
Virginia Polytechnic Institute and State University
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy - No. 13030 - Page 1
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy
1.0 Purpose
This policy establishes requirements for the safe, secure, and compliant use of
recombinant or synthetic nucleic acid molecules and/or biohazardous materials. These
requirements are intended to protect university personnel, the public, as well as the
environment.
2.0 Policy and Principles
Virginia Tech is actively committed to preserving the health and safety of its students,
staff, and faculty, and also to protecting the environment and the community. It is
recognized that use of potentially biohazardous materials and organisms containing
recombinant or synthetic nucleic acid molecules is necessary in many Virginia Tech
research and teaching laboratories. To ensure the safe handling of these organisms,
Virginia Tech requires that all research and instruction involving recombinant or synthetic
nucleic acid molecules and/or biohazardous materials is conducted at Virginia Tech shall
be conducted in accordance with federal and state laws.
To ensure compliance with the NIH Guidelines, Virginia Tech has established an
Institutional Biosafety Committee (IBC) tasked with: (i) developing and implementing
policies for the safe conduct of recombinant or synthetic nucleic acid molecule research,
and safe handling and use of biohazardous materials; (ii) reviewing PI-submitted protocols,
lab biosafety manuals, and other documentation regarding the handling, use, and storage of
regulated materials; (iii) reviewing the results of periodic lab biosafety inspections by the
Biosafety Officer [BSO]; (iv) ensuring that appropriate training is provided and
documented for the IBC Chair and members, BSO and other containment experts (when
applicable), Principal Investigators, laboratory staff, and students regarding laboratory
safety and implementation of the NIH Guidelines; and, (v) reviewing incidents and/or
policy violations.
2.1 Scope
This policy, its amendments and additions, applies to all university personnel (faculty,
staff, and students), as well as visitors, engaged in instructional activities and/or research
involving recombinant or synthetic nucleic acid molecules, biohazardous agents, materials
and toxins that are:
• University- or externally-funded and/or sponsored.
• Conducted by University personnel and/or visitors.
• Conducted using the University’s property, equipment, and facilities.
• Received, stored, used, transferred or disposed of at any of the University facilities.
No. 13030
Policy Effective Date:
1/28/2014
Last Revision Date:
1/7/2020
Policy Owner:
Dan Sui
Policy Author: (Contact
Person)
Laurel Miner
Affected Parties:
Undergraduate
Graduate
Faculty
Staff
Other
1.0 Purpose
2.0 Policy and Principles
3.0 Scope
4.0 Procedures
5.0 Definitions
6.0 References
7.0 Approval and
Revisions

Virginia Polytechnic Institute and State University
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy - No. 13030 - Page 2
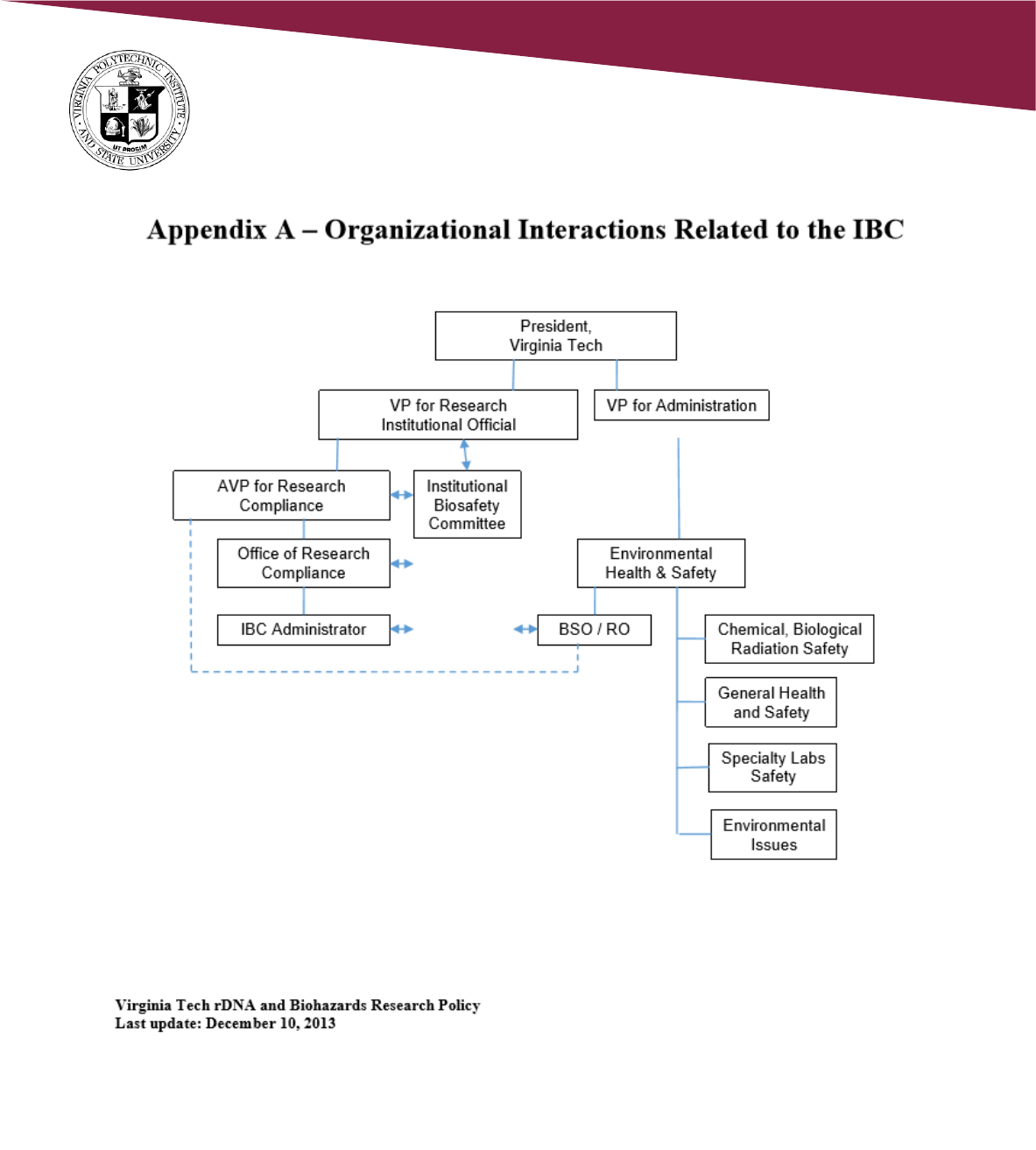
2.2 Oversight
2.2.1 Senior Vice President for Research and Innovation – “Institutional Official”
The Senior Vice President for Research and Innovation is the university official with final responsibility for
ensuring that all research and instructional activities involving the handling and use of recombinant or synthetic
nucleic acid molecules and/or potentially biohazardous materials is in compliance with all applicable laws,
regulations, guidelines, and policies. The Senior Vice President for Research and Innovation assists the University
President in maintaining continuing relationships with state and federal regulatory agencies which deal with
regulated activities included in this policy.
The Senior Vice President for Research and Innovation will appoint members to the IBC and will appoint the Chair
of the IBC.
2.2.2 Division of Scholarly Integrity and Research Compliance (SIRC)
The Division of Scholarly Integrity and Research Compliance is the functional administrative unit that is charged
with supporting the IBC in fulfilling its responsibility for ensuring both institutional and individual researcher
compliance with federal and state laws, regulations, policies, and guidelines for research involving recombinant or
synthetic nucleic acid molecules, infectious biological or synthetic agents, biologically derived materials and toxins
at Virginia Tech.
The Division of Scholarly Integrity and Research Compliance (SIRC) is an administrative unit under the
supervision of the Associate Vice President for SIRC, who reports to the Senior Vice President for Research and
Innovation, the designated Institutional Official for regulatory compliance. The Division of Scholarly Integrity and
Research Compliance has executive responsibility for the implementation of all Virginia Tech policies involving
the use, in research and instruction, of recombinant or synthetic nucleic acid molecules, infectious biological or
synthetic agents, biologically derived materials and toxins. The IBC Program is an administrative unit within the
Division of Scholarly Integrity and Research Compliance, responsible for the administrative support of the IBC.
2.2.2.1 Associate Vice President for Scholarly Integrity and Research Compliance
The AVP for Scholarly Integrity and Research Compliance reports to the Senior Vice President for Research and
Innovation and oversees the operation and management of the Division of Scholarly Integrity and Research
Compliance, including the administrative support provided to the IBC. The Associate Vice President for Scholarly
Integrity and Research Compliance acts as a non-voting consultant for the IBC, when needed.
2.2.2.2 IBC Program
The IBC Program includes the administrative staff responsible for ensuring that IBC policies and practices are
followed, and working with the BSO to ensure compliance with NIH Guidelines, the BMBL, Select Agents and
Toxins regulations, OSHA regulations, and best practices to ensure institutional compliance with applicable federal
laws, regulations, and policies [listed in the References section below]. The IBC Program consists of the IBC
Program Director and IBC Administrator, a Senior IBC Program Coordinator, and IBC Program Coordinators, as
needed. These individuals have frequent and varied contacts inside and outside of the organization as required to
establish parameters/metrics for program success, e.g., developing procedures, coordinating service delivery,
promoting program(s) goals and objectives in addition to providing technical advice.
The IBC Program Director attends IBC meetings and acts as a liaison for the IBC, with support from the
AVP for Scholarly Integrity and Research Compliance and the IO.

Virginia Polytechnic Institute and State University
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy - No. 13030 - Page 3
IBC Program staff review all protocols that are submitted to the IBC Program to ensure that the submissions
are complete, and that the information provided by the researchers is consistent with the IBC requirements,
NIH Guidelines requirements and Virginia Tech regulations. They are also responsible for guiding
researchers through the IBC process, assisting researchers with federal and Virginia Tech requirements,
reviewing annual review submissions, conducting in-person annual review meetings, providing guidance to
researchers and personnel related to IBC protocols, and providing guidance related to other areas involving
biosafety compliance and training.
2.2.3 Environmental Health and Safety (EHS)
EHS is the administrative unit in which the University Biosafety Officer (BSO)/Responsible Official (RO) resides.
EHS promotes a positive, integrated safety culture for the university community, advocates safe and healthy living,
learning, and working environments, and helps departments comply with regulations and mandates. The University
BSO and the Associate BSOs are under the supervision of the Assistant Vice President for EHS, who reports to the
Associate Vice President for Safety and Security.
The BSO and the AVP for EHS are ex officio members of the IBC. In addition to BSO oversight, other EHS
program areas associated with lab and personnel safety include, but are not limited to: bloodborne pathogens,
chemical safety, radiation safety, respiratory protection, and the Occupational Health Assurance Program.
2.2.3.1 Assistant Vice President for Environmental Health and Safety
The AVP for EHS reports directly to the Associate Vice President for Safety and Security and oversees all
operation and management of EHS programs. The AVP for EHS is an ex officio member of the IBC.
2.2.3.2 University Biosafety Officer/Responsible Official (BSO/RO)
The BSO/RO directs and manages the University Biosafety Program which includes general biosafety as well as
the Select Agent and Toxin program. The BSO/RO develops, implements, and coordinates program requirements to
enhance the university’s biosafety-related objectives and ensure compliance with all applicable regulations,
guidelines, policies, and directives.
Specific to the IBC, the BSO/RO has the following duties and responsibilities:
• conducting periodic inspections to ensure that laboratory standards are rigorously followed;
• reporting to the IBC and the institution any significant problems, violations of the NIH Guidelines, and any
significant research-related accidents or illnesses of which the BSO/RO becomes aware unless the BSO/RO
determines that a report has already been filed by the Principal Investigator;
• developing emergency plans for handling accidental spills and personnel contamination and investigating
laboratory accidents involving recombinant or synthetic nucleic acid molecule research or biohazardous
materials; providing advice on laboratory security; and,
• providing technical advice to Principal Investigators (PIs) and the IBC Committee on safety procedures
To meet objectives of the IBC and general university biosafety requirements, the BSO/RO works closely with the
SIRC and the IBC Administrator as well as many other university units.

Virginia Polytechnic Institute and State University
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy - No. 13030 - Page 4
2.2.4 Institutional Biosafety Committee (IBC) - NIH Guidelines Section IV-B-2
On behalf of Virginia Tech, the Institutional Biosafety Committee is responsible for:
• reviewing use of recombinant or synthetic nucleic acid molecules and/or potentially biohazardous material
work conducted at the institution for compliance with, among other requirements, the NIH Guidelines, the
review includes: –
o independent assessment of the biosafety containment levels required by the NIH Guidelines and
BMBL for the proposed research;
o assessment of the facilities, procedures, practices, and training and expertise of personnel involved
with use of these materials; and
o ensuring compliance with all surveillance, data reporting, and adverse event reporting requirements
required by the NIH Guidelines.
• notifying the Principal Investigator of the results of the IBC's review and the protocol’s approval status
• lowering containment levels for certain experiments as specified in the NIH Guidelines
• setting containment levels for Experiments Involving Whole Animals, and Experiments Involving Whole
Plants
• periodically reviewing applicable work conducted at the institution to ensure compliance with the NIH
Guidelines
• adopting emergency plans covering accidental spills and personnel contamination reporting any significant
problems with or violations of the NIH Guidelines and any significant research-related accidents or
illnesses to the appropriate institutional official and National Institutes of Health Office of Biotechnology
Activities (NIH/OBA) within 30 days
• performing such other functions as may be delegated to the IBC under the NIH Guidelines.
2.2.4.1 The IBC’s Authority
The IBC has the authority to approve, require modifications in, disapprove, or halt all research activities that fall
within its jurisdiction as specified by federal regulations, state law, and institutional policy. The IBC has the
authority to require appropriate training of PIs, lab staff, and students, and to prohibit individuals who have not
completed training from working under an approved protocol. The IBC acts as a surrogate for the federal
government in ensuring local regulatory compliance.
2.2.4.2 IBC Meetings
IBC meetings to review protocols and amendments are generally held monthly, on the 2nd Tuesday of each month,
with additional meetings scheduled as needed.
2.2.5 Researchers and Instructors
2.2.5.1 Protocol Submission for IBC Review and Approval
IBC protocol submissions, whether they are new IBC protocol submissions, modifications or renewals, must be
submitted to the ORC IBC Administrator by the Principal Investigator for review and approval by the IBC. IBC
review and approval is required, before study initiation, for studies which fall under NIH Guidelines Sections III-A,
III-B, III-C, and III-D. For experiments/activities which fall under NIH Guidelines Section III-E, protocol
submission to the IBC may be simultaneous with project initiation. For experiments/activities which are classified

Virginia Polytechnic Institute and State University
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy - No. 13030 - Page 5
as Exempt, as defined in NIH Guidelines Section III-F, the IBC Chair or designee will confirm the PI’s assertion
that the activities are Exempt.
2.2.5.1.1 Confidentiality
Protocol submission forms will be considered confidential, to the extent permitted by Commonwealth of Virginia
law, except insofar as the dissemination of information regarding research projects or activities and IBC
deliberations, decisions, and recommendations to appropriate Institutional officials is required to effectuate or
support the policies or interests of the Institution. The NIH Guidelines require that most IBC meetings where
protocols involving rDNA are reviewed be open to the public, and thus discussions that occur during meetings
cannot be considered as confidential.
2.2.5.2 Responsibilities of Researchers and Instructors
The responsibilities of Virginia Tech researchers/PIs/instructors when using recombinant or synthetic nucleic acid
molecules and/or potentially biohazardous materials includes, but is not limited to, the following:
• ensuring that activities are not initiated or subsequently modified prior to IBC review and approval
• reporting any significant problems, violations of the NIH Guidelines, or any significant research-related
accidents and illnesses to the BSO/RO (where applicable), Greenhouse/Animal Facility Director (where
applicable), IBC, and other appropriate authorities (if applicable) within 30 days, to facilitate prompt
reporting to NIH/OBA by the BSO/RO
• ensuring that she/he is adequately trained in good microbiological techniques, and that she/he has
appropriately trained research staff and students in those techniques
• ensuring adherence to IBC approved emergency plans for handling accidental spills and personnel
contamination
• complying with shipping requirements for recombinant or synthetic nucleic acid molecules and/or
potentially biohazardous materials
• acquiring proper permits for obtaining/transporting exotic or regulated plants or pathogenic organisms and
Select Agents
• making an initial determination of the required levels of physical and biological containment in accordance
with the NIH Guidelines and BMBL
• selecting appropriate microbiological practices and laboratory techniques to be used for the research
• communicating any proposed changes, or any problem encountered, to the IBC
• supervising the safety performance of staff to ensure that the required safety practices and techniques are
employed
• investigating and reporting any significant problems pertaining to the operation and implementation of
containment practices and procedures in writing to the UBO/RO (where applicable), Greenhouse/Animal
Facility Director (where applicable), IBC, NIH/OBA, and other appropriate authorities
• correcting work errors and conditions that may result in the release of recombinant or synthetic nucleic acid
molecule materials, or exposure of lab personnel to biohazardous agents or toxins
• ensuring the integrity of the physical containment (e.g., biological safety cabinets) and the biological
containment, ensuring that biosafety cabinets have been certified annually
• being ultimately responsible for compliance with all IBC approved protocols/modifications.

Virginia Polytechnic Institute and State University
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy - No. 13030 - Page 6
3.0 Procedures
3.1 Initial IBC Review
Principal Investigators (PIs) and instructors seeking initial IBC approval must send a completed Protocol
Application to the IBC Program in accordance with the IBC policy on protocol submission. A Protocol Application
consists of several forms that capture information about the specific research/teaching activities. In addition to
documentation, facility inspections, documentation review, training and applicable occupational health
requirements must be completed prior to IBC approval of the protocol.
3.1.1 Pre-interview (Optional)
Any PI or instructor planning to submit a Protocol Application may contact the IBC Program Director for
assistance with the protocol submission and IBC processes.
3.1.2 Select Agents and Toxins
In addition to required IBC documentation, specific documentation for the use of select agents and toxins is
required. This information is gathered by the BSO/RO. The IBC Program staff will alert the BSO/RO of any
anticipated use of select agents or toxins. Select Agents and biological toxins cannot be obtained/procured or used
without BSO review and approval.
3.2 Amending Protocols
An amendment to an approved protocol includes, but is not limited to, changes to staff, location of experiment,
gene of interest, nature of the inserted nucleic acids, host cells, animals used, vectors, cell lines, and cultures.
Protocols may be amended in accordance with the IBC policy on protocol submission.
3.3 Annual Review
All protocols that have been previously approved by the IBC require an annual review to assess any changes that
have been made during the previous year. This review also verifies that all work has been conducted in accordance
with the approved protocol. The IBC contacts the Principal Investigator or instructor prior to the anniversary date of
initial protocol approval with instructions.
3.4 Protocol Renewal
All full committee protocols must be renewed by the PI every three years.
4.0 Definitions
NIH Guidelines – The “NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules
(NIH Guidelines)” specify the practices for constructing and handling of: (i) recombinant nucleic acid molecules,
(ii) synthetic nucleic acid molecules, including those that are chemically or otherwise modified but can base pair
with naturally occurring nucleic acid molecules, and (iii) cells, organisms, and viruses containing such molecules.
The guidelines are applicable to all recombinant or synthetic nucleic acid research within the United States (U.S.)
or its territories, regardless of the source of funding for the research.
Institutional Biosafety Committee (IBC) – The compliance oversight committee required by the NIH Guidelines.
The expertise and membership of the IBC must be reflective of the research conducted at an institution, e.g.,

Virginia Polytechnic Institute and State University
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy - No. 13030 - Page 7
including plant or animal experts, a Biological Safety Officer (BSO), or other expertise as appropriate, and must
also include at least two unaffiliated members who can represent the interests of the community surrounding the
registered institution.
IBC Research or Teaching Protocol (Protocol) – Information provided by the Principal Investigator (PI) in
defined submission forms that describes: (i) the use of recombinant DNA or synthetic nucleic acid molecules and
the cells, organisms, and viruses containing such molecules; (ii) the applicable NIH Guidelines; (iii) the training,
experience, and expertise of the PI in handling and use the specified agents under specified containment criteria;
(iv) the training of staff included in the protocol; (v) a description and floorplan of facilities and equipment to
assess whether containment practices are appropriate; (vi) lab Biosafety Manual.
Biohazardous Materials – Infectious biological or synthetic agents, biologically derived materials and toxins that
present a risk or potential risk to the health of humans, animals, or plants either directly through exposure or
infection or indirectly through damage to the environment. Categories of potentially infectious biological materials
may include the following:
• human, animal, and plant pathogens (bacteria, parasites, fungi, viruses, prions)
• toxins of biological origin
• human and non-human primate cells and unfixed tissues
• animal or plant pathogens and products, specifically genetically engineered organisms and veterinary
biologics
• select agents and toxins
• infected animals and animal tissues and infected plants.
Biological Safety Officer (BSO) – This individual is required by the NIH Guidelines, and has the following duties
and responsibilities: to conduct periodic inspections to ensure that laboratory biosafety standards are rigorously
followed; to report to the IBC and the institution any significant problems, violations of the NIH Guidelines, and
any significant research related accidents or illnesses; to develop emergency plans for handling accidental spills
and/or personnel contamination, and for investigating lab accidents involving rDNA research; to provide advice on
laboratory security; to provide technical advice to PIs and the IBC on research safety procedures.
BMBL – The CDC/NIH handbook, “Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th
Edition”, provides a code of practice for biosafety, addressing the safe handling and containment of infectious
microorganisms and hazardous biological materials. The BSO and the IBC use the BMBL to assess containment
practices and personal protective equipment (PPE) required for activities proposed in a PI’s IBC protocol.
Office of Science Policy (OSP) – The NIH Office of Science Policy promotes science, safety, and ethics
in biotechnology through advancement of knowledge, enhancement of public understanding, and
development of sound public policies. OBA accomplishes its mission through analysis, deliberation, and
communication of scientific, medical, ethical, legal, and social issues. An institution that is
conducting research subject to the NIH Guidelines must have an IBC, and that IBC must be registered
with and approved by OSP, demonstrating that the IBC has knowledge of local institutional
characteristics, e.g., adequate investigator training, laboratory conditions, and operating procedures.
Responsible Official (RO) – The RO is the designated individual at the institution with the authority and
responsibility to act on behalf of the institution to ensure compliance with the requirements of APHIS and HHS

Virginia Polytechnic Institute and State University
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy - No. 13030 - Page 8
regulations governing the possession and use of Select Agents and Select Agent Toxins. The RO ensures that
annual inspections are conducted and that deficiencies are corrected. Currently, the BSO is the RO at Virginia
Tech
USDA APHIS – The United States Department of Agriculture Animal and Plant Health Inspection Service
(USDA-APHIS) regulates genetically engineered (GE) organisms and certain GE organisms that may pose a risk to
plant or animal health. APHIS uses the term biotechnology to mean the use of recombinant or synthetic nucleic acid
molecules technology, or genetic engineering to modify living organisms. Permits are required for the importation,
transit, domestic interstate movement and environmental release of organisms that impact plants and animals.
HHS CDC – The Department of Health and Human Services (HHS) Centers for Disease Control and Prevention is
the agency dedicated to protecting health and promoting quality of life through the prevention and control of
disease, injury, and disability. The CDC is also the enforcement agency for HHS-regulated select agents and toxins.
Select Agents and Toxins – Specific biological agents and toxins identified by the Department of Health and
Human Services (HHS) and the United States Department of Agriculture (USDA) as having the potential to pose a
severe threat to the public, animal or plant health, or to animal or plant products. Regulated material also includes:
• Nucleic acids that can produce infectious forms of any of the select agent viruses
• Recombinant and/or synthetic nucleic acids that encode for the functional form(s) of any of the toxins if the
nucleic acids:
o Can be expressed in vivo or in vitro, or
o Are in a vector or recombinant host genome and can be expressed in vivo or in vitro.
• Genetically modified select agents and toxins
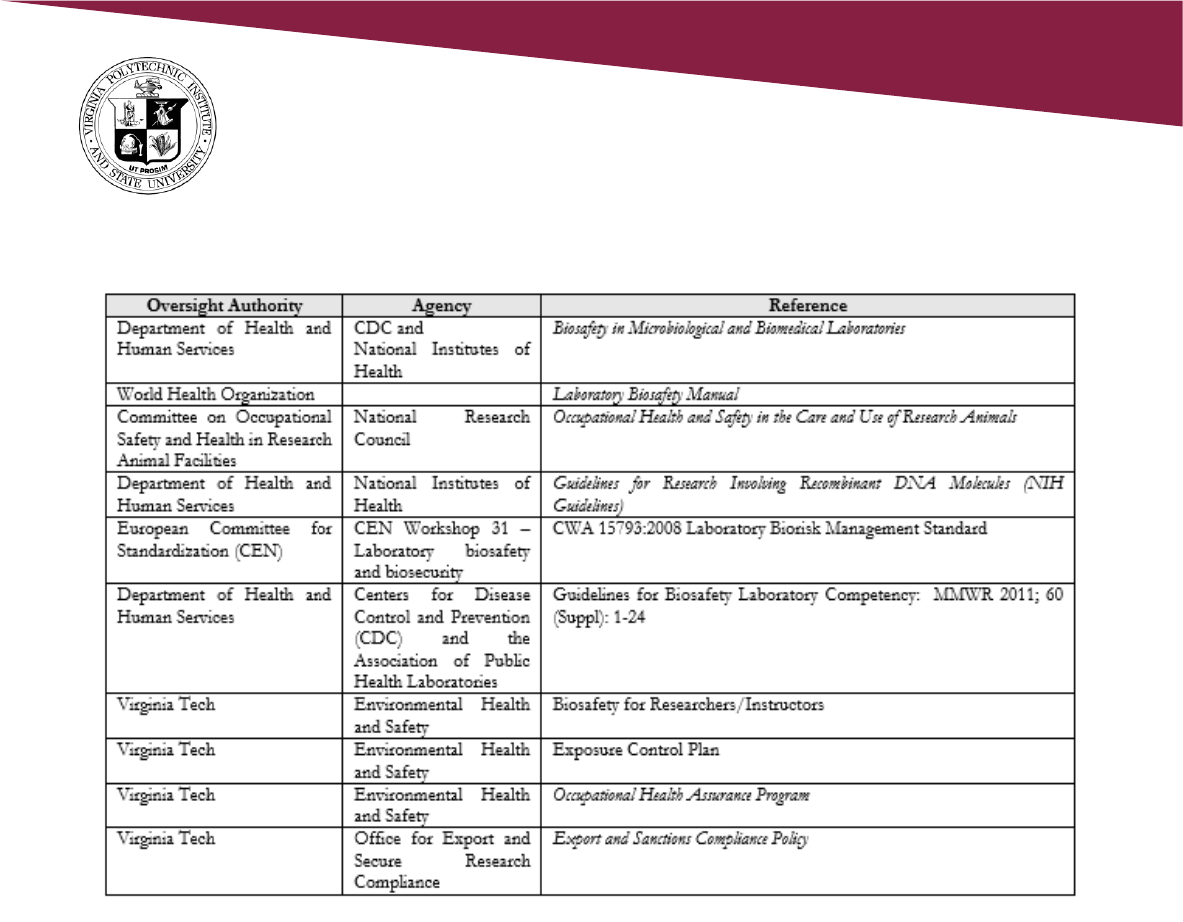
5.0 References
Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition
https://www.cdc.gov/labs/BMBL.html
NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules:
https://osp.od.nih.gov/buitechnology/nih-Guidelines

Virginia Polytechnic Institute and State University
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy - No. 13030 - Page 9
5.1 Regulations
Virginia Polytechnic Institute and State University
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy - No. 13030 - Page 10
5.2 Standards of Practice/University Requirements
6.0 Approval and Revisions
Approved January 28, 2014 by Virginia Tech Institutional Biosafety Committee (IBC)
Approved January 29, 2014 by Vice President for Research, Robert W. Walters.
Approved May 15, 2014 by University President, Charles W. Steger.
• Revision 1
▪ Technical revision and title changes throughout
▪ Included provision for in-person annual review meetings in section 2.2.2.2
Policy review and technical changes recommended by the Virginia Tech Institutional Biosafety Committee
and by IBC Administrator, Regina Allen on November 12, 2019.
Approved January 7, 2020 by Vice President for Policy and Governance, Kim O’Rourke.
Virginia Polytechnic Institute and State University
Virginia Tech Recombinant and Synthetic Nucleic Acid and Biohazard Research Policy - No. 13030 - Page 11
