano. Escuela Agrìcola Panamericana – El
Zamorano, Honduras.
Espino, R.R.C., Jamalualdin, S.H., Silayoi, B. and Nasution, R.E. (1992) Musa L. (edible
cultivars). In: Verheij, E.W.M. and Coronel, R.E. (eds) Plant Resources in South East
Asia, No. 2, Edible Fruits and Nuts. Prosea, Bogor, Indonesia.
Galán Saúco, V. (1992) Los Frutales Tropicales en los Subtropicos. II Plátano (banano).
Mundi Prensa, Madrid (in Spanish).
Gowen, S. (ed.) (1995) Bananas and Plantains. Chapman and Hall, London.
Israeli, Y. and Lahav, E. (1986) Banana. In: Monselise, S.P. (ed.) CRC Handbook of Fruit
Set and Development. CRC Press, Boca Raton, Florida, pp. 45–73.
Lahav, E. and Turner, D.W. (1983) Fertilizing for High Yield Banana. Bulletin No. 7,
International Potash Institute, Bern, Switzerland.
Lardizabal, R. and Gutiérrez, H. (2006) Producción de plátano de alta densidad. Programa
de Diversifi cación Económica Rural (USAID-RED) Ofi cinas de FHIA, La Lima,
Honduras.
Lebot, V., Aradhya, K.M., Manshardt, R. and Meilleur, B. (1993) Genetic relationships
among cultivated bananas and plantains from Asia and the Pacifi c. Euphytica 67,
163–175.
Lodh, S.B., Ravel, P., Selvaraj, V. and Kohli, R.R. (1971) Biochemical changes with
growth and development of Dwarf Cavendish banana. Indian Journal of Horticulture
28, 38–45.
Loyola Santos, J.L., Shephard, K. and Alves, E.J. (1986) Propagacao rápida da bananeira.
Informe Agropecuario Belo Horizonte 12(133), 33–38.
Marriot, J., Robinson, M. and Karikari, S. (1981) Starch and sugar transformation during
the ripening of plantains and bananas. Journal of Science of Food and Agriculture 32,
1021–1026.
Martín-Prevel, P. (1990) Past, present, and future of tropical fruit nutrition with special
reference to banana. Acta Horticulturae 275, 523–534.
Murray, D.B. (1961) Shade and fertilizer relations in the banana. Tropical Agriculture 38,
123–132.
Novak, F.J. (1992) Musa (banana and plantains). In: Hammerschlag, F.A. and Litz, R.E.
(eds) Biotechnology of Perennial Fruit Crops. CAB International, Wallingford, UK, pp.
449–488.
Ortiz, R. (2008) Musaceae, Musa spp. (banana and plantain). In: Janick, J. and Paull, R.
E. (eds) The Encyclopedia of Fruits and Nuts. CAB International, Wallingford, UK, pp.
513–522.
Reynolds, P.K. (1951) Earliest evidence of banana culture. Journal of American Oriental
Society 71(4) (Suppl.).
Risède, J.M., Chabrier, C., Dorel, M., Dambas, T., Achard, R. and Quénerhevé, P. (2010)
Integrated management of banana nematodes: lessons from a case study in the
French West Indies. Banana Case Study, Guide Number 4, Banana Cropping System
Research Unit, CIRAD, France.

378 References
Robinson, J.C. and Human, N.B. (1988) Forecasting of banana harvest (‘Williams’)
in the subtropics using seasonal variation in bunch development rate and bunch
mass. Scientia Horticulturae 34, 249–263.
Robinson, J.C. and Nel, D.J. (1985) Comparative morphology, phenology and production
potential of banana cultivars ‘Dwarf Cavendish’ and ‘Williams’ in the Eastern
Transvaal Lowveld. Scientia Horticulturae 25, 149–161.
Robinson, J.C. and Nel, D.J. (1990) Competitive inhibition of yield potential in a
‘Williams’ banana plantation due to excessive sucker growth. Scientia Horticulturae
43, 225–236.
Rowe, P. and Rosales, F.E. (1996) Bananas and plantains. In: Janick, J. and Moore, J.N.
(eds) Fruit Breeding, Vol. I, Tree and Tropical Fruits. John Wiley & Sons, New York, pp.
167–211.
Simmonds, N.W. (1982) Bananas, 2nd edn. Tropical Agricultural Series, Longman,
London.
Smith, M.K. (1988) A review of factors infl uencing the genetic stability of
micropropagated bananas. Fruits 43, 219–223.
Stover, R.H. and Simmonds, N.W. (1987) Bananas. Longman, London.
Turner, D.W. (1970) Daily variation in banana leaf growth. Australian Journal of
Experimental Agriculture and Animal Husbandry 10, 231–234.
Turner, D.W. (1994) Bananas and plantains. In: Scha er, B. and Andersen, P.C. (eds)
Handbook of Environmental Physiology of Fruit Crops. Vol. II, Subtropical and Tropical
Crops. CRC Press, Boca Raton, Florida, pp. 37–64.
Vuylsteke, D., Ortiz, R., Pasberg-Gauhl, C., Gauhl, F., Gold, C., Ferris, S. and Speizer, P.
(1993) Plantain and banana research at the International Institute of Tropical
Agriculture. HortScience 28, 873–874, 970–971.
Wainwright, H. (1992) Improving the utilization of cooking bananas and plantains.
Outlook on Agriculture 21, 177–181.
Wenkam, N.S. (1990) Foods of Hawaii and the Pacifi c Basin, Fruits and Fruit Products, Raw,
Processed, and Prepared, Vol. 4, Composition. Research Extension Series 110, College
of Tropical Agriculture and Human Resources, University of Hawaii, Honolulu,
Hawaii.
Wong, S., Kiew, R., Argent, G., Set, O., Lee, S.K. and Gan, Y.Y. (2002) Assessment of
the validity of the Sections in Musa (Musaceae) using ALFP. Annals of Botany 90,
231–238.
CHAPTER 9. LITCHI AND LONGAN
Litchi
Alexander, D.McE., Scholefi eld, P.B. and Frodsham, A. (1982) Litchi. In. Some Tree
Fruits for Tropical Australia. Commonwealth Scientifi c and Industrial Research
Organization, Canberra, Australia, p. 31.
Anonymous (1985) An Album of Guangdong Litchi Varieties in Full Color. Guangdong
Province Scientifi c Technology Commission. Guangdong, China.
Aradhya, M.K., Zee, F.T. and Manshardt, R.M. (1995) Isozyme variation in lychee (Litchi
chinensis Sonn.). Scientia Horticulturae 63, 21–25.

References 379
Campbell, C.W. and Knight, R.J. Jr (1987) Production of litchis. In: Cultivation and
Production of Tropical Fruits. 23rd Congress, NORCOFEL, September 1983, Canary
Islands (in Spanish), pp. 215–221.
Chapman, K.R. (1984) Sapindaceae. In: Page, P.E. (compiler) Tropical Tree Fruits for
Australia. Information Series Q183018, Queensland Department of Primary
Industry, Brisbane, Australia. Chapter 26, pp.179–191.
Chen, W.S. and Ku, M.L. (1988) Ethephon and kinetin reduce shoot length and increase
fl ower bud formation in lychee. HortScience 23, 1078.
Galán Saúco, V. (1989) Litchi Cultivation (Menini, U.G. FAO Coordinator). FAO Plant
Production and Plant Protection Paper No. 83. FAO, Rome (in Spanish).
Galán Saúco, V. (1990) Los frutales tropicales en las subtropicos. I. Aquacate, mango, litchi,
longan. Mundiprensa, Madrid (in Spanish).
Gro , G.W. (1921) The Lychee and Lungan. Orange Judd Co., New York.
Hieke, S., Menzel, C.M., Doogan, V.J. and Ludders, P. (2002) The relationship between
yield and assimilate supply in lychee (Litchi chinensis Sonn.). Journal of Horticultural
Science and Biotechnology 77, 326–332.
Huang, H.B. and Qiu, Y.X. (1987) Growth correlations and assimilate partitioning
in arillate fruit of Litchi chinensis Sonn. Australian Journal of Plant Physiology 14,
181–186.
Huang, H.B., Jiang, S.Y. and Xie, C. (1983) The initiation of aril and ontogeny of fruit in
Litchi chinensis Sonn. Journal of the South China Agriculture College 4, 78–83.
Joubert, A.J. (1986) Litchi. In: Monselise, S.P. (ed.) CRC Handbook of Fruit Set and
Development. CRC Press, Inc., Boca Raton, Florida, pp.233–246.
Kadman, A. and Gazit, S. (1970) Flowering and Fruiting of Litchi. Division of Subtropical
Horticulture, Volcani Institute of Agricultural Research, Bet Dagan, Israel,
pp.120–122.
Khan, I., Misra, R.S. and Srivastava, R.P. (1976) E ects of plant growth regulators on the
fruit drop, size and quality of litchi cultivar Rose Scented. Progressive Horticulture 8,
61–69.
Limangkura, L. (1966) Studies on the nature of ovule abortion in the Gro variety of
lychee (Litchi chinensis Sonn.). MS thesis, Department of Horticulture, University of
Hawaii, Honolulu, Hawaii.
Lin, T.S., Shau, S.M., Yen, C.R., Lin, T.C. and Chang, J.W. (1991) E ects of fruits, leaves,
light intensity and pruning on fl owering of litchi (Litchi chinensis Sonn.). Second
Symposium on Forcing Culture of Horticultural Crops. Taichung District Agricultural
Improvement Station, Taiwan, Special Publication No. 23 (Chinese, English
summary), pp.127–136.
Menzel, C.M. (1983) The control of fl oral initiation in lychee: a review. Scientia
Horticulturae 21, 201–215.
Menzel, C.M. (1985) Propagation of lychee: a review. Scientia Horticulturae 25, 31–48.
Menzel, C.M. and Paxton, B.F. (1986a) A screening technique for lychee genotypes
adapted to warm climates. Acta Horticulturae 175, 59–61.
Menzel, C.M. and Paxton, B.F. (1986b) The e ect of cincturing on fl owering of lychee in
subtropical Queensland. Acta Horticulturae 175, 233–235.
Menzel, C.M. and Simpson, D.R. (1987) E ect of cincturing on growth and fl owering
of lychee over several seasons in subtropical Queensland. Australian Journal of
Experimental Agriculture 27, 733–738.
Menzel, C.M. and Simpson, D.R. (1988) E ect of temperature on growth and fl owering of
litchi (Litchi chinensis Sonn.) cultivars. Journal of Horticulture Science 63, 349–360.

380 References
Menzel C.M. and Simpson, D.R. (1990) Performance and improvement of lychee
cultivars: a review. Fruit Varieties Journal 44, 197–215.
Menzel, C.M. and Simpson, D.R. (1991) A description of lychee cultivars. Fruit Varieties
Journal 45, 45–56.
Menzel, C.M. and Simpson, D.R. (1994) Lychee. In: Scha er, B. and Andersen, P.C. (eds)
Handbook of Environmental Physiology of Fruit Crops Vol II. Subtropical and Tropical
Crops. CRC Press, Boca Raton, Florida, pp. 123–144.
Menzel, C.M., Watson, B.J. and Simpson, D.R. (1988) The lychee in Australia. Queensland
Agriculture Journal 114, 19–27.
Menzel, C.M., Rasmussen, T.S. and Simpson, D.R. (1989) E ect of temperature and leaf
water stress on growth and fl owering of litchi (Litchi chinensis Sonn.). Journal of
Horticultural Science 64, 739–752.
Mo, B.C. (ed.) (1992) Litchi High Production Cultivation and Technology. Guangxi Science
and Technology Publishers, Nanning, China ( in Chinese).
Morton, J.F. (1987) Fruits of Warm Climates. Morton, Miami, Florida.
Mustard, M.J. (1954) Fundamentals of panicle di erentiation. Proceedings of the Florida
Lychee Growers’ Association 1, 7–8.
Nakasone, H.Y. and Paull, R.E (1998) Tropical Fruits. CAB International, Wallingford,
UK.
Nakata, S. (1953) Girdling as a Means of Inducing Flower Bud Initiation in Litchi. Progress
Note 95, University of Hawaii, Hawaii Agriculture Experiment Station, Honolulu,
Hawaii.
Nakata, S. and Suehisa, R. (1969) Growth and development of Litchi chinensis as a ected
by soil-moisture stress. American Journal of Botany 56, 1121–1126.
Nakata, S. and Watanabe, Y. (1966) E ects of photoperiod and night temperature on the
fl owering of Litchi chinensis. Botanical Gazette 127, 146–152.
Pandey, R.S. and Yadava, R.P.S. (1970) Pollination of litchi (Litchi chinensis Sonn.)
by insects with special reference to honey bees. Journal of Apiculture Research 9,
103–105.
Paull, R.E. and Chen, N.J. (1987) E ect of storage temperature and wrapping on quality
characteristics of litchi fruit. Scientia Horticulturae 33, 223–236.
Paull, R.E., Chen, N.J., Deputy, J., Huang, H.B., Cheng, G.W. and Gao, F.F. (1984) Litchi
growth and compositional changes during fruit development. Journal of the
American Society for Horticultural Science 109, 817–821.
Paull, R.E., Reyes, M.E.Q. and Reyes, M.U. (1995) Litchi and rambutan insect
disinfestation: treatments to minimize induced pericarp browning. Postharvest
Biology and Technology 6, 139–148.
Prasad, A. and Jauhari, C.S. (1963) E ect of 2,4,5-T and alpha NAA on ‘drop stop’ and
size of litchi fruits. Madras Agricultural Journal 50, 28–29.
Singh, L.B. and Singh, U.P. (1954) The Litchi (Litchi chinensis Sonn.). Superintendent of
Printing and Stationery, Lucknow, India.
Singh, U.S. and Lal, R.K. (1980) Infl uence of growth regulators on setting, retention
and weights of fruit in two cultivars of litchi. Scientia Horticulturae 12, 321–326.
Storey, W.B. (1973)
The Lychee. California Avocado Society Yearbook 1972–1973,
pp.75–86.
Storey, W.B., Hamilton, R.A. and Nakasone, H.Y. (1953) Gro – a New Variety of
Lychee. Circular 39, University of Hawaii, Hawaii Agriculture Experiment Station,
Honolulu, Hawaii.

References 381
Viruel, M.A. and Hormaza, J.I. (2004) Development, characterization and variability
analysis of microsatellites in lychee (Litchi chinensis Sonn., Sapindaceae). TAG
Theoretical and Applied Genetics 108, 896–902.
Wenkam, N.S. (1990) Foods of Hawaii and the Pacifi c Basin. Fruits and Fruit Products: Raw,
Processed and Prepared. Vol. 4. Composition. Research Extension Series 110, College
of Tropical Agriculture and Human Resources, Honolulu, Hawaii.
Wills, R.B.H., Lim, J.S.K. and Greenfi eld, H. (1986) Composition of Australian foods. 31.
Tropical and subtropical fruit. Food Technology Australia 38, 118–123.
Yen, C.R. (1995) Litchi. In: Taiwan Agricultural Encyclopedia Crops, 2nd edn. Agricultural
Publisher Council, Taipei, Taiwan (in Chinese), pp. 33–42.
Yuan, R.C. and Huang, H.B. (1988) Litchi fruit abscission: its patterns, e ect of shading,
and relation to endogenous abscisic acid. Scientia Horticulturae 36, 281–292.
Longan
Chapman, K.R. (1984) Sapindaceae. In: Page, P.E. (compiler) Tropical Tree Fruits for
Australia. Information Series Q183018, Queensland Department of Primary
Industry, Brisbane, Australia, Chapter 26, pp.179–191.
Chian, G.Z., Hee, Y.Q., Lin, Z.Y., Liu, M.Y. and Feng, X.B. (1996) Longan High Production
Cultivation Techniques. Guangxi Science Technology Publishers. Nanning, China (in
Chinese).
Galán Saúco, V. (1990) Los frutales tropicales en las subtropicos. I. Aquacate, mango, litchi,
longan. Mundiprensa, Madrid (in Spanish).
Gro , G.W. (1921) The Lychee and Lungan. Orange Judd Co., New York.
Ke, G.W. (1990) Development of fl esh and seed of longan. Symposium on Litchi and
Longan. Guangxi Subtropical Fruit Research and Development O ce, Nanning,
China, pp. 176–178.
Lian, T.K. and Chen, L.L. (1965) A preliminary observation on the fl ower bud initiation
of longan in Foochow. Acta Horticulture Sinica 4, 13–18.
Liu, X.H. and Ma, C.L. (2001) Production and research of longan in China. Acta
Horticulturae 558, 73–82.
Matsumoto, T.K., Nagao, M.A. and Mackey, B. (2007) O -season fl ower induction
of longan with potassium chlorate, sodium chlorite and sodium hypochlorite.
HortTechnology 17, 296–300.
Menzel, C.M., Watson, B.J. and Simpson, D.R. (1988b) Longans – a place in Queensland’s
horticulture? Queensland Agriculture Journal 115, 251–265.
Nakasone, H.Y. and Paull, R.E. (1998) Tropical Fruits. CAB International, Wallingford,
UK.
Subhadrabandhu, S. and Yapwattanaphun, C. (2001) Regulation o -season fl owering
of longan in Thailand. Acta Horticulturae 558, 193–198.
Wenkam, N.S. (1990) Foods of Hawaii and the Pacifi c Basin. Fruits and Fruit Products: Raw,
Processed and Prepared. Vol. 4. Composition. Research Extension Series 110, College
of Tropical Agriculture and Human Resources, Honolulu, Hawaii.
Wills, R.B.H., Lim, J.S.K. and Greenfi eld, H. (1986) Composition of Australian foods. 31.
Tropical and subtropical fruit. Food Technology Australia 38, 118–123.
Yen, C.R. and Chang, J.W. (1991) Variation of fruit characters among longan varieties
in Taiwan. Journal of the Chinese Society for Horticultural Science 37, 21–34.

382 References
Yen, C.R., Chau, C.N., Chang, J.W. and Tzeng, J.C. (2001) E ects of chemicals on fl owering
in longan. Journal of the Chinese Society for Horticultural Science 47, 195–200.
Yen, C.R., Tzeng, J.C., Chau, C.N. and Chang, J.W. (2005) Longan production in Taiwan.
Acta Horticulturae 665, 61–66.
Zee, F., Chan, H.T. Jr and Yen, C.R. 1998. Lychee, longan, rambutan and pulasan. In:
Shaw, P.E., Chan, H.T. Jr and Nagy, S. (eds) Tropical and Subtropical Fruits. AgScience,
Inc. Auburndale, Florida, pp. 290–335.
CHAPTER 10. MANGO
Adlan, H.A. (1965) Floral morphology as related to the fruit drop in mango (Mangifera
indica L.). MSc thesis, Department of Horticulture, University of Hawaii. Honolulu,
Hawaii.
Akamine, E.K. (1976) Problems in shipping fresh Hawaiian tropical and subtropical
fruits. Acta Horticulturae 57, 157–161.
Azzouz, S., Said, G.A. and Abdella, M.Y. (1989) Studies on the control of malformation
in some mango cultivars. Zagazig Journal of Agricultural Research 16, 43–47.
Bondad, N.D. and Apostol, C.J. (1979) Induction of fl owering and fruiting in immature
mango shoots with KNO
3
. Current Science 48, 591–593.
Campbell, C.W. (1961) Comparison of yields of polyembryonic and monoembryonic
mangos. Proceedings of the Florida State Horticultural Society 74, 363–365.
Campbell, C.W. (1988) Progress in mango cultivation. Proceedings of the Interamerican
Society of Tropical Horticulture 32, 8–19.
Campbell, R.J. and Campbell, C.W. (1992) Commercial Florida mango cultivars. Acta
Horticulturae 341, 55–59.
Chang, M.T. and Lion, M.F. (1987) Delay fruit harvest by pinching mango infl orescence.
In: Chang, L.R. (ed.) Proceedings of the Symposium on Forcing Culture of Horticultural
Crops. Special Publication No. 10, Taichung District Agricultural Improvement
Station, Taichung, Taiwan, pp. 119–128.
Crane, J.H. and Campbell, C.W. (1991) The Mango. Fruit Crops Fact Sheet FC-2, Florida
Cooperative Extension Service, Gainesville, Florida.
Cull, B.W. (1991) Mangos crop management. Acta Horticulturae 291, 154–173.
Davenport, T.L. (1993) Floral manipulation in mangoes. In: Chia, L.E. and Evans,
D.O. (eds) Proceedings on the Conference on Mango in Hawaii. Cooperative Extension
Service, University of Hawaii, Honolulu, Hawaii, pp. 54–60.
Davenport, T.L. (2003) Management of fl owering in three tropical and subtropical fruit
tree species. HortScience 38(7), 1331–1335.
Degani, C., El-Batsri, R. and Gazit, S. (1990) Enzyme polymorphism in mango. Journal of
the American Society for Horticultural Science 115, 844–847.
Duarte, O. (2002) El mango, una opción para Honduras? A Case Study. CCPA, Escuela
Agrícola Panamericana – El Zamorano, Honduras.
Galán Saúco, V. (1979) Actual situation, problems and prospects of mango development
in the Canary Islands. Acta Horticulturae 102, 7–14.
Galán Saúco, V. (1996) Mango world production (outside Israel, Egypt and India). Acta
Horticulturae 455, 1–6.
Galán Saúco, V. (2009) El cultivo del mango. Coedición: Instituto Canario de
Investigaciones Agrarias, La Laguna, Tenerife, España y Ediciones Mundi-Prensa,
Madrid (in Spanish).

References 383
Gazit, S. and Kadman, A. (1980) 13-1 mango root stock selection. HortScience 15, 669.
Hamilton, R.A., Chia, C.L. and Evans, D.O. (1992) Mango Cultivars in Hawaii.
InformationText Series 042, HITAHR, University of Hawaii, Honolulu, Hawaii.
Hayes, W.B. (1966) Fruit Growing in India, 3rd revised edn. Indian Universities Press,
Allahabad, India.
Huete, M. and Arias, S. (2007) Manual para la Producción de Mango. U.S. A.I.D. – RED.
Ofi cinas de la FHIA, La Lima, Cortés, Honduras.
Issarakraisila, M., Considine, J.A. and Turner, D.W. (1993) E ects of temperature on
pollen viability in mango cv. Kensington. Acta Horticulturae 341, 112–124.
Ito, P.J., Hamilton, R.A. and Rapoza, H. (1992) ‘Exel’, a High Quality Dessert Mango.
Commodity Fact Sheet 169 MAN-3(B) Fruit, HITAHR, University of Hawaii,
Manoa, Hawaii.
Joel, D.M. (1978) The secretory ducts of mango fruits, a defense system e ective against
Mediterranean fruit fl y. Israel Journal of Botany 27, 44–45.
Johnson, G.I. and Coates, L.M. (1993) Postharvest diseases of mango. Postharvest News
and Information 4, 27N–34N.
Knight, R.J. Jr (1985) Criteria for evaluating important characters in mango (Mangifera
indica L.) germplasm. In: Technology for Agricultural Development: Joint Proceedings
of the 21st Caribbean Food Crops Society and the 32nd Annual Meeting of the American
Society of Horticultural Science, Tropical Region. ASHSTR, Port of Spain, Trinidad,
pp. 57–60.
Knight, R.J. and Schnell, RJ. (1993) Mango (Mangifera indica L.) introduction and
evaluation in Florida and its impact on the world industry. Acta Horticulturae 341,
125–135.
Krishna, H. and Singh, S.K. (2007) Biotechnological advances in mango (Mangifera
indica L.) and their future implication in crop improvement – a review. Biotechnology
Advances 25, 223–243.
Lakshminarayana, S., Gomez-Cruz, A. and Martinez-Romero. S. (1985) Preliminary
study of a new preharvest stem-end rot and associated microfl ora in mango.
HortScience 20, 947–948.
Lovey, B.R., Robinson, S.P., Brophy, J.J. and Chacko, E.K. (1992) Mango sapburn
components of fruit sap and their role in causing skin damage. Australian Journal of
Plant Physiology 19, 449–457.
Malo, S.E. (1972) Mango culture in Florida. Acta Horticulturae 24, 149–154.
Malo, S.E. (1976) Recent advances and possibility of mango culture in tropical America
with emphasis on the Florida situation. Acta Horticulturae 57, 47–52.
Malo, S.E. and Campbell, C.W. (1978) Studies on mango fruit breakdown in Florida.
Proceedings of the American Society for Horticultural Science – Tropical Region 22,
1–15.
Mendoza, D.B. and Wills, R.B.H. (eds) (1984) Mango: Fruit Development. Postharvest
Physiology and Marketing in ASEAN. ASEAN Food Handling Bureau, Kuala Lumpur,
Malaysia.
Mostert, P.G. and Abercrombie, R.A. (1998) Soil requirements and soil preparation. In:
de Villiers, E.A. (ed.) The Cultivation of Mangoes. Institute of Tropical and Subtropical
Crops, ACR, LNR, Nelspruit, South Africa, pp. 19–46.
Mukherjee, S.K. (1950) Cytological investigation of the mango and the allied Indian
species. Proceedings of the National Institute of Science India 16, 281–303.

384 References
Nagao, M.A. and Nishina, M.S. (1993) Use of potassium nitrate on mango fl owering. In:
Proceedings of a Conference on Mango in Hawaii. College of Tropical Agriculture and
Human Resources, University of Hawaii, Manoa, Honolulu, Hawaii, pp. 61–66.
Nakasone, H.Y. (1979) Observations and Comments on Tropical Fruit Cultivation. FIRA,
Bank of México, México City (in Spanish).
Nakasone, H.Y., Bowers, F.A.I. and Beaumont, J.H. (1955) Terminal growth and
fl owering behavior of the ‘Pirie’ mango (Mangifera indica L.) in Hawaii. Proceedings
of the American Society of Horticultural Science 66, 183–191.
Nuñez Elisea, R. and Davenport, T.L. (1995) E ect of leaf age, duration and cool
temperature treatment and photoperiod on bud dormancy release and fl oral
initiation in mango. Scientia Horticulturae 62, 63–73.
Oosthuyse, S.A. and Jacobs, G. (1995) Relationship between branching frequency,
and growth, cropping, and structural strength of 2 year old mango trees. Scientia
Horticulturae 64, 85–93.
Pennock, W., Torres-Sepulveda, A., Lopez-Garcia, J., Reyes-Soto, I., Valle-Lamboy, S.,
Cedeno-Maldonado, A. and Jackson, G. (1972) Yield and fruit size comparison
in the fi rst size crops of 16 mango varieties. Journal of Agriculture Puerto Rico 56,
343–365.
Pinto, A.C.Q. and Sharma, D.K. (1984) Studies on growth behavior and sex expression
in some exotic introductions of mango (Mangifera indica L.) in Brazil. Proceedings of
the American Society for Horticultural Science, Tropical Region 28, 25–27.
Pollard, G.V. and Alleyne, E.H. (1986) Insect pests as constraints to the production of
fruits in the Caribbean. In: Brathwaite, C.W.D., Marte, R. and Porsche, E. (eds) Pests
and Diseases as Constraints in the Production and Marketing of Fruits in the Caribbean.
Technical Events Series A2/TT-8 6-001, IICA, Bridgetown, Barbados, pp. 31–61.
Pope, W.T. (1929) Mango Culture in Hawaii. Hawaii Agricultural Experiment Station
Bulletin 58, Honolulu, Hawaii.
Ram, S. and Sirohi, S.C. (1991) Feasibility of high density orcharding in Dashehari
mango. Acta Horticulturae 291, 207–212.
Reuveni, O., Kuepper, W. and Wiebel, J. (1991) Rooting mango cuttings under
intermittent mist, Acta Horticulturae 291, 174–181.
Scha er, B., Whiley, A.W. and Crane, J.H. (1994) Mango. In: Scha er, B. and Andersen,
E. C. (eds) Handbook of Environmental Physiology of Fruit Crops. Vol. II. Subtropical
and Tropical Crops. CRC Press, Boca Raton, Florida, pp. 165–197.
Shawky, I., Zidan, Z., El-Tomi, A. and Dashan, D. (1980) Flowering malformation in
relation to vegetative growth of ‘Taimour’ mangoes. Egypt Journal of Horticulture
7, 1–8.
Singh, L.B. (1960) The Mango – Botany, Cultivation and Utilization. Interscience
Publishers, New York.
Tandon, D.K. and Kalra, S.K. (1983) Changes in sugars, starch, and amylase activity
during development of mango fruit cv. Dashehari. Journal of Horticultural Science
58, 449–453.
Turnball, C.G.N., Anderson, K.L. and Winston, E.C. (1996) Infl uence of gibberellin
treatment on fl owering and fruiting patterns in mango. Australian Journal of
Experimental Agriculture
36, 603–611.
Wainwright, H. and Burbage, M.B. (1989) Physiological disorders in mango (Mangifera
indica L.) fruit. Journal of Horticultural Science 64, 125–135.

References 385
Wenkam, N.S. (1990) Foods of Hawaii and the Pacifi c Basin. Fruits and Fruit Products, Raw,
Processed, and Prepared. Vol. 4, Composition. Research Extension Series 110. College
of Tropical Agriculture and Human Resources, University of Hawaii, Honolulu,
Hawaii.
Whiley, A.W. and Saranah, J. (1981) Mango evaluation. In: Biennial Research Report No. 2
for 1979–80. Maroochy Horticultural Research Station, Queensland, Department
of Primary Industries and Forestry, Brisbane, Australia, p. 47.
Whiley, A.W. and Scha er, B. (1997) Stress physiology. In: Litz, R.E. (ed.) The Mango:
Botany, Production and Uses. CAB International, Wallingford, UK, pp. 147–176.
Whiley, A.W., Saranah, J.B., Rasmussen, T.S., Winston, E.C. and Wolstenholme, B.N.
(1988) E ect of temperature on growth of ten cultivars of mango with relevance
to production in Australia. Proceedings of the 4th Australian Conference on Tree and
Nut Crops, pp. 176–185.
Whiley, A.W., Mayers, P.E., Bartley, J.B. and Saranah, J.B. (1993) Breeding mangoes for
Australian conditions. Acta Horticulturae 341, 136–145.
Yee, W. (1958) The Mango in Hawaii. Agriculture Extension Service Circular No. 388,
University of Hawaii, Honolulu, Hawaii.
Young, T.W. and Koo, R.C.J. (1974) Increasing yield of ‘Parvin’ and ‘Kent’ mangos on
Lakewood sand by increasing nitrogen and potassium fertilizer. Proceedings of the
Florida State Horticultural Society 87, 380–384.
Young, T.W. and Sauls, J.W. (1979) The Mango Industry in Florida. Florida Cooperative
Extension Service Bulletin 189, Florida Cooperative Extension Service, Institute of
Food and Agricultural Sciences, University of Florida, Gainesville, Florida.
CHAPTER 11. PAPAYA
Agnew, G.W.J. (1968) Growing quality papayas in Queensland. Queensland Agriculture
Journal 94, 24–36.
Airi, S.K., Gill, S.S. and Singh, S.N. (1986) Clonal propagation of papaya (Carica papaya
L.). Journal of Agriculture Research, Punjab 23, 237–239.
Allan, P. (1964) Papaws grown from cuttings. Farming in South Africa 39(11), 35–40.
An, J.F. and Paull, R.E. (1990) Storage temperature and ethylene infl uences on ripening
of papaya fruit. Journal of the American Society for Horticultural Science 115,
949–953.
Anonymous (2003) FAOSTAT Database. Food and Agriculture Organization of the United
Nations, Rome (accessed 17 February 2010: http://faostat.fao.org/site/339/
default.aspx).
Awada, M. (1958) Relationships of Minimum Temperature and Growth Rate with Sex
Expression of Papaya Plants (Carica papaya L.). Hawaii Agriculture Experiment
Station Technical Bulletin 38, Honolulu, Hawaii.
Awada, M. (1961) Soil moisture tension in relation to fruit types of papaya plants.
Hawaii Farm Science 10(2), 7–8.
Awada, M. (1977) Relations of introgen, phosphorus, and potassium fertilization to
nutrient composition of the petiole and growth of papaya. Journal of the American
Society for Horticultural Science 102, 413–418.

386 References
Awada, M. and Ikeda, W.S. (1957) E ects of Water and Nitrogen Application on
Composition, Growth, Sugars in Fruits, Yield and Sex Expression of the Papaya
Plants (C. papaya L.). Hawaii Agriculture Experiment Station Bulletin 33, Honolulu,
Hawaii.
Awada, M. and Long, C. (1980) Nitrogen and potassium fertilization e ects on fruiting
and petiole composition of 24- to 48-month old papaya plants. Journal of the
American Society for Horticultural Science 105, 505–507.
Awada, M., Suehisa, R.H. and Kanehiro, Y. (1975) E ects of lime and phosphorus on
yield, growth, and petiole composition of papaya. Journal of the American Society for
Horticultural Science 100, 294–298.
Awada, I.P., Suehisa, R.H. and Padgett, M.M. (1979) E ects of drip irrigation and
fertilization on vegetative growth, fruit yield, and mineral composition of the
petioles and fruits of papaya. University of Hawaii, Agricultural Experiment
Station, Honolulu, Hawaii.
Badillo, V.M. (1993) Caricaceae segundo esquema. Alcance 43, Universidad Central de
Venezuela, Facultad de Agronomia, Caracas, Venezuela (in Spanish).
Badillo, V.M. (2000) Carica L. vs. Vasconcella St. Hil. (Caricaceae): con la rehabilitación de
este último. Ernstia 10, 74–79.
Chan, H.T. Jr, Hibbard, K.L., Goo, T. and Akamine, E.K. (1979) Sugar composition of
papaya during fruit development. HortScience 14, 140–141.
Chan, Y.K. (1984) Studies on carpellody of stamens in papaya (C. papaya L.). MARDI
Research Bulletin 12, 157–162.
Chan, Y.K. (1992) Progress in breeding of F
1
papaya hybrids in Malaysia. Acta
Horticulturae 292, 41–49.
Chan, Y.K. (1993) Memperkenalkan ‘Eksotika’ II: varieti betik hibrid yang lebih baik.
[Introducing the ‘Eksotika’ II: an improved hybrid papaya]. Teknology Buah-buahan
MARDI 9, 25–28.
Chan, Y.K. (2001) Heterosis in Eksotika × Sekaki papaya hybrids. Journal of Tropical
Agriculture and Food Science 29, 139–144.
Chan, Y.K. and Tan, L.K. (1990) Drying treatments in relation to seed germination of
four papaya varieties. MARDI Research Journal 18, 1–7.
Chan, Y.K. and Toh, W.K. (1984) Growth studies on some vegetative characters of
papaya (Carica papaya L.). MARDI Research Bulletin 12, 46–54.
Chen, N.M. and Paull, R.E. (1986) Development and prevention of chilling injury in
papaya fruit. Journal of the American Society for Horticultural Science 111, 639–643.
Chittiraichelvan, R. and Shanmugavelu, K.G. (1979) Studies of the growth and
development of the fruit of CO-2 papaya (Carica papaya L.) II. Changes in biochemical
constituents. Indian Journal of Horticulture 36, 42–48.
Clemente, H. and Marler, T.E. (1996) Drought stress infl uences gas-exchange responses
of papaya leaves to rapid changes in irradiance. Journal of the American Society for
Horticultural Science 121, 292–295.
Conover, R.A., Litz, R.E. and Malo, S.E. (1986) ‘Carifl ora’ a papaya for South Florida and
the Caribbean. HortScience 21, 1072.
Drew, R.A. (1988) Rapid clonal propagation of papaya in vitro from mature fi eld-grown
trees. HortScience 23, 609–611.
Fitch, M.M.M., Manshardt, R.M., Gonsalves, D., Slightom, J.L. and Sanford, J.C. (1992)
Virus resistant papaya plants derived from tissues bombarded with the coat protein
gene of papaya ringspot virus. Biotechnology 10, 1466–1472.

References 387
Hamilton, R.A. (1954) A quantitative study of growth and fruiting in inbred and
crossbred progenies from two Solo papaya strains. Hawaii Agriculture Experiment
Station Technical Bulletin 20.
Higgins, J.S. and Holt, V.S. (1914) The Papaya in Hawaii. Hawaii Agriculture Experiment
Station Technical Bulletin 32, Honolulu, Hawaii.
Hofmeyr, J.D.J. (1938) Determination of sex in Carica papaya. Farming in South Africa 13,
332.
Ito, P.J. (1976) The e ect of leaf pruning on yield and quality of ‘Solo’ papayas in
Hawaii. Proceedings of the Tropical Region, American Society for Horticultural Science
20, 46–50.
Kuhne, F.A. and Allan, P. (1970) Seasonal variation in fruit growth of Carica papaya L.
Agroplantae 2, 99–104.
Lange, A.H. (1961) The e ects of temperature and photoperiod on the growth of Carica
papaya. Ecology 42, 481–486.
Litz, R.E. and Conover, R.A. (1978) In vitro propagation of papaya. HortScience 13,
241–242.
Manshardt, R.M. and Wensla , T.F. (1989a) Zygotic polyembryony in interspecifi c hybrids
of Carica papaya and C. caulifl ora. Journal of the American Society for Horticultural
Science 114, 684–689.
Manshardt, R.M. and Wensla , T.F. (1989b) Interspecifi c hybridization of papaya with
other Carica species. Journal of the American Society for Horticultural Science 114,
689–694.
Marler, T.E. (1994) Papaya. In: Scha er, B. and Andersen, P.C. (eds) Handbook of
Environmental Physiology of Fruit Crops Vol. 2. Subtropical and Tropical Crops. CRC
Press, Boca Raton, Florida, pp. 216–224.
Marler, T.E. and Discekici, H.M. (1997) Root development of ‘Red Lady’ papaya plants
grown on a hillside. Plant & Soil 195, 37–42.
Mekako, H.U. and Nakasone, H.Y. (1975) Interspecifi c hybridization among 6 Carica
species. Journal of the American Society for Horticultural Science 100, 237–242.
Morshidi, M. (1996) Genetic variability in Carica papaya and related species. PhD
dissertation, University of Hawaii at Manoa, Honolulu, Hawaii.
Mosqueda-Vazquez, R., Aragaki, M. and Nakasone, H.Y. (1981) Screening of Carica
papaya L. seedlings for resistance to root rot caused by Phytophthora palmivora Butl.
Journal of the American Society for Horticultural Science 106, 484–497.
Nakasone, H.Y. and Aragaki, M. (1973) Tolerance to Phytophthora fruit and root rot in
Carica papaya L. Proceedings Tropical Region American Society for Horticultural Science
17, 176–185.
Nakasone, H.Y. and Storey, W.B. (1955) Studies on the inheritance of fruiting height
of Carica papaya
L. Proceedings of the American Society for Horticultural Science 66,
168–182.
Paull, R.E. and Chen, N.J. (1990) Heat shock response in fi eld grown ripening papaya
fruit. Journal of the American Society for Horticultural Science 115, 623–631.
Qiu, Y.X., Nishina, M.S. and Paull, R.E. (1995) Papaya fruit growth, calcium uptake,
and fruit ripening. Journal of the American Society for Horticultural Science 120,
246–253.
Quintana, M.E.C. and Paull, R.E. (1993) Mechanical injury during postharvest handling
of ‘Solo’ papaya fruit. Journal of the American Society for Horticultural Science 118,
618–622.

388 References
Seo, S.T., Farias, G.J. and Harris, E.J. (1982) Oriental fruit fl y: ripening of fruit and its
e ect on index of infestation of Hawaiian papayas. Journal of Economic Entomology
75, 173–178.
Storey, W.B. (1938) Segregations of sex types in Solo papaya and their application to
the selection of seed. Proceedings of the American Society for Horticultural Science 35,
83–85.
Storey, W.B. (1941a) Part I. The botany and sex relationships of the papaya. In: Papaya
Production in the Hawaiian Islands. University of Hawaii, Hawaii Agriculture
Experiment Station Bulletin 87, Honolulu, Hawaii.
Storey, W.B. (1941b) Genetics of sex determination in papaya. Hawaii Agriculture
Experiment Station Annual Report 1940, 52–53.
Storey, W.B. (1958) Modifi cation of sex expression in papaya. Horticulture Advance 2,
49–60.
Storey, W.B. (1969) Papaya (Carica papaya L.). In: Ferwerda, P. and Wit, F. (eds) Outline
of Perennial Crop Breeding in the Tropics. H. Veenman and Zonen, Wageningen, The
Netherlands, pp. 389–407.
Subhadrabandhu, S. and Nontaswatsri, C. (1997) Combining ability analysis of some
characters of introduced and local papaya cultivars. Scientia Horticulturae 71,
203–212.
Van Droogenbroeck, B., Breyne, P., Goetghebeur, P., Romeijn-Peeters, E., Kyndt, T. and
Gheysen, G. (2002) AFLP analysis of genetic relationships among papaya and
its wild relatives (Caricaceae) from Ecuador. Theoretical and Applied Genetics 105,
289–297.
Wenkam, N.S. (1990) Foods of Hawaii and Pacifi c Basin Fruits and Fruit Products: Raw,
Processed, and Prepared. Vol. 4. Composition. Research and Extension Series 110,
College of Tropical Agriculture and Human Resources, Honolulu, Hawaii.
Yee, W. (1970) Papayas in Hawaii. Circular 436, Cooperative Extension Service, University
of Hawaii, Honolulu, Hawaii.
CHAPTER 12. PINEAPPLE
Aldrich, W.W. and Nakasone, H.Y. (1975) Day versus night application of calcium
carbide for fl ower induction in pineapple. Journal of the American Society for
Horticultural Science 100, 410–413.
Antoni, M.G. and Leal, F.J. (1980) Species of the genus Ananas: origin and geographic
distribution. Tropical Region, American Society for Horticultural Science 24, 103–106
(in Spanish).
Antoni, S., Grazia, M. and Leal, F. (1980) Key to the identifi cation of commercial varieties
of pineapple (Ananas comosus). Proceedings of the Tropical Region, American Society
for Horticultural Science 24, 107–112 (in Spanish).
Aradhya, M.K., Zee, F. and Manshardt, R.M. (1994) Isozyme variation in cultivated and
wild pineapple. Euphytica 79, 87–99.
Armstrong, J.W. and Vargas, R.I. (1982) Resistance of pineapple variety 59-650 to fi eld
populations of Oriental fruit fl ies and Melon fl ies (Diptera: Tephritidae). Journal of
Economic Entomology 75, 781–782.
Bartholomew, D.P. (1977) Infl orescence development of pineapple (Ananas comosus (L.)
Merr). induced to fl ower with ethephon. Botanical Gazette 138, 312–320.

References 389
Bartholomew, D.P. and Criley, R.A. (1983) Tropical fruit and beverage crops. In: Nichells
L.G. (ed.) Handbook of Plant Growth Regulating Chemicals, Vol. II. CRC Press, Boca
Raton, Florida.
Bartholomew, D.P. and Kadzimin, S.B. (1977) Pineapple. In: Alvim, P.T. and Kozlowski,
T.T. (eds) Ecophysiology of Tropical Crops. Academic Press Inc., New York,
pp.113–156.
Bartholomew, D.P. and Malézieux, E. (1994) Pineapple. In: Scha er, B. and Andersen,
P. (eds) Handbook of Environmental Physiology of Fruit Crops. CRC Press Inc., Boca
Raton, Florida, pp. 243–291.
Bartholomew, D.P. and Paull, R.E. (1986) Pineapple. In: Monselise, S.P. (ed.) CRC Handbook
of Fruit Set and Development. CRC Press, Boca Raton, Florida, pp. 371–388.
Bartholomew, D.P., Malézieux, E., Sanewski, G.M. and Sinclair, E. (2003) Infl orescence,
and fruit development and yield. In: Bartholomew, D.P., Paull, R. and Rohrbach,
K.G. (eds) The Pineapple: Botany, Production and Uses. CABI Publishing, Wallingford,
UK, pp. 167–202.
Brewbaker, J.L. and Gorrez, D.D. (1967) Genetics of incompatibility in monocotyledonous
genera Ananas (pineapple) and Gasteria. American Journal of Botany 54, 611–616.
Chan, Y.K. and Lee, C.K. (1985) The hybrid 1 Pineapple: a new canning variety developed
at MARDI. Teknologi Buah-buahan (Malay) 1, 24–30.
Chan, Y.K., Coppens d’Eeckenbrugge, G. and Sanewski, G.M. (2003) Breeding and
variety improvement. In: Bartholomew, D.P., Paull, R. and Rohrbach, K.G. (eds)
The Pineapple: Botany, Production and Uses. CABI Publishing, Wallingford, UK, pp.
33–55.
Collins, J.L. (1933) Morphological and cytological characters of triploid pineapples.
Cytologia 4, 248–256.
Collins, J.L. (1948) Pineapples in ancient America. Science Monthly 65, 372–377.
Collins, J.L. (1949) History, taxonomy and culture of the pineapple. Economic Botany 3,
335–359.
Collins, J.L. (1960) The Pineapple: Botany, Cultivation and Utilization. Interscience
Publishers Inc., New York.
Collins, J.L. and Kerns, K.R. (1938) Mutations in the pineapple. Journal of Heredity 29,
162–172.
Coppens d’Eeckenbrugge, G. and Leal, F. (2003) Morphology, anatomy and taxonomy.
In: Bartholomew, D.P., Paull, R. and Rohrbach, K.G. (eds) The Pineapple: Botany,
Production and Uses. CABI Publishing, Wallingford, UK, pp. 13–32.
Coppens d’Eeckenbrugge, G., Leal, F. and Duval, M.F. (1997) Germplasm resources of
pineapple. Horticultural Reviews 21, 133–175.
Ekern, P.C. (1968) Phyllotaxy of pineapple plants and fruit. Botanical Gazette 129,
92–94.
Evans, D.O., Stanford, W.G. and Bartholomew, D.P. (1988) Pineapple. Commodity Fact
Sheet PIN-3(A), College of Tropical Agriculture and Human Resources, Hawaii
Institute of Tropical Agriculture and Human Resources, Manoa, Hawaii.
Friend, D.C. and Lydon, J. (1979) E ect of daylength on fl owering, growth and CAM of
pineapple (Ananas comosus (L.)). Botanical Gazette 140, 280–283.
Gowing, D.P. (1961) Experiments on the photoperiodic response in pineapple. American
Journal of Botany 48, 16–21.
Hepton, A. (2003) Culture system. In: Bartholomew, D.P., Paull, R. and Rohrbach, K.G.
(eds) The Pineapple: Botany, Production and Uses. CABI Publishing, Wallingford, UK,
pp. 109–142.

390 References
Hepton, A. and Hodgson, A.S. (2003) Processing. In: Bartholomew, D.P., Paull, R. and
Rohrbach, K.G. (eds) The Pineapple: Botany, Production and Uses. CABI Publishing,
Wallingford, UK, pp. 281–290.
Krauss, B.H. (1948) Anatomy of the vegetative organs of the pineapple, Ananas comosus
(L) Merr. I. Introduction, organography, the stem, and the lateral branches or
axillary buds. Botanical Gazette 110, 159–217.
Krauss, B.H. (1949) Anatomy of the vegetative organs of the pineapple, Ananas comosus
(L) Merr. II. The leaf. Botanical Gazette 110, 333–404.
Lacoeuilhe, J.J. (1975) Etudes sur le contrÔle du cycle de l’ananas en Côte d’Ivoire. Fruits
30, 307–312.
Leal, F.J. and Soule, J. (1977) ‘Maipure’, a new spineless group of pineapple cultivars.
HortScience 12, 301–305.
Lim, W.H. (1985) Diseases and Disorders of Pineapples in Peninsular Malaysia. MARDI
Report #97, Kuala Lumpur, Malaysia.
Loison-Cabot, C. (1992) Origin, phylogeny and evolution of pineapple species. Fruits
47, 25–32.
Malézieux, E. and Bartholomew, D.P. (2003) Plant nutrition. In: Bartholomew, D.P.,
Paull, R.E. and Rohrbach, K.G. (eds) The Pineapple: Botany, Production and Uses.
CABI Publishing, Wallingford, UK, pp.143–165.
Malézieux, E., Zhang, J.B., Sinclair, E.R. and Bartholomew, D.P. (1994) Predicting
pineapple harvest date in di erent environments, Amiga computer simulation
models. Agronomy Journal 86, 609–617.
Malézieux, E., Cote, F. and Bartholomew, D.P. (2003) Crop environment, and vegetative
physiology and growth. In: Bartholomew, D.P., Paull, R.E. and Rohrbach, K.G. (eds)
The Pineapple: Botany, Production and Uses. CABI Publishing, Wallingford, UK, pp.
69–107.
Nightingale, G.T. (1942) Nitrate and carbohydrate reserves in relation to nitrogen
nutrition of pineapple. Botanical Gazette 103, 409–456.
Okimoto, M.C. (1948) Anatomy and histology of the pineapple infl orescence and fruit.
Botanical Gazette 110, 217–230.
Paull, R.E. (1993) Pineapple and papaya. In: Seymour, G., Taylor, J. and Tucker, G. (eds)
Biochemistry of Fruit Ripening. Chapman & Hall, London, pp. 291–323.
Paull, R.E. and Chen, C.-C. (2003) Postharvest physiology, handling, and storage of
pineapple. In: Bartholomew, D.P., Paull, R.E. and Rohrbach, K.G. (eds) The Pineapple:
Botany, Production and Uses. CABI Publishing, Wallingford, UK, pp. 253–279.
Paull, R.E. and Reyes, M.E.Q. (1996) Preharvest weather conditions and pineapple fruit
translucency. Scientia Horticulturae 66, 59–67.
Paull, R.E. and Rohrbach, K.G. (1985) Symptom development of chilling injury
in pineapple fruit. Journal of the American Society for Horticulture Science 110,
100–105.
Py, C., Lacoeuilhe, J.J. and Teisson, C. (1987) The Pineapple. Cultivation and Uses. G.-P.
Maisonneuve, Paris.
Rodriguez, A.G. (1932) Infl uence of smoke and ethylene on the fl owering of pineapple
(Ananas sativas Shult). Journal of the Department of Agriculture, Puerto Rico 26,
5–18.
Rohrbach, K.G. (2003) History, distribution and world production. In: Bartholomew,
D.P., Paull, R.E. and Rohrbach, K.G. (eds) The Pineapple: Botany, Production and Uses.
CABI Publishing, Wallingford, UK, pp. 1–12.

References 391
Rohrbach, K.G. (Undated) Pineapple. The Plant and its Culture. Hawaii Institute of Tropical
Agriculture and Human Resources, Honolulu, Hawaii.
Rohrbach, K.G. and Apt, W.J. (1986) Nematode and disease problems of pineapple. Plant
Disease 70, 81–87.
Rohrbach, K.G. and Johnson, M. (2003) Pests, diseases and weeds. In: Bartholomew,
D.P., Paull, R.E. and Rohrbach, K.G. (eds) The Pineapple: Botany, Production and Uses.
CABI Publishing, Wallingford, UK, pp. 203–251.
Rohrbach, K.G. and Schmitt, D.P. (1994) Pineapple. In: Plotz, R.C., Zentmyer, G.A.,
Nishijima, W.T. and Rohrbach K.G. (eds) Compendium of Tropical Fruit Diseases. APS
Press, St. Paul, Minnesota, pp. 45–55.
Sanford, W.G. (1962) Pineapple crop log-concept and development. Better Crops Plant
Food 46, 32–43.
Sanford, W.G. (1964) Factors infl uencing the interpretation of the crop log. In. Bould,
C., Prevot, P. and Magness, J.R. (eds) Plant Analysis and Fertilizer Problems. W.F.
Humphrey Press, Geneva, New York, pp. 255–267.
Sether, D.M. and Hu, J.S. (2002) Closterovirus infection and mealybug exposure are
necessary for the development of mealybug wilt of pineapple disease. Phytopathology
92, 928–935.
Sideris, C.P. and Krauss, B.H. (1936) The classifi cation and nomenclature of groups of
pineapple leaves, sections of leaves and sections of stems based on morphological
and anatomical di erences. Pineapple Quarterly 6, 135–147.
Soler, A. (1992) Metabolisme de l’ethephon dans l’épiderme de l’ananas (Ananas comosus
(L.) Merr.). Fruits 47, 471–477.
Teisson, C. (1979a) Le brunissement interne de l’ananas. I. Historique. II. Matériel et
méthodes. Fruits 34, 245–261.
Teisson, C. (1979b) Le brunissement interne de l’ananas. III. Symptomatologie. IV.
Approche biochimique du phénomène. Fruits 34, 315–339.
Wenkam, N.S. (1990) Foods of Hawaii and the Pacifi c Basin Fruits and Fruit Products: Raw
Processed, and Prepared Vol. 4. Composition. College of Tropical Agriculture and
Human Resources Research Extension Series No. 110, Honolulu, Hawaii.
This page intentionally left blank

393
INDEX
Page numbers in bold refer to fi gures, tables and maps.
abscission 5, 232
acetylene 120, 352
allelopathy 37
Annona species
breeding
cultivars 134–136
genetics 133–134
problems 134
commercially important species 123–124
defoliation 97
diseases 144–146
ecology 127–129
family 123
fertilization 143–144
fl owers 130–131
fruit 132–133
composition 151
growth regulators for fruit setting 132
harvesting 148–149
postharvest handling 149–150
ripening 150
use 150
growth and management practices
fi eld preparation for planting 139
pruning 76, 141–142
sweetsop and atemoya 141
transplanting and spacing 139–140
weed management 147–148
insect pests 146–147
irrigation 140
origin, distribution and
morphology 124–126
pollination 131–132, 134
by hand 99
humidity and temperature
requirements 127
propagation
methods 136–137, 139
rootstock and scion compatibility 138,
139
aphids: barriers against 60
apomixes 64
aroma: e ects of temperature 109
atemoya see Annona species
auxins 98
avocado
breeding
cultivars 164, 165
genetics 164–165
methods 166
selection 166–167
commercially important species 153–154
diseases 176–178
ecology 156–158
family 153
fertilization 175–176
fl owers 160–162
fruit 163–164
composition 184
harvesting 180–182
postharvest handling 182–183
use 183
growth and management practices
fi eld preparation for planting 173
integrated crop management
(ICM) 177–178
pruning 175
transplanting and spacing 173–174
weed management 179–180
insect pests 178–179, 180
irrigation 174–175
morphology 159

394 Index
avocado – continued
origin and distribution 154–156
phenological cycle 160
pollination 162–163
propagation
cuttings 171
grafting 171–173
seedlings 170–171
rootstocks 167–168
tree characteristics 158, 160
bacteria, pathogenic 93
bagging 100
banana and plantain
breeding
cultivars 186, 188, 196, 197–198
genetics 195
problems 195–196
selection 196
diseases 209, 210–211, 212
drainage 42–43
ecology 187–189, 190
fertilization 204–205, 207
fl owers 192–193
fruit
composition 219
harvesting 214–215
marketing 217–218
postharvest handling 215–216
ripening 217, 218
soil nutrients removed 206
use 218–220
genus 185–187
growth and development 194, 195
e ect of pseudostem height 207
growth and management practices
bunch covers 208
cableways 202
fi eld preparation for planting 200
high-density annual planting 201–202,
207
pruning and bunch propping 203
sucker management and leaf
removal 203–204
transplanting and spacing 200–201
weed management 214
windbreaks 214
irrigation 84, 202–203
morphology
fruit 193–194
plant 189–190, 191, 192
origin and distribution 187
pests 212, 213, 214
propagation 67, 68, 199–200
pruning 78
barriers
against pests 60, 90, 91
against wind 57–59, 214, 295, 321
beds, raised 42
bees 99
beetles: pollinators 131
beta-carotene 7
biodiversity 1
birds 60
boron 315
branching 72
breakdown, internal 287
budding: propagation method 68
bunches (banana and plantain)
covers 208
propping 203
bunchy top (disease) 316
cableways 202
carambola
pollination 99
vulnerability to wind 25, 27
carbohydrate 6
carpellody 299, 301, 305
cherimoya see Annona species
chlorfl urenol 346
climate
change 31
classifi cation 15–17
day length 19–20
defi nition 11
e ect of altitude 18
rainfall see rainfall
solar radiation 15
subtropics 18–19
temperature 20–23
types in tropics 17–18
winds, frost and hail 24–27
Codex Alimentarius: nutrient claims 7
condensation: during storage 112
consumers: expectations and knowledge 8, 9
containers 71
corms 69, 199–200
cultivars
Annona species 134–136
avocado 164, 165, 169–170
banana 186, 188, 196, 197–198
litchi and longan 237–238, 239–241
mango 265, 266–267, 268–269, 287

Index 395
papaya 309–311
pineapple 329, 330, 343–345
cuttings 66
cyclones 25, 26
defoliation 97
dichogamy 98, 99
diseases 93–94
Annona species 144–146
avocado 176–178
banana and plantain 209, 210–211, 212
litchi and longan 246–247
mango 280, 281, 282
papaya 315–316, 317–318, 319
resistance 304, 309
pineapple 353, 355, 356
resistance 343
postharvest 117–118
disinfection: of pruning tools 77–78
disinfestation, postharvest 117, 250, 282,
322
distribution: spread of fruit through migration
of people 2
drainage 41–43
energy: content in fruit 6
ethephon 231, 275, 352, 359
ethylene 105, 106, 353
for postharvest ripening 119–120, 183,
217, 286
evapotranspiration 84
evolution, fruit 4–5
fatigue, soil 37
fertilizers
application
diluted solutions 88
foliar 88–89, 350
solids 87–88
timing 87
foliar nutrient analysis 143, 144
needs and dosages 84, 86
soil and plant analysis 85, 87
species requirements
Annona 143–144
banana and plantain 204–205, 207
litchi and longan 244–245
mango 274–275
papaya 315
pineapple 350–351, 352
fi eld capacity (FC) 82
fl avour: e ects of temperature 109
fl ooding 24, 41
fl owering
Annona species 130–131
avocado 160–162
banana and plantain 192–193
e ect of photoperiod and
temperature 19–20, 256
induction 96, 98, 232–233, 275–278,
351–353
litchi and longan 228–230
mango 256, 275–278
pineapple 89, 339–340, 351–353, 360
species characteristics 72
folate 7
follicle 5
forums, international 8–9
fruit fl ies 90, 282–283
fungi 93, 209
gibberellins 98
anti-gibberellins 277, 278
girdling 95–97, 160, 231, 277
grafting 68
green manures 53
ground covers 53
growth: regulators 97–98, 132, 277–278
guava
fl owers 19
pruning 76
vulnerability to wind 25
HACCP 118–119
handling, postharvest 116–117
Annona species 149–150
avocado 182–183
banana and plantain 215–216
generalized scheme 102
litchi and longan 249–250
mango 285–287
objective 103
pineapple 360–361
postharvest physiological
disorders 362–363
harvesting 116
Annona species 148–149
avocado 180–182
banana and plantain 214–215
litchi and longan 248–249
mango 285
papaya 321–322
pineapple 359–360

396 Index
herbicides 55–56
horticulture: limitations in tropics 9–10
humidity, relative for storage 111, 112–113
imports 8
injuries
chilling 106–107, 113, 286–287, 323,
362
mechanical 113–114
insects 90–92
pest target species
Annona 146–147
avocado 178–179
banana and plantain 212, 213
litchi and longan 247–248
mango 282–283, 284, 285
papaya 319–320
pineapple 357, 358
postharvest disinfestation 117, 250, 282,
322
integrated crop management (ICM) 177–178
integrated pest management (IPM) 95
intercrops 50–51
International Society of Horticultural Science
(ISHS) 9
International Tropical Fruit Network
(TFNet) 9
irradiation 250, 321
irrigation
and plantation layout 43
control 82–84
determination of demand 24
individual species needs
Annona 140
avocado 174–175
banana and plantain 202–203
litchi and longan 243
mango 271–272
papaya 313–314
low-pressure 80–81
of steep land 39
pineapple 348–350
sprinkler 81–82
sub-irrigation 82
surface 79–80
water quality 36–37
kinetin 231
Köppen, Wladimir: climate classifi cation 15,
16
kudzu 52
layering 67
leaves
defoliation 97
foliar nutrient analysis 143, 144
papaya 296
pineapple 338
removal of dead leaves 204
limb braces 25
liming 36
litchi and longan
breeding
cultivars 237–238, 239–241
genetics 236
selection 236, 239
diseases 246–247
ecology 223–225, 226
factors a ecting fruiting cycle 224
family 221–222
fl owers 226–230
fruit
composition 251
erratic bearing 245–246
growth and development 233–235
harvesting 248–249
postharvest handling 249–250
retention after setting 231
seedless fruit 231–232
use 250–251
growth and management practices
chemical regulators 230–233
fertilization 244–245
fi eld preparation 242
girdling 97, 231
pruning 243–244
transplantation and spacing 242–243
weed management 248
insect pests 247–248
limb braces 25
moisture requirements 23–24
morphology 227, 229
tree 225–226
origin and distribution 222
pollination 230
propagation 241–242
temperature requirements 22
logistics 120–121
longan see litchi and longan
losses, postharvest 101–102
macadamia 99
mango
breeding

Index 397
cultivars 265, 266–267, 268–269, 287
genetics 264
problems 264–265
diseases 280, 281, 282
ecology 21, 23–24, 254–256
family 252
fertilization 274–275
fl owers 257–259
induction 97, 275–278
fruit
composition 289
growth and development 263–264
harvesting 283
marketing 288
morphology 261, 263
postharvest handling 285–287
use 288–290
yields 257, 278–280
growth and management practices
chemical regulators 261–263
fi eld preparation 271
pruning 272–274
weed management 285
growth retardants 98
induction of fl owering 98
insect pests 282–283, 284, 285
irrigation 271–272
marketing and production 268
morphology 253
origin and distribution 252, 254
pollination 259–260
polyembryony 260–261
propagation 270–271
pruning 75
rootstocks 269
tree characteristics 257
mangosteen
tolerant to fl ooding 24
manure, green 53
marcottage 67
market analysis: in establishment of new
orchards 33–34
mites 92, 180, 319–320, 357
monsoon 25
morphology
atemoya 126
cherimoya 124–125
pond apple 126
sweetsop 125–126
mulching 54
nematodes 94, 212, 213, 214, 321, 355, 356
control by soil fumigation 347
nitrates: for fl owering induction 277–278
nurseries: management 69–70
nutrients, dietary
contents in fruits 6–7, 151, 184, 219,
251, 289, 325, 364
e ects of storage 108–109
orchards
establishment
drainage 41–43
irrigation water quality 36–37
land preparation 39–41
land selection 34, 35
mountainous land 39
planning 37–39
plantation layout 43–44, 45
planting see planting
previous land usage 37
role of market analysis 33–34
soil evaluation 36
fl oor management 52–54
organic fruit 120
paclobutrazol 277, 278
papaya
breeding
cultivars 309–311
genetics 303–307
objectives 307–309
transgenic varieties 307
diseases 315–316, 317–318, 319
resistance 304, 309
ecology 292–295
fl owers 296, 298–299, 301
types 300
fruit 302–303, 304
characteristics 305–307
composition 325
harvesting 321–322
marketing 323–324
postharvest handling 322–323
use 324–325
world production 324
growth and management practices
fertilization 315
fi eld preparation 312
orchard protection 321
propagation 311–312
pruning 314–315
transplanting and spacing 313
weed management 321

398 Index
papaya – continued
growth characteristics 298
intolerant to fl ooding 24
irrigation 313–314
leaves 296
morphology 297
origin and distribution 292
pests
insects and mites 319–320
nematodes 321
pH tolerance 30, 293
pollination 301–302
stem 295–296
taxonomy and nomenclature 291
vulnerability to wind 25
passion fruit
pollination 99
vulnerability to wind 25
peach
problems with orchard replantation 37
permanent wilting point (PWP) 83
pests 10
barriers 60
e ects of climate change 31
insects see insects
integrated pest management 95
nematodes 94
postharvest disinfestation 117
vertebrates 95
pH, soil 30–31, 36, 293
phosphorus
defi ciency in soil 27, 29
photoperiod 13, 19, 128, 189, 256, 335–336
physiology, postharvest
chilling injury 106–107
ethylene production 105, 106
moisture loss 107–108, 109
respiration 104–105, 106, 107
unique characteristics of tropical
fruits 103–104
pineapple
breeding
cultivars 329, 330, 343–345
genetics 342
selection 342–343
diseases 353, 355, 356
resistance 343
ecology 24, 330–336
fl owering induction 98, 351–353, 354
fruit
composition 364
e ect of precocious fl owering on
production 360
growth and development 340–341
harvesting 359–360
morphology 337
postharvest handling 360–361
postharvest physiological
disorders 362–363
standards 361
use 363–365
weight at harvest versus plant weight at
forcing 354
growth and management practices
fertilization 350–351, 352
fi eld preparation for planting 346–347
planting 347–348
soil fumigation 347
spacing 348, 349
weed management 357, 359
irrigation 348–350
leaves 338
morphology 328
fl owers 19–20
fruit 337
roots 337–338
stem 336–337
origin and distribution 329
pests
insects and mites 357, 358
nematodes 347, 355, 356
propagation 63, 67, 68, 345–346
taxonomy and nomenclature 327–328
wind damage 25
plantain see banana and plantain
planting
associated crops and intercrops 50–51
high-density annual 201–202, 207
layout 43–44, 45
material 48–50
procedure 46–48, 70, 347–348
spacing 44, 46, 47
Annona species 140
avocado 173–174
banana and plantain 200–201
litchi and longan 242–243
papaya 313
pineapple 348, 349
pluviometers 83
pollination 162–163, 166, 230, 260–261,
301–302
by beetles 131
b
y hand 98–99, 128, 131–132
cross-pollination 131, 134
temperature and humidity
requirements 127

Index 399
polyembryony 260–261
potassium
content in fruit 7
production, fruit
2007 world statistics 4
world trends and projections 7–8
propagation
di erences betwen sexually and asexually
propagated trees 69
importance to trade 63
litchi and longan 241–242
methods
cuttings 66
for various crops 65, 66, 136–137, 139,
199–200, 241–242, 270–271,
311–312, 345–346
grafting and budding 68
layering 67
sexual (seed) 64–65
tissue culture 67, 200, 312
natural propagative structures 68–69
to ensure quality 63
protein 6
pruning
Annona species 141–142
avocado 175
banana and plantain 203
cuts and tools 77–78
formation pruning 71, 73, 74
litchi and longan 243–244
mango 272–274
papaya 314–315
production pruning 74–76
rationale 70–71
sanitary and maintenance 76
structural regeneration 76–77, 78
to change a variety 77
quality
defi nition 101
major logistical issue 120–121
objective of postharvest handling 101
of fruit
consumer expectations 8
of plants 48–49
radiation, solar 15, 20, 158, 189, 190,
294–295, 334–335, 336
temporary shade 98–100
rain gauges 83
rain shelters 100
rainfall
amount available to plants 23
patterns in the tropics 13, 14, 23
quantity and distribution 23–24
species preferences
Annona species 127
avocado 156–157
banana and plantain 187–188
litchi and longan 223
mango 254–255
papaya 293
pineapple 331–332
rainforest 17
regulators, chemical 97–98, 230–231,
261–263, 346
respiration, postharvest 104–105, 106, 107
ripening 119–120, 183, 217, 286
pre-harvest 359
rodents 95
roots
development in containers 70
e ect of soil on development 40
pineapple 337–338
rootstocks
Annona species 138, 139
avocado 167–168
mango 269
rotation, crop 37
safety, food 118–119
salinity 156, 293
plant tolerance 38, 168
sap burn 287
savannah
climate 17–18
scoring 95–97, 277
seedlings 69–70
seeds
use in production 64–65
use in selection 63
selection
seeds versus vegetative material 63
shrink 101
smudging 275
sodium naphthalene acetate (SNA) 230–231
soil
analysis 85
classifi cation and characteristics 27–31
defi ciencies 27, 29, 30–31
distribution of soil types in tropics 28, 29
e ect of fl ooding 41
e ect on root development 40

400 Index
soil – continued
evaluation 36
fatigue 37
fumigation 347
land selection for orchards 34, 35
moisture content 23
problems in tropics 10
species preferences
Annona species 127
avocado 156
banana 127
litchi and longan 223
mango 254
papaya 292–293
pineapple 331
standards 116, 286, 361
storage
chilling injury 106–107, 113, 286–287,
323, 362
controlled and modifi ed
atmosphere 114–116, 250, 286, 323
e ects on fl avour and aroma 109
mechanical injury 113–114
moisture loss 107–108, 109, 112–113,
113
on-tree 180–181
potential 110, 111
relative humidity 111, 112–113
temperature 108, 110–111, 183, 361
vitamin C loss 108–109
storms 26
subsoiling 40
subtropics
use for tropical fruit crops 21
suckers 68–69, 199–200, 336–337
management 203–204
sweetsop see Annona species
taxonomy 3
temperature
chilling injury 106–107, 113, 286–287,
323, 362
cold resistance 167
e ect on fl owering 20, 161, 257
e ects on fl avour and aroma 109
factors of infl uence 20–21
for storage 108, 110–111, 183, 249, 250
in tropics 11, 13
postharvest cooling 117
species preferences 21–23
Annona species 127–128
avocado 157–158
banana and plantain 188–189
litchi and longan 224–225, 226
mango 255–256
papaya 293–294
pineapple 332–334
tensiometers 81, 83
TFNet (International Tropical Fruit
Network) 9
thiourea 277
Thornthwaite, C.W.: climate classifi cation 15
time domain refl ectometry (TDR) 83
tissue culture 67, 200, 312
traps, insect 90, 91
trees: as windbreaks 57–58, 59
tropics
characteristics 1
defi nition and characteristics of region 11,
13–15
distribution of tropical and subtropical
regions 12
uniconazole 277, 278
urea 231
viruses and viroids 94, 316, 319
vitamin C 7
loss on storage 108–109
weeds: management 54–56
Annona species 147–148
avocado 179–180
banana and plantain 214
litchi and longan 248
mango 285
papaya 321
pineapple 357, 359
winds 24–27, 56–57
susceptible species
Annona species 128
banana and plantain 189, 214
mango 257
windbreaks 57–59, 214, 295, 321


