REPORT TO CONGRESS
Prescription Drug Pricing Report
August 6, 2019
U.S. Department of Health and Human Services
Prepared by:
Office of the Assistant Secretary for Planning and Evaluation (ASPE)
2
Table of Contents
Page
Section 1. Introduction
5
Section 2. Medicare Part B
9
Section 3. Medicare Part D
18
Section 4. Medicaid
27
Section 5. Drugs Benefiting from Government Grants or Research Subsidies
35
Appendix. Top 10 Drugs by Total Cost, Spending per Unit, and Prescription
Frequency in Medicare Part B, Medicare Part D, and Medicaid
41
3
Tables
Table 2-1 Medicare Fee-for-Service Part B Program Spending for Drug Benefits, 2006-2017
Table 2-2 Medicare Fee-for-Service Part B Program Spending per Enrollee and User for Drug
Benefits, 2006-2017
Table 2-3 Top 10 Highest-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
(2008, 2011, 2014, 2017)
Table 2-4 Top 10 Highest-Cost Prescribed Drugs, Medicare Part B, Ranked by Spending per
Unit (2011, 2014, 2017)
Table 2-5 Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number
of Services (2008, 2011, 2014, 2017)
Table 3-1 Medicare Part D Total Program Spending and Benefit Spending, 2006-2017
Table 3-2 Medicare Part D Prescription Gross Drug Costs (GDC): 2007-2017
Table 3-3 Top 10 Highest-Cost Drugs, Medicare Part D, Ranked by Total Spending (2008,
2011, 2014, 2017)
Table 3-4 Top 10 Highest-Cost Drugs, Medicare Part D, Ranked by Spending per Unit (2008,
2011, 2014, 2017)
Table 3-5 Top 10 Most Frequently Prescribed Drugs, Medicare Part D, Ranked by Number of
Claims (2008, 2011, 2014, 2017)
Table 4-1 Medicaid Prescription Drug Gross Spending, 2006-2017
Table 4-2 Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Total Spending (2008,
2011, 2014, 2017)
Table 4-3 Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Spending per Unit (2014,
2017)
Table 4-4 Top 10 Most Frequently Prescribed Drugs, Medicaid, Ranked by Number of Claims
(2008, 2011, 2014, 2017)
Table 5-1 Prescription Drugs Approved for Sale by the Food and Drug Administration from
2013 to 2017, and 2017 Spending by Program Where Applicable
Table A-1 Top 10 High-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
(All years)
4
Table A-2 Top 10 Highest-Cost Prescribed Drugs, Medicare Part B, Ranked by Spending per
Unit (All years)
Table A-3 Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total
Number of Services (All years)
Table A-4 Top 10 High-Cost Prescribed Drugs, Medicare Part D, Ranked by Total Spending
(All years)
Table A-5 Top 10 Highest-Cost Drugs, Medicare Part D, Ranked by Spending per Unit (All
years)
Table A-6 Top 10 Most Frequently Prescribed Drugs, Medicare Part D, Ranked by Number of
Claims (All years)
Table A-7 Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Total Spending (All years)
Table A-8 Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Spending per Unit (All
years)
Table A-9 Top 10 Most Frequently Prescribed Drugs, Medicaid, Ranked by Number of Claims
(All years)
Figures
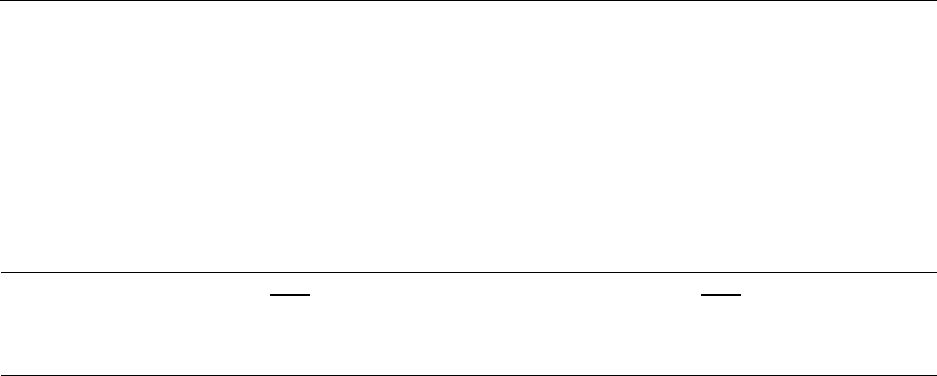
Figure 1-1 Annual Percentage Change in Prescription Drug Spending by Program, 2007-2017
Figure 3-1 Medicare Part D Total Spending per Enrollee and Per User, 2006-2017
5
SECTION 1: INTRODUCTION
The Secretary of Health and Human Services (HHS) has been directed to submit a drug pricing
report containing information requested by the House Committee on Appropriations. In
response, the Assistant Secretary for Planning and Evaluation (ASPE) developed this report
containing data and analyses related to prescription drug spending between 2006
1
and 2017 as
well as on prescription drugs benefiting from public funding for biomedical research since 2013.
The sections on prescription drug spending provide comparative gross prescription drug
spending and prices as well as the top 10 highest-cost drugs and the top 10 most frequently
prescribed drugs for each of the following: (1) The Medicare program under part B of title XVIII
of the Social Security Act; (2) The Medicare prescription drug program under part D of title
XVIII of the Social Security Act; (3) The Medicaid program under title XIX of the Social
Security Act. The section on public funding for biomedical research provides the list of drugs
that have been approved for sale by the Food and Drug Administration (FDA) in the past five
years that have benefited significantly from government grants or research subsidies in either the
pre-clinical or clinical stages of development, as well as spending in Medicare and Medicaid for
each of those drugs.
This report does not include: (1) prescription drug spending or prices pertaining to programs of
the Department of Veterans Affairs; (2) spending and prices net of rebates for individual drugs
within Medicare Part D or Medicaid, or spending net of rebates in aggregate for Medicaid; or (3)
a breakdown of the comparative prices net of rebates for each of the 10 most frequently
prescribed drugs or the 10 highest-cost drugs between ambulatory settings and retail settings.
Rebate data and prices net of rebates are excluded because this information is generally
considered proprietary and is subject to a variety of disclosure restrictions under Federal law.
Prescription Drug Spending in Medicare Part B, Medicare Part D, and Medicaid
Prescription drug spending continues to increase in the United States.
2
The Office of the Actuary
within the Centers for Medicare and Medicaid Services (CMS) estimates that in 2019, $360.3
billion will be spent on retail prescription drugs, rising from $265.2 billion in 2013.
3
The May
2018 Trump Administration blueprint for lower drug prices described a new, more transparent
drug pricing system that would lower high prescription drug prices and bring down out-of-pocket
1
The committee request data on drug pricing back to 2008. In several analyses, this report presents data starting
with 2006; several policy changes began in 2006 including the implementation of Medicare Part D and the first year
that most hospital outpatient departments began using ASP methodology for payments under Medicare Part B.
2
According to IQVIA, the outlook to 2022 is for 2–5% net spending growth, with 1–4% growth in retail and mail-
order prescription drugs. This growth, driven primarily by the large number of new medicines, many of which will
be specialty and orphan drugs, will be offset by the impact of losses of brand exclusivity. Available from:
https://www.iqvia.com/institute/reports/medicine-use-and-spending-in-the-us-review-of-2017-outlook-to-2022
3
Sisko AM, Keehan SP, Poisal JA, et al. National Health Expenditure Projections, 2018–27: Economic And
Demographic Trends Drive Spending And Enrollment Growth. Health Affairs. 3 (2019). Available from:
https://www-healthaffairs-org.ezproxyhhs.nihlibrary.nih.gov/doi/pdf/10.1377/hlthaff.2018.05499. The $360.3
billion estimate for 2019 includes $116.0 billion for Medicare (including Part B and Part D) and $35.8 billion for
Medicaid. Both estimates are net of rebates. See Table 11 in https://www.cms.gov/Research-Statistics-Data-and-
Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/Proj2018Tables.zip.

6
costs. The blueprint includes four strategies for putting American patients first: bringing down
out-of-pocket-costs, boosting competition, strengthening negotiation, and creating incentives for
lower list prices.
Trends in prescription drug utilization and spending vary across the Medicare Part B, Medicare
Part D, and Medicaid programs. Differences in utilization and spending reflect underlying
variation in eligibility for each program, including age, disability, income, and medical need. The
Medicare Program provides health insurance coverage for individuals aged 65 years and older as
well as certain younger individuals with disabilities or with End-Stage Renal Disease. The
Medicaid program is a joint Federal-State program that provides coverage for disabled
individuals and individuals and families with low incomes; some individuals with incomes above
these limits may also qualify due to high medical expenses.
In addition to populations served, these programs and different parts of these programs also vary
in the coverage offered for prescription drugs. Medicare Part D and Medicare Part B provide
coverage for different types of prescription drugs based on site of service: in general, Medicare
Part D provides coverage for self-administered prescription drugs and Medicare Part B covers
drugs that are administered in physician’s offices or hospital outpatient departments. In
Medicaid, prescription drug coverage is an optional benefit, currently offered across sites of
service by all states and the District of Columbia.
Prescription drug expenditures are projected to continue rising during the coming decade,
placing increasing fiscal pressures on commercial, federal, and state budgets.
4
,
5
Despite the
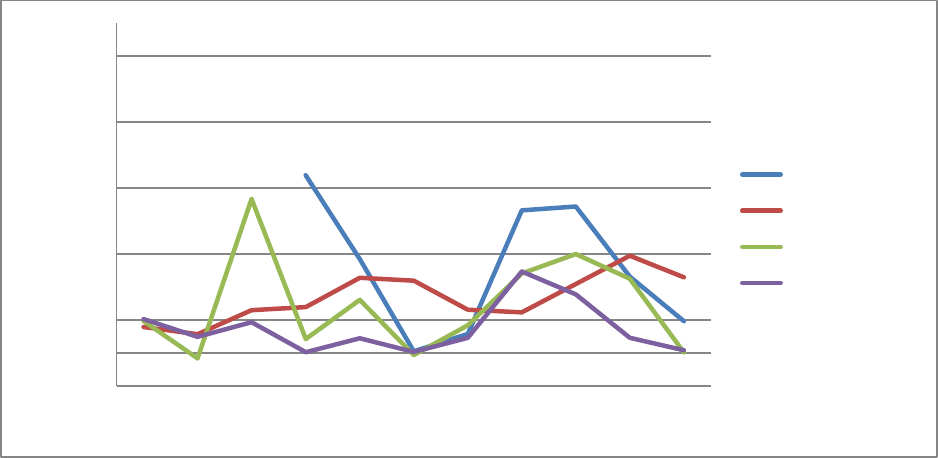
slowdown in the last 2 years as shown in Figure 1-1, over the longer period from 2006 to 2017,
program spending for prescription drug has been growing annually at 7 percent for Medicare Part
D, 8 percent for Medicare Part B, and 17 percent for Medicaid.
6
Increases in prescription drug
spending are not expected to be uniform across government programs, however, in part due to
differences in eligibility and coverage across programs. Another important factor underlying
differential projected increases in prescription drug spending is variation in use of purchasing
arrangements, utilization management strategies, and value-based approaches by the different
government programs.
4
Observations on trends in prescription drug spending. ASPE Issue Brief (3/8/2016). https://aspe.hhs.gov/pdf-
report/observations-trends-prescription-drug-spending
5
Sisko AM, Keehan SP, Poisal JA, et al. National Health Expenditure Projections, 2018–27: Economic And
Demographic Trends Drive Spending And Enrollment Growth. Health Affairs. 3 (2019). Available from:
https://www-healthaffairs-org.ezproxyhhs.nihlibrary.nih.gov/toc/hlthaff/0/0
6
Medicaid data for years before 2010 are not shown in Figure 1-1 because they do not include spending by
Managed Care Organizations and may be subject to data errors. The high rate of increase in Medicaid spending in
2014 and 2015 is associated with Medicaid expansion under the Affordable Care Act and the launch of expensive
new drugs such as Sovaldi and Harvoni for hepatitis C.
7
Figure 1-1 Annual Percentage Change in Prescription Drug Spending by Program, 2007-2017
Prescription Drug Development and Public Funding
Prescription drugs can effectively treat many acute and chronic diseases leading to improvements
in quality of life, life expectancy, and overall population health. However, development of new
prescription drugs is expensive, uncertain, and slow. The high costs of new drug development
require the prospect of financial returns to encourage sponsors to continue investing in
innovation. To encourage investment, sponsors of certain drugs approved by the Food and Drug
Administration (FDA) are granted exclusive rights to market their drug for a period of time. In
addition, new drugs may benefit significantly from government grants or research subsidies in
either the pre-clinical or clinical stages of development. For example, the Orphan Drug Act
provides incentives, including grants, tax credits, and an additional period of market exclusivity
to encourage investment in treatments for rare diseases or conditions. Because new medicines
can improve the health of individuals and the population more broadly, the incentives for
innovation described above are important. At the same time, policy makers must balance these
incentives with assuring that the new medicines are affordable and reflect their value in terms of
improving patient health outcomes.
Congressional Request for this Drug Pricing Report
“The Committee directs the Secretary of Health and Human Services to submit a report to the
Committee on Appropriations of the House of Representatives not later than 120 days after the
date of the enactment of the Bill to which this Committee Report pertains regarding price
changes of prescription drugs since 2008. The report should include comparative prescription
drug prices (net of rebates) paid by the following programs for the 10 most frequently prescribed
drugs and the 10 highest-cost drugs for each of the following: (1) The Medicare program under
part B of title XVIII of the Social Security Act. (2) The Medicare prescription drug program
under part D of title XVIII of the Social Security Act. (3) The Medicaid program under title XIX
of the Social Security Act. (4) The Department of Veterans Affairs. The report should also
provide a breakdown of the comparative prices (net of rebates) for each of the 10 most frequently
-0.05
0.05
0.15
0.25
0.35
0.45
2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017
Percentage Change
Medicaid
Medicare Part B
Medicare Part D
NHE Rx
8
prescribed drugs and the 10 highest-cost drugs between ambulatory settings and retail settings.
Under Medicare Part D, the report should detail gross Part D drug costs and net Part D drug costs
and the Direct and Indirect Remuneration for the 10 most frequently prescribed drugs and the 10
highest-cost drugs. In addition, the report should include total annual costs due to prescription
drugs to the Medicare program under part B of title XVIII of the Social Security Act, the
Medicare prescription drug program under part D of title XVIII of such Act, and the Medicaid
program under title XIX of such Act. Finally, the report should list the drugs that have been
registered for sale by the Food and Drug Administration (FDA) in the past five years that have
benefited significantly from government grants or research subsidies in either the pre-clinical or
clinical stages of development, as well as the price (net of rebates) and total spending in
Medicare and Medicaid for each of those drugs.”
9
SECTION 2: MEDICARE PART B
This section presents information about prescription drugs in Medicare Part B between 2006 and
2017. It presents data pertaining to trends in overall spending for prescription drugs and the top
10 highest-cost drugs and top 10 most frequently prescribed drugs in Medicare Part B. The data
presented in this section include only spending in fee-for-service Medicare Part B and exclude
Medicare Advantage spending.
Program Overview
Medicare is a federal health insurance program created in 1965 for people ages 65 and older; it
was expanded in 1972 to cover people under age 65 with permanent disabilities and end-stage
renal disease (ESRD). Medicare Part B, also known as the Supplementary Medical Insurance
(SMI) program, helps pay for physician, outpatient, some home health, and preventive services.
Part B is financed through a combination of general revenues, premiums paid by beneficiaries,
interest and other sources. Premiums are automatically set to cover 25 percent of spending in the
aggregate, while general revenues subsidize 73 percent and the remaining 2 percent is financed
through interest and other sources. Higher-income beneficiaries pay a larger share of spending,
ranging from 35 percent to 80 percent of Part B costs.
Certain types of drugs including infusible and injectable drugs and biologics administered in
physician offices and hospital outpatient departments, as well as certain other drugs provided by
pharmacies and suppliers (e.g., inhalation drugs and certain oral anticancer, oral antiemetic, and
immunosuppressive drugs) are covered by Part B.
7
Providers purchase these Part B drugs and
Medicare payments are made directly to these providers.
Through the passage of the Medicare Prescription Drug, Improvement, and Modernization Act of
2003 (MMA)
8
, beginning in 2005 payments for Part B drugs generally are tied to health care
providers’ acquisition costs by paying for a drug’s average sales price (ASP) plus a 6 percent
add-on (106 percent of ASP) as computed by CMS using quarterly sales price and volume of
sales data.
9
The Secretary was provided discretion for drugs administered in hospital outpatient
settings, to determine payment based on average acquisition costs or similarly to how payment is
made in a physician’s office. The Secretary has used ASP based pricing for most Part B drugs
provided in hospital outpatient departments since 2006.
7
Steven Sheingold, Elena Marchettie-Bowick, Nguyen Nguyen and Robin Yabroff, Medicare Part B Drugs:
Pricing and Incentives, ASPE, March 8, 2016. Available from:
https://aspe.hhs.gov/system/files/pdf/187581/PartBDrug.pdf
8
Medicare Prescription Drug, Improvement, and Modernization Act of 2003. 2003. retrieved from:
https://www.congress.gov/108/plaws/publ173/PLAW-108publ173.pdf
9
Medicare payments on the claims data reflect the 2 percent reduction due to the sequester in effect during the
period from April 2013 through September 2027.

10
Medicare Part B Spending and Spending Trends
Overall spending and spending trends
In Calendar Year (CY) 2017, total Medicare expenditures were $710 billion, of which, $314
billion were for total Part B benefit.
10
The total fee-for-service Part B benefit was $193.5 billion
after netting out spending for Medicare Advantage and administrative expenses (Table 2-1). As
shown in Table 2-1, fee-for-service Part B drug program spending grew from $10.1 billion in
2006 to $24.3 billion in 2017, representing an average annual growth rate of 8.3 percent.
11
Comparatively, total fee-for-service Part B benefit spending grew at 3.4 percent annually over
the same period. Part B drug spending has particularly increased since 2014, with annual average
growth of 11.9 percent compared to 5.6 percent for total Part B benefit spending.
Table 2-1 Medicare Fee-for-Service Part B Program Spending for Drug Benefits, 2006-2017
12
Total Part B Benefit
Part B Drug Program
Year
Spending
($B)
Annual Growth
Spending
($B)
Annual Growth
Part B Drugs' Share of
Part B Benefit
2006
134.4
-
10.1
-
7.5%
2007
137.5
2.3%
10.5
3.3%
7.6%
2008
132.2
-3.9%
10.8
3.7%
8.2%
2009
149.2
12.9%
11.5
6.5%
7.7%
2010
154.5
3.6%
12.3
6.8%
8.0%
2011
162.6
5.2%
13.7
11.3%
8.4%
2012
170.5
4.9%
15.2
10.7%
8.9%
2013
171.3
0.5%
16.2
6.8%
9.5%
2014
176.3
2.9%
17.2
6.2%
9.8%
2015
182.1
3.3%
19.0
10.2%
10.4%
2016
186.1
2.2%
21.8
14.6%
11.7%
2017
193.5
4.0%
24.3
11.3%
12.6%
Average
Annual
2006-17
3.4%
8.3%
Source: Total Part B benefit spending from Trustees Reports 2007-2018 (Table III. C5 for 2007-2011 and Table III.
C1 for 2012-2018) netting out spending in Medicare Advantage (MA numbers provided by the Office of the CMS
Office of the Actuary The data presented in this table include only spending in fee-for-service Medicare Part B and
exclude Medicare Advantage spending.
10
Table II.B1, p. 11. Medicare Trustees Report (2018). 2018 Annual Report Of The Boards Of Trustees Of The
Federal Hospital Insurance And Federal Supplementary Medical Insurance Trust Funds. Retrieved from:
https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-
Reports/ReportsTrustFunds/Downloads/TR2018.pdf
11
Program spending excludes beneficiary liability and third party payments. Total FFS Part B spending (Medicare
program, beneficiary liability and third party payments) grew from $12.7 B. in 2006 to $30.6 B. in 2017.
12
Medicare payments on the claims data reflect the 2 percent reduction due to the sequester in effect during the
period from April 2013 through September 2027.

11
Table 2-2 Medicare Fee-for-Service Part B Program Spending per Enrollee and User for Drug
Benefits, 2006-2017
Total Part B Benefit
Part B Drug Program
Year
Fee-for-Service
Enrollees (M)
Spending per
Enrollee ($)
Annual
Growth
Payment per
Enrollee ($)
Annual
Growth
2006
33.1
4,064
-
305
-
2007
32.4
4,240
4.3%
324
6.0%
2008
32.0
4,136
-2.5%
338
4.3%
2009
31.8
4,691
13.4%
362
7.0%
2010
32.2
4,800
2.3%
382
5.7%
2011
32.5
4,998
4.1%
421
10.2%
2012
32.9
5,184
3.7%
462
9.8%
2013
33.1
5,174
-0.2%
489
5.9%
2014
33.2
5,315
2.7%
519
6.0%
2015
33.3
5,475
3.0%
571
10.2%
2016
33.7
5,528
1.0%
648
13.3%
2017
33.6
5,762
4.2%
724
11.8%
Source: Total Part B benefit spending from Trustees Reports 2007-2018 (Table III. C5 for 2007-2011 and Table III.
C1 for 2012-2018); Enrollment from Trustees Report 2018 Table V. B3. The data presented in this section include
only spending in fee-for-service Medicare Part B and exclude Medicare Advantage spending.
Spending concentration for top ten drugs
A relatively small number of Part B drugs account for a significant share of the spending.
13
Table
2-3
14
presents the top 10 drugs in term of Medicare Part B drug program payment over the last
ten years. For brevity, we present only 2008, 2011, 2014, and 2017 here, with tables for all years
included in Appendix Table A-1. Concentrated spending for a relatively small number of drugs
has been consistent for the past decade with the top 10 highest-cost drugs accounting for 45 to 50
percent of total Part B spending on drugs.
In 2017, the top 10 highest-cost drugs accounted for $14.0 billion in Part B payments including
beneficiary cost-sharing, or 46 percent of $30.3 billion in total Part B spending for all drugs. At
nearly $2.5 billion in Medicare Part B payments, aflibercept accounted for more spending than
any other drug in 2017. Medicare Part B payments including beneficiary cost-sharing for
aflibercept was $961.29 per unit in 2017, or $1,922.58 for a standard adult dose of 2 mg. We
defined unit based on the Healthcare Common Procedure Coding System (HCPCS) billing unit,
which, in many cases, is the lowest dispensable amount or the lowest denomination (e.g., one pill
or a standardized volume for liquids) and may not be the common dose.
15
Rituximab, which has
13
Spending numbers presented are program spending, net of beneficiary cost sharing, and include sequester.
14
The drug spending presented in Table 2-3 includes claims by critical access hospitals, Maryland hospitals and
third party claims that were excluded in the data underlying the CMS dashboard even without imposing the
restriction that a drug must have been present in two consecutive years (for calculating annual change). Source:
CMS Office of Enterprise Data and Analytics (OEDA).
15
Per the above example, a unit of aflibercept is 1mg, although the common dose for aflibercept is 2mg. On the
claims data the unit is the MTUS_CNT.
12
historically been in the top two of the top ten list over the last decade, accounts for the second
most Medicare Part B payments with approximately $1.8 billion. Table 2-4 presents the top 10
highest-cost prescribed drugs in Medicare Part B ranked by spending per unit for 2011, 2014,
and 2017 with all years 2011-2017 presented in Appendix Table A-2
As displayed in Table 2-5, the top 10 most frequently prescribed drugs in Medicare Part B, are
relatively inexpensive and typically account for less than 10 percent of total Part B drug
spending. In 2017, spending on the top 10 most frequently prescribed drugs was $2.6 billion, or
9% of $30.3 billion in total Part B spending for all drugs, although aflibercept, the 10
th
most
prescribed drug accounted for $2.5 billion in Part B spending. The remaining nine drugs each
accounted for at most $25.0 million in spending and had average per unit spending of less than
$11. Spending and pricing data for all years are included in Appendix Table A-3.
Table 2-3 Top 10 Highest-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
2008
2011
Rank
Description
Spending
per Unit
($)
Total
Spending
($M)
Description
Spending
per Unit
($)
Total
Spending
($M)
1
Rituximab cancer
treatment (Rituxan)
516.88
1,151.1
Ranibizumab injection
(Lucentis)
405.36
1,428.9
2
Bevacizumab
injection (Avastin)
57.40
944.7
Rituximab injection
(Rituxan)
611.58
1,373.0
3
Infliximab injection
(Remicade)
55.85
814.8
Bevacizumab injection
(Avastin)
60.45
1,012.5
4
Injection,
pegfilgrastim
(Neulasta) 6mg
2,206.63
798.8
Injection, pegfilgrastim
(Neulasta) 6mg
2,643.78
1,020.5
5
Ranibizumab injection
(Lucentis)
397.09
735.8
Infliximab injection
(Remicade)
61.49
960.5
6
Darbepoetin alfa, non-
esrd (Aranesp)
2.91
676.7
Oxaliplatin (Eloxatin)
9.42
498.3
7
Oxaliplatin (Eloxatin)
9.42
457.5
Pemetrexed injection
(Alimta)
53.07
464.4
8
Epoetin alfa, non-esrd
(Epogen/Procrit)
9.26
462.3
Darbepoetin alfa, non-
esrd (Aranesp)
3.15
435.4
9
Docetaxel (Taxotere)
327.85
396.7
Trastuzumab injection
(Herceptin)
70.60
410.7
10
Gemcitabine HCl
(Gemzar)
132.43
330.8
Docetaxel injection
(Taxotere)
18.70
395.9
Medicare Part B Spending, Top 10
6,769.2
8,000.1
Medicare Part B Spending, All Drugs
13,614.3
17,191.6
2014
2017
Rank
Description
Spending
per Unit
($)
Total
Spending
($M)
Description
Spending
per Unit
($)
Total
Spending
($M)
1
Rituximab injection
(Rituxan)
693.39
1,552.5
Aflibercept injection
(Eylea)
961.29
2,473.4
2
Ranibizumab injection
(Lucentis)
390.64
1,336.0
Rituximab injection
(Rituxan)
819.18
1,815.3
13
3
Aflibercept injection
(Eylea)
964.44
1,302.0
Injection, nivolumab
(Opdivo)
25.95
1,516.5
4
Injection,
pegfilgrastim
(Neulasta) 6mg
3,312.87
1,235.7
Injection, pegfilgrastim
(Neulasta) 6mg
4,188.25
1,461.7
5
Infliximab injection
(Remicade)
71.72
1,223.5
Infliximab not biosimil
(Remicade) 10mg
85.01
1,413.9
6
Bevacizumab injection
(Avastin)
65.17
1,091.4
Denosumab injection
(Prolia)
16.75
1,296.6
7
Denosumab injection
14.30
799.3
Bevacizumab injection
(Avastin)
73.26
1,100.2
8
Trastuzumab injection
(Herceptin)
80.78
581.0
Inj pembrolizumab
(Keytruda)
46.15
1,062.2
9
Pemetrexed injection
(Alimta)
59.50
575.4
Ranibizumab injection
(Lucentis)
372.44
1,039.1
10
Bortezomib injection
(Velcade)
$36.00
489.3
Trastuzumab injection
(Herceptin)
94.87
814.1
Medicare Part B Spending, Top 10
10,186.1
13,993.0
Medicare Part B Spending, All Drugs
21,597.8
30,294.0
Source: Analysis of carrier, durable medical, and outpatient claims data 2006-2017. Data include Part B covered
drugs administered in physicians' offices and furnished by suppliers, covered drugs in hospital outpatient
departments; and reflect only Part B drugs paid under the average sales price plus 6 percent (ASP). HCPCS codes
and prices for carrier and DM were obtained from the CMS ASP file, those for OP come from the CMS Addendum
B file. Lines with denied payments or Medicare as secondary payer were dropped. Total Spending and Spending per
Unit are net of beneficiary cost-sharing and include the sequester.
Table 2-4 Top 10 Highest-Cost Prescribed Drugs, Medicare Part B, Ranked by Spending per
Unit
2011
2014
Rank
Description
Spending
per Unit
($)
Total
Spending
($M)
Description
Spending
per Unit
($)
Total
Spending
($M)
1
Sipuleucel-t,
minimum of 50
million autologous
cd54+ cells activated
with pap-gm-csf,
including
leukapheresis and all
other preparatory
procedures, per
infusion
32,857.47
89.9
Sipuleucel-t, minimum
of 50 million
autologous cd54+ cells
activated with pap-gm-
csf, including
leukapheresis and all
other preparatory
procedures, per
infusion (Provenge)
33,864.70
173.2
2
Fluocinolone
acetonide, intravitreal
implant
16,253.07
1.4
Injection, pegaspargase,
per single dose vial
(Oncaspar)
5,952.34
1.1
3
Injection, porfimer
sodium, 75 mg
5,041.82
0.9
Injection, pegfilgrastim,
6 mg (Neulasta)
3,270.26
1,172.9
4
Leuprolide acetate
implant, 65 mg
4,378.09
0.3
Histrelin implant
(vantas), 50 mg
(Vantas)
2,915.55
5.9
5
Ganciclovir, 4.5 mg,
long-acting implant
3,485.88
0.4
Injection, basiliximab,
20 mg (Simulect)
2,525.33
0.7

14
6
Histrelin implant
(vantas), 50 mg
2,945.12
17.1
Injection, crotalidae
polyvalent immune fab
(ovine), up to 1 gram
(Crofab)
2,409.87
1.7
7
Injection,
pegaspargase, per
single dose vial
2,680.69
0.3
Injection, vincristine
sulfate liposome, 1 mg
(Marqibo)
1,849.51
1.2
8
Injection,
pegfilgrastim, 6 mg
2,615.20
972.0
Injection, digoxin
immune fab (ovine),
per vial (Digifab)
1,490.59
0.7
9
Injection, denileukin
diftitox, 300
micrograms
1,585.58
7.0
Injection, carmustine,
100 mg (Bicnu)
1,431.81
1.3
10
Injection, reteplase,
18.1 mg
1,425.91
0.5
Injection, pentostatin,
10 mg (Nipent)
1,425.10
0.8
Medicare Part B Spending, Top 10
1,089.9
1,359.5
Medicare Part B Spending, All Drugs
17,191.6
21,597.8
2017
Rank
Description
Spending
per Unit
($)
Total
Spending
($M)
1
Sipuleucel-t,
minimum of 50
million autologous
cd54+ cells activated
with pap-gm-csf,
including
leukapheresis and all
other preparatory
procedures, per
infusion (Provenge)
38,716.24
202.5
2
Injection,
pegaspargase, per
single dose vial
(Oncaspar)
9,666.53
2.3
3
Injection,
pegfilgrastim, 6 mg
(Neulasta)
4,142.60
1,400.1
4
Injection, basiliximab,
20 mg (Simulect)
3,309.41
1.1
5
Injection, carmustine,
100 mg (Bicnu)
3,278.80
1.9
6
Histrelin implant
(vantas), 50 mg
(Vantas)
3,127.67
3.0
7
Injection, digoxin
immune fab (ovine),
per vial (Digifab)
2,933.29
0.9
8
Injection, crotalidae
polyvalent immune
fab (ovine), up to 1
gram (Crofab)
2,766.58
1.3
9
Injection, vincristine
sulfate liposome, 1 mg
(Marqibo)
2,325.16
1.1

15
10
Injection, pentostatin,
10 mg (Nipent)
1,925.04
0.8
Medicare Part B Spending, Top 10
1,399.3
Medicare Part B Spending, All Drugs
30,294.0
Note: Medicare Part B Spending per Unit data only available for years 2011-2017.
Source: Analysis of carrier, durable medical, and outpatient claims data 2006-2017. Data include Part B covered
drugs administered in physicians' offices and furnished by suppliers, covered drugs in hospital outpatient
departments; and reflect only Part B drugs paid under the average sales price plus 6 percent (ASP). HCPCS codes
and prices for carrier and DM were obtained from the CMS ASP file, those for OP come from the CMS Addendum
B file. Lines with denied payments or Medicare as secondary payer were dropped. Total Spending and Spending per
Unit include beneficiary cost-sharing and include the sequester.
Table 2-5 Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number
of Services
2008
2011
Rank
Description
Spending
per Unit
($)
Total
Spending
($M)
Description
Spending
per Unit
($)
Total
Spending
($M)
1
Vitamin b12 injection
(Cobal-1000, Cobolin-
M)
0.28
0.9
Vitamin b12 injection
(Cobal-1000, Cobolin-
M)
0.36
1.0
2
Dexamethasone
sodium phos
(Hexadrol)
0.09
1.9
Triamcinolone acet inj
NOS (Kenalog)
1.62
17.1
3
Ondansetron hcl
injection (Zofran)
0.43
7.8
Dexamethasone sodium
phos (Hexadrol)
0.09
1.8
4
Triamcinolone
acetonide inj
(Kenalog)
1.44
13.3
Albuterol non-comp unit
0.06
28.2
5
Albuterol ipratrop
non-comp (Duoneb)
0.66
157.8
Albuterol ipratrop non-
comp (Duoneb)
0.24
45.0
6
Normal saline solution
infus
0.29
0.8
Methylprednisolone 40
MG inj (Medrol)
2.76
6.7
7
Methylprednisolone
40 MG inj (Medrol)
4.40
10.1
Methylprednisolone 80
MG inj (Depo-Medrol)
6.91
11.9
8
Epoetin alfa, non-esrd
(Epogen/Procrit)
9.26
462.3
Normal saline solution
infus
0.26
0.5
9
Albuterol non-comp
unit
0.04
15.7
LOCM 300-399mg/ml
iodine,1ml
0.17
15.3
10
Methylprednisolone
80 MG inj (Depo
Medrol)
8.54
14.2
Epoetin alfa, non-esrd
(Epogen/Procrit)
9.96
354.8
Medicare Part B Spending, Top 10
684.8
482.3
Medicare Part B Spending, All Drugs
13,614.3
17,191.6
2014
2017
Rank
Description
Spending
per Unit
($)
Total
Spending
($M)
Description
Spending
per Unit
($)
Total
Spending
($M)
1
Triamcinolone acet inj
nos (Kenalog)
1.75
19.5
Triamcinolone acet inj
nos(Kenalog)
1.80
24.1

16
2
Dexamethasone
sodium phos
(Hexadrol)
0.13
2.6
Dexamethasone sodium
phos (Hexadrol)
0.12
2.3
3
Vitamin b12 injection
(Cobal-1000)
2.02
4.7
Vitamin b12 injection
(Cobal-1000)
2.83
6.8
4
Methylprednisolone
40 mg inj (Medrol)
2.94
7.8
Methylprednisolone 40
mg inj (Medrol)
5.70
16.2
5
Albuterol non-comp
unit
0.05
18.1
Albuterol non-comp unit
0.05
13.6
6
Albuterol ipratrop
non-comp (Duoneb)
0.18
26.9
Methylprednisolone 80
mg inj (Medrol)
10.88
18.8
7
Methylprednisolone
80 mg inj
5.60
9.7
Albuterol ipratrop non-
comp (Duoneb)
0.15
19.9
8
Locm 300-399mg/ml
iodine,1ml
0.18
15.8
Locm 300-399mg/ml
iodine,1ml
0.12
11.2
9
Betamethasone
(Celestone Soluspan)
acet&sod phosp
5.59
14.6
Betamethasone
acet&sod phosp
6.74
20.1
10
Ceftriaxone sodium
injection (Rocephin)
0.67
2.6
Aflibercept injection
(Eylea)
961.29
2,473.4
Medicare Part B Spending, Top 10
122.3
2,606.4
Medicare Part B Spending, All Drugs
21,597.8
30,294.0
Source: Analysis of carrier, durable medical, and outpatient claims data 2006-2017. Data include Part B covered
drugs administered in physicians' offices and furnished by suppliers, covered drugs in hospital outpatient
departments; and reflect only Part B drugs paid under the average sales price plus 6 percent (ASP). HCPCS codes
and prices for carrier and DM were obtained from the CMS ASP file, those for OP come from the CMS Addendum
B file. Lines with denied payments or Medicare as secondary payer were dropped. Total Spending and Spending per
Unit include beneficiary cost-sharing and include the sequester.
Data and Methods
The Medicare claims data used in the analyses of spending and trends in spending include Part
B
16
covered drugs administered in physicians’ offices and furnished by suppliers (carrier and
durable medical equipment (DME) claims files) and covered drugs in hospital outpatient
departments (outpatient claims files) from 2006 to 2017. Many of the analyses start with
calendar year 2006 because it is the first year that most hospital outpatient departments were paid
using the ASP methodology.
Medicare Part B drugs are identified by the HCPCS codes in the claims data. Analyses are
restricted to Part B drugs paid under the methodology described in section 1847A of the Social
Security Act.
17
As a result, the analyses exclude vaccines; blood products with P* codes (but
16
Part B drugs that are separately paid, i.e., not bundled and paid under a bundled system.
17
Typically, this means ASP, but may also include WAC or AMP based payments. In fact, WAC-based payment
occurs in limited situations, like when a drug is new, and AMP-based payment occurs infrequently when AMP
exceeds ASP by a threshold percentage and other safeguards are met. The pricing files do not always indicate which
source is used for a payment amount.

17
include blood clotting with J & Q codes); claims in the DME file with an AWP flag
18
; and
enteral and parenteral drugs that have B* codes.
Claims with HCPCS codes that represent ESRD
19
drugs as well as claims with HCPCS codes
that do not represent drugs were dropped from the analyses. Codes and prices for carrier and
DME were obtained from the CMS ASP files, while those for hospital outpatient departments
come from the CMS Addendum B files. Claim lines with denied payments or Medicare as
secondary payer were dropped from the analyses. Medicare payments include Medicare
program payments and beneficiary cost sharing, and include the effects of the budget
sequestration beginning in 2013, which reduced Medicare spending rates by a fixed 2 percent per
year.
20
The top 10 highest cost drugs and top 10 most frequently prescribed drugs present average
spending per billing unit. We define unit based on the combination of HCPCS unit per
beneficiary per date, which, in many cases, is the lower of the lowest dispensable amount or the
lowest denomination (e.g., one pill or a standardized volume for liquids) and may not be the
common dose.
21
18
Claims in the durable medical equipment (DME) file with an AWP flag include infusion drugs which used to
appear in the ASP Drug Pricing Files. This indicator was not maintained in the ASP Drug Pricing files and its use
has been discontinued (because of the change in DME infusion payment resulting from the Cures Act).
19
ESRD drugs were mostly bundled into the ESRD facility composite rates by 2014.
20
The budget sequestration in 2013 refers to the automatic spending cuts to United States federal government
spending in particular categories of outlays that were initially set to begin on January 1, 2013, as an austerity fiscal
policy as a result of Budget Control Act of 2011 (BCA), and were postponed by two months by the American
Taxpayer Relief Act of 2012 until March 1 when this law went into effect. The nine-year cuts (2013-2021) are split
evenly (by dollar amounts, not by percentages) between the defense and non-defense categories. Some major
programs like Social Security, Medicaid, federal pensions and veteran's benefits are exempt. By a special provision
in the BCA, Medicare spending rates were reduced by a fixed 2 percent per year. That is providers and health
insurance plans will be paid 98 cents on the dollar under Medicare for the entire nine-year period 2013-2021.
As the sequester applies to federal payment only (80 percent of total payment while beneficiaries still pay the full 20
percent copay), the effective federal payment under ASP+6% is ASP+(1.06*(1-2%*80%))) or ASP+4.3%., The bi-
partisan budget package that keeps the government funded through March 23, 2018 approved by Congress and
signed into law on February 9, 2018 extended the mandatory Medicare sequestration cut until 2027.
21
An example where the billing unit is lower than the dispensable amount is bevacizumab. This drug is available in
100mg and 400mg vials, so the lowest dispensable amount (without pharmacy/outsourcer repackaging) is 100mg.
The lowest denomination is 10mg, hence the HCPCS billing unit is 10mg. Payment in Oct 2017 was about $75 per
10mg, and Medicare’s share was about $60; these figures correspond to the unit being equal to the HCPCS code
descriptor amount.

18
SECTION 3: MEDICARE PART D
This section presents information about prescription drugs in Medicare Part D between 2006 and
2017. Specifically, in response to the information requested by the House Committee on
Appropriations, this section presents data pertaining to trends in overall spending for prescription
drugs and the top 10 highest-cost drugs and top 10 most frequently prescribed drugs in Medicare
Part D.
22
For many of the analyses contained in this chapter, measures of drug spending are constructed
from Part D Prescription Drug Event (PDE) records to include payments to the pharmacy by the
Part D plan sponsor and the beneficiary’s out-of-pocket liability. These measures are referred to
as gross drug costs (GDC). In some cases we estimate Medicare program spending for Part D,
which differs from gross drug costs to the extent that rebates and other price concessions affect
plan premiums but are not reflected in prices paid at the pharmacy.
23
Program Overview
The Medicare Modernization Act of 2003 (MMA) authorized Medicare Part D as a voluntary
drug benefit for Medicare beneficiaries, and the Part D program was implemented in January
2006. Private plans compete for enrollees by providing and managing the drug benefit.
24
Each
enrollee in either Part A or Part B is also entitled to enroll in a Part D prescription drug plan. In
addition, some Medicare Advantage plans also cover the Part D benefit. These plans are known
as MA-PDs. Similar to Part B, enrollment in Part D is voluntary and the enrollee pays a monthly
premium.
25
In 2017, total enrollment in Medicare was 58.0 million, of which enrollment in Part
D was 44.5 million.
26
Enrollees in Part D pay a monthly premium, in addition to cost sharing and
typically costs up to a deductible for their drugs. Low-income beneficiaries (LIS) pay lower or
no premiums, cost sharing, or deductibles. Under Part D, private plan sponsors submit annual
premium bids for providing the benefit. Medicare subsidizes 74.5 percent of the national average
22
Rebate data at the drug level and even at the therapeutic class level are considered proprietary data and therefore
not available for this report.
23
Total Government payment is estimated as the sum of Premium Subsidies (PG), Reinsurance (RI), Low-Income
Premium Subsidies (LIPS), and Low-Income Cost Sharing (LICS), with the government paying 74.5% of premiums
and beneficiaries paying 25.5%.
24
The Medicare Part D drug benefit is administered through private prescription drug plans, which each separately
design and manage benefits and pay claims. Private prescription drug plans use purchasing arrangements and
utilization management, including negotiation of prices with manufacturers and pharmacies, formularies, step
therapy, quantity limitations, and prior authorization. All formularies must include “all (with specified exceptions)”
drugs in the immunosuppressant, antidepressant, antipsychotic, anticonvulsant, antiretroviral, and antineoplastic
classes to ensure patient access to these protected classes of drugs. The current exceptions are that the formulary
does not have to include all therapeutic equivalents (i.e., generics) and can use safety edits to limit quantities (see 42
CFR 423.120(b)(2)(vi)).
25
Starting in 2011, higher income enrollees pay higher premiums, as in Part B.
26
Medicare Trustees Report 2018 Table II.B.1, p. 11 Medicare Trustees Report (2018). 2018 Annual Report Of The
Boards Of Trustees Of The Federal Hospital Insurance And Federal Supplementary Medical Insurance Trust Funds.
Retrieved from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-
Reports/ReportsTrustFunds/Downloads/TR2018.pdf
19
premium and provides additional assistance for premiums and out-of-pocket costs to LIS
beneficiaries. In CY 2017, total Medicare benefit payment is $710 billion, of which, $100
billion (or 14 percent) is for the Part D benefit.
27
Medicare Part D Program Spending and Program Spending Trends
Medicare Part D program spending per enrollee
28
rose about 3.4 percent annually between CY
2006 to CY 2017.
29
In recent years, program spending per enrollee grew from $1,782 per
enrollee in 2013 to $2,312 per enrollee in 2016, before decreasing to $2,249 in 2017 (Figure 3-
1). As the number of enrollees increased about 3.5 percent annually, or 45 percent in total from
2006 to 2017, total spending increased 7.0 percent annually from 2006 to 2017 (Table 3-1).
Figure 3-1 Medicare Part D Total Spending per Enrollee and Per User, 2006-2017
Source: Spending and Enrollment from Trustees Report 2018, Tables III. D3 and V.B3; Users from Acumen
analysis of claims data for ASPE
27
Medicare Trustees Report 2018, Table II.B1, p. 10. Medicare Trustees Report (2018). 2018 Annual Report Of The
Boards Of Trustees Of The Federal Hospital Insurance And Federal Supplementary Medical Insurance Trust Funds.
Retrieved from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-
Reports/ReportsTrustFunds/Downloads/TR2018.pdf Medicare benefit payment is program payment for the benefits
net of rebates.
28
Federal spending (Medicare Part D net program spending) is based on a percent of premiums which in turn reflect
the rebates plans expect to receive. Federal spending is estimated as the sum of Premium Subsidies, Reinsurance,
Low-Income Premium Subsidies, Low-Income Cost Sharing, and, risk corridor payments. CMS pays plans a
monthly prospective payment for each enrollee (the direct subsidy). This payment is first adjusted by the enrollee’s
case mix and other subsidy factors, namely low-income status and longterm institutionalized status. A second
adjustment to the plan’s approved bid is the subtraction of the enrollee’s premium. (See the following section on
how premiums are calculated.) CMS also provides plans with interim prospective payment adjustments for
individual reinsurance and low-income subsidies. The agency reconciles actual levels of enrollment, risk factors,
levels of incurred allowable drug costs (after rebates and other discounts), reinsurance amounts, and low-income
subsidies after the end of each year.
29
Annual compound growth rate of total program spending per enrollee and enrollment growth computed from
United States Centers for Medicare & Medicaid Services. Medicare Trustees Reports, 2018. June 2018. Part D total
spending from Table III.D3 (p. 105) and Part D enrollment from Table V.B3 (p. 181).
2,116
2,083
1,950
2,293
2,257
2,305
2,139
1,986
2,103
2,308
2,465
2,377
1,551
1,583
1,513
1,807
1,786
1,878
1,786
1,782
1,928
2,148
2,312
2,249
1,500
1,600
1,700
1,800
1,900
2,000
2,100
2,200
2,300
2,400
2,500
2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017
Total D Spending per User ($) Total D Spending per Enrollee ($)

20
In 2017, total program spending was estimated to be $100.0 billion, while net benefit spending
was $100.1 billion (Table 3-1). The difference reflects federal administrative costs.
30
Table 3-1 Medicare Part D Total Program Spending and Benefit Spending, 2006-2017
Total Part D Enrollees
(M)
Part D Total Program Spending
($B)
Part D Benefits Spending
($B)
Year
Enrollment
(M)
Annual
Growth
Spending ($B)
Annual
Growth
Spending
($B)
Annual
Growth
2006
30.6
47.4
47.1
2007
31.4
2.7%
49.7
4.9%
48.8
3.6%
2008
32.6
3.8%
49.3
-0.8%
49.0
0.4%
2009
33.6
3.2%
60.8
23.3%
60.5
23.5%
2010
34.8
3.4%
62.1
2.1%
61.7
2.0%
2011
35.7
2.7%
67.1
8.1%
66.7
8.1%
2012
37.4
4.8%
66.9
-0.3%
66.5
-0.3%
2013
39.1
4.4%
69.7
4.2%
69.3
4.2%
2014
40.5
3.6%
78.1
12.1%
77.7
12.1%
2015
41.8
3.2%
89.8
15.0%
89.5
15.2%
2016
43.2
3.4%
99.9
11.2%
99.5
11.2%
2017
44.5
2.9%
100.0
0.1%
100.1
0.6%
Average Annual
2006-17
3.5%
7.0%
7.1%
Source: Spending and Enrollment from Trustees Report 2018, Tables III. D3 and V. B3
Given that the total drug cost obtained from the 2017 claims data was over $154.2 billion (Table
3-2); this implies that Medicare spending ($100.0 billion) was about 65 percent of the gross drug
cost in 2017. This difference reflects primarily beneficiary cost sharing and rebates, and to a
lesser extent administrative cost and profits of plan sponsors, low-income subsidies, net risk
corridor payment, coverage gap discount, and the timing of reconciliation payments, among
other factors.
Medicare Part D Gross Drug Costs and Costs Trends
In CY 2017, total gross drug costs (GDC) for the Medicare Part D drugs is estimated to be
$154.2 billion (Table 3-2).
31
This reflects a 6.0 percent increase from the previous year’s $145.4
billion in GDC. In recent years, the growth in GDC has slowed from its peak in 2013 through
2015, when GDC exhibited double digit growth each year. The higher rates of growth in 2013 to
2015 in total GDC were primarily the result of increases in both utilization (number of users,
claims, and days) and unit cost (per user, per script, and per day) driven in large part because of
spending for drugs used to treat hepatitis C and even faster growth in prices for existing brand-
30
For 2017, total program spending is lower than benefit payments because of a larger-than-usual downward
adjustment of $0.3 billion for prior-year allocations among Part A, Part B, and Part D (2018 Trustees, Table III.D3)
31
Estimate based on Medicare Part D events (PDE) 2007-17 files by Acumen for ASPE

21
name drugs.
32
Over the entire 2007-17 period, total gross drug cost increased by 9.6 percent
annually, while Medicare benefit spending (net of rebates and cost-sharing) grew at 7.4 percent
(Table 3-1). The divergence likely reflects the growth in manufacturer rebates over time.
33
Moreover, the period from 2007 to 2017 encompasses two sub-periods: the period from 2007 to
2012 saw increasing entry of generic drugs into the market, while the subsequent period from
2013 to 2017 experienced the arrivals of expensive drugs such as the hepatitis C and specialty
drugs. As shown in Table 3-2, although the annual average growth of GDC over the whole
2007-17 period was 9.6 percent, the annual rate during 2007-12 was 7.7 percent, increasing to
11.5 percent during 2012 to 2017.
Table 3-2 Medicare Part D Prescription Gross Drug Costs (GDC): 2007-2017
Total Gross Drug Cost
Users
Drug Cost Per User
Year
Gross Drug
Cost ($B)
Annual
Growth
Users (M)
Annual
Growth
Cost per User ($)
Annual
Growth
2007
$61.9
-
23.9
-
$2,594
-
2008
$68.2
10.2%
25.3
5.9%
$2,699
4.1%
2009
$73.5
7.8%
26.5
4.9%
$2,773
2.7%
2010
$77.4
5.3%
27.5
3.8%
$2,813
1.5%
2011
$84.6
9.3%
29.1
5.8%
$2,908
3.4%
2012
$89.5
5.8%
31.3
7.5%
$2,862
-1.6%
2013
$103.3
15.4%
35.1
12.2%
$2,944
2.9%
2014
$121.0
17.1%
37.1
5.8%
$3,258
10.7%
2015
$136.8
13.1%
38.9
4.7%
$3,517
8.0%
2016
$145.4
6.3%
40.5
4.2%
$3,589
2.0%
2017
$154.2
6.0%
42.1
3.8%
$3,666
2.2%
Average Annual
2007-17
9.6%
5.8%
3.5%
2007-12
7.7%
5.6%
2.0%
2012-17
11.5%
6.1%
5.1%
Source: Analysis of Medicare Part D Events data 2007-2017 by Acumen for HHS/ASPE.
32
IQVIA Institute for Human Data Science. Medicine use and spending in the U.S.: a review of 2017 and outlook to
2022. Parsippany (NJ): IQVIA Institute for Human Data Science; 2018 Apr.
33
IMS Institute. Medicine Use and Cost Trends: Overall Market and Oncology. Discussion with ASPE. July 11,
2016.

22
Top 10 Drugs by Total Spending and by Number of Claims
Over the last 10 years, the top 10 highest-cost drugs in Part D account for approximately 20
percent of total Part D GDC. Table 3-3 presents the top 10 drugs in term of Medicare Part D
GDC over the last ten years. For brevity, we present only 2008, 2011, 2014, and 2017 here, with
tables for all years included in Appendix Table A-4.
In 2017, the top 10 highest-cost drugs accounted for $25.5 billion in Part D GDC, or 17 percent
of $154.2 billion in total Part D GDC. At $3.3 billion in Medicare Part D GDC, Revlimid, used
to treat blood cancers, accounted for more spending than any other drug in 2017. Medicare Part
D GDCs for Revlimid was $626.98 per unit in 2017 (Table A-4 shows that the GDC per user for
Revlimid was $88,442 in 2017). A unit refers to the lowest dispensable amount (e.g. one pill or a
standardized volume for liquids). In addition, Medicare beneficiaries using these high cost drugs
face high patient liabilities despite the catastrophic coverage provisions of Part D. More detailed
spending and pricing data for the top highest-cost drugs for all years are included in Appendix
Table A-4. Table 3-4 presents the top 10 highest-cost prescribed drugs in Medicare Part D
ranked by spending per unit for 2008, 2011, 2014, and 2017 with all years 2008-2017 presented
in Appendix Table A-5
As shown in Table 3-5, the top 10 most frequently prescribed drugs in Medicare Part D (as
calculated by number of claims), are relatively inexpensive and typically account for less than 10
percent of total Part D GDC. In 2017, spending on the top 10 most frequently prescribed drugs
was $4.1 billion, or 3% of $154.2 billion in total Part D GDC. Spending and pricing data for all
years are included in Appendix Table A-6.
23
Table 3-3 Top 10 Highest-Cost Drugs, Medicare Part D, Ranked by Total Spending
2008
2011
Rank
Drug Name
GDC Per
Unit ($)
GDC ($M)
Drug Name
GDC Per
Unit ($)
GDC ($M)
1
Lipitor
$2.95
$2,397.8
Plavix
$6.18
$3,656.7
2
Plavix
$3.83
$2,305.1
Lipitor
$4.57
$2,672.9
3
Nexium
$4.73
$1,487.0
Seroquel
$7.28
$2,045.3
4
Seroquel
$4.78
$1,462.2
Nexium
$5.89
$1,970.1
5
Aricept
$5.18
$1,326.1
Advair Diskus
$3.81
$1,664.9
6
Zyprexa
$12.65
$1,229.0
Zyprexa
$19.88
$1,625.3
7
Advair Diskus
$2.94
$1,213.3
Abilify
$18.97
$1,469.6
8
Actos
$4.82
$1,063.0
Crestor
$4.44
$1,416.3
9
Prevacid
$4.58
$947.2
Actos
$7.01
$1,294.1
10
Abilify
$14.13
$837.1
Spiriva
$7.48
$1,288.4
Medicare Part D GDC, Top 10
$14,267.8
$19,103.6
Medicare Part D GDC, All Drugs
$68,223.6
$84,639.2
2014
2017
Rank
Drug Name
GDC Per
Unit ($)
GDC ($M)
Drug Name
GDC Per
Unit ($)
GDC ($M)
1
Sovaldi
$1,016.87
$3,102.2
Revlimid
$626.98
$3,312.8
2
Nexium
$7.82
$2,658.3
Eliquis
$6.41
$3,078.9
3
Crestor
$6.07
$2,541.2
Januvia
$12.86
$2,786.1
4
Abilify
$28.67
$2,524.9
Lantus Solostar
$24.82
$2,632.4
5
Advair Diskus
$4.94
$2,273.8
Xarelto
$12.76
$2,611.8
6
Spiriva
$9.43
$2,156.2
Harvoni
$1,119.62
$2,555.8
7
Lantus
Solostar
$21.74
$2,014.7
Lyrica
$6.66
$2,516.9
8
Januvia
$9.67
$1,773.8
Advair Diskus
$6.22
$2,374.8
9
Lantus
$21.52
$1,724.2
Humira Pen
$2,235.96
$2,015.7
10
Revlimid
$450.86
$1,670.5
Spiriva
$12.15
$1,662.0
Medicare Part D GDC, Top 10
$22,439.8
$25,547.2
Medicare Part D GDC, All Drugs
$121,001.4
$154,229.6
Source: Analysis of Medicare claims data (carrier, outpatient, and Prescription Drug Event) by Acumen for ASPE.
24
Table 3-4 Top 10 Highest-Cost Drugs, Medicare Part D, Ranked by Spending per Unit
2008
2011
Rank
Drug Name
GDC Per
Unit ($)
GDC ($M)
Drug Name
GDC Per
Unit ($)
GDC ($M)
1
Retisert
19784.33
0.1
Lucentis
38985.58
1.1
2
Somatuline Depot
7648.06
0.3
Eylea
37720.00
0.0
3
Animas 2020
5663.20
0.0
Ilaris
16278.40
0.8
4
Arcalyst
5198.06
1.1
Stelara
10756.61
31.7
5
Viadur
4905.50
0.1
Somatuline Depot
8342.85
3.5
6
Vantas
4738.90
0.5
Neulasta
5952.62
28.3
7
H.P. Acthar
4547.26
6.9
Mozobil
5703.27
1.1
8
Fabrazyme
3443.73
1.4
Sylatron 4-Pack
5567.04
0.1
9
Neulasta
3399.84
19.5
Jevtana
5528.20
1.2
10
Herceptin
2728.76
2.3
H.P. Acthar
5303.69
49.5
Medicare Part D GDC, Top 10
$32.2
$117.4
Medicare Part D GDC, All Drugs
$68,223.6
$84,639.2
2014
2017
Rank
Drug Name
GDC Per
Unit ($)
GDC ($M)
Drug Name
GDC Per
Unit ($)
GDC ($M)
1
Eylea
38360.36
2.9
Eylea
38341.55
13.0
2
Lucentis
36442.45
2.7
Lucentis
37542.48
3.2
3
Gattex
28077.72
46.7
Gattex
35331.78
164.8
4
Ilaris
16381.65
4.1
Spinraza
25650.18
1.2
5
Stelara
14510.12
156.9
Krystexxa
19152.45
13.6
6
Jetrea
11850.00
0.0
Lemtrada
18062.64
7.7
7
Somatuline Depot
11421.89
10.2
Jetrea
16868.29
0.0
8
Krystexxa
7744.50
0.8
Ilaris
16621.41
8.1
9
Neulasta
7384.42
57.8
Somatuline Depot
15515.58
25.7
10
H.P. Acthar
6497.68
391.1
Signifor Lar
11541.03
2.7
Medicare Part D GDC, Top 10
$673.1
$239.9
Medicare Part D GDC, All Drugs
$121,001.4
$154,229.6
Source: Analysis of Medicare claims data (carrier, outpatient, and Prescription Drug Event) by Acumen for ASPE.
25
Table 3-5 Top 10 Most Frequently Prescribed Drugs, Medicare Part D, Ranked by Number of
Claims
2008
2011
Rank
Drug Name
GDC Per
Unit ($)
GDC ($M)
Drug Name
GDC Per
Unit ($)
GDC ($M)
1
Lisinopril
$0.25
$345.0
Simvastatin
$0.31
$594.3
2
Simvastatin
$0.57
$694.1
Lisinopril
$0.19
$322.3
3
Furosemide
$0.11
$131.8
Hydrocodone-
Acetaminophen
$0.18
$430.1
4
Hydrocodone-
Acetaminophen
$0.18
$312.5
Amlodipine Besylate
$0.30
$383.1
5
Levothyroxine Sodium
$0.24
$207.8
Omeprazole
$0.50
$674.7
6
Amlodipine Besylate
$0.45
$401.5
Levothyroxine
Sodium
$0.22
$256.5
7
Lipitor
$2.95
$2,397.8
Furosemide
$0.11
$133.5
8
Omeprazole
$0.86
$695.6
Metformin Hcl
$0.12
$214.4
9
Hydrochlorothiazide
$0.12
$94.1
Metoprolol Tartrate
$0.09
$126.1
10
Atenolol
$0.11
$111.7
Hydrochlorothiazide
$0.12
$95.2
Medicare Part D GDC, Top 10
$5,391.9
$3,230.2
Medicare Part D GDC, All Drugs
$68,223.6
$84,639.2
2014
2017
Rank
Drug Name
GDC Per
Unit ($)
GDC ($M)
Drug Name
GDC Per
Unit ($)
GDC ($M)
1
Lisinopril
$0.13
$281.4
Atorvastatin Calcium
$0.28
$808.9
2
Levothyroxine Sodium
$0.33
$631.5
Levothyroxine
Sodium
$0.36
$836.1
3
Amlodipine Besylate
$0.16
$303.6
Amlodipine Besylate
$0.11
$267.1
4
Simvastatin
$0.19
$346.5
Lisinopril
$0.10
$256.2
5
Hydrocodone-
Acetaminophen
$0.26
$676.2
Omeprazole
$0.21
$394.3
6
Omeprazole
$0.29
$527.6
Gabapentin
$0.14
$494.5
7
Atorvastatin Calcium
$0.44
$747.2
Furosemide
$0.09
$139.7
8
Furosemide
$0.10
$135.6
Simvastatin
$0.13
$219.2
9
Metformin Hcl
$0.08
$203.8
Hydrocodone-
Acetaminophen
$0.24
$498.4
10
Gabapentin
$0.20
$491.2
Metformin Hcl
$7.41
$188.2
Medicare Part D GDC, Top 10
$4,344.5
$4,102.6
Medicare Part D GDC, All Drugs
$121,001.4
$154,229.6
Source: Analysis of Medicare claims data (carrier, outpatient, and Prescription Drug Event) by Acumen for ASPE.

26
Data and Methods
Medicare Part D PDE data were used from 2007 to 2017 to calculate annual total gross drug
costs, price, and utilization. Although the Medicare Part D program started in 2006, data in the
initial year are not considered reliable for analyses. As a result, 2007 was the first year used for
evaluating trends.
The analysis is based on all drugs reported in the PDE data, excluding non-covered claims and
compound drugs. Additional information was obtained from the Health Plan Management
System (HPMS), Medispan, and First Data Bank.
34
The unit of analysis for the study is the National Drug Code (NDC), a unique product identifier.
The NDC is a unique 10-digit, 3-segment numeric identifier assigned to each medication
identifying the labeler or vendor, product (specific strength, dosage form, and formulation for a
particular firm), and trade package (package forms and sizes).
The top 10 highest cost drugs and top 10 most frequently prescribed drugs present average
spending per dosage unit. Units refer to both the number of claims or the quantity dispensed in
the PDE data. Quantity dispensed describes how many dosage units of the medication were
dispensed in the current drug event (normally, it is the number of units, grams, milliliters, other.
If compounded item, total of all ingredients will be supplied as Quantity Dispensed). Since drugs
are available in multiple strengths and dosage forms, the average spending per dosage unit at the
brand name and generic name level is weighted to account for variation in claims volume for
specific brand name, generic name, strength, dosage form, routes of administration, and
manufacturer levels.
34
The Health Plan Management System (HPMS) provides such information as plan type, cost share tier level, and
utilization management (quantity limit, prior authorization, step therapy). The First Data Bank (FDB) and Medispan
provide such information as generic and brand name, dosage form, strength, and route of administration. In
addition, Medispan provides information on drug class and protected class.
27
SECTION 4: MEDICAID
This section presents information about prescription drugs in Medicaid between 2006 and 2017.
It presents data pertaining to trends in overall spending for prescription drugs and the top 10
highest-cost drugs and top 10 most frequently prescribed drugs in Medicaid.
Program overview
Medicaid, created alongside Medicare in 1965, provides comprehensive health coverage,
including prescription drug benefits, to disabled and low-income individuals and families. Unlike
Medicare, Medicaid is administered by states in accord with federal statutes and regulations.
Financial responsibility for Medicaid is apportioned between the federal government and the
states according to the applicable Federal Medical Assistance Percentage (FMAP). Although
prescription drug coverage is legally an optional rather than a mandatory Medicaid benefit, all
states and the District of Columbia have elected to provide this coverage.
About 74 million people were enrolled in Medicaid in 2017.
35
Most enrollees receive services
under some form of managed care.
36
States can carve prescription drugs out of managed care but
fewer do so since the Affordable Care Act (ACA) extended Medicaid prescription drug rebates
to cover Managed Care Organizations (MCOs) as well as Fee for Service (FFS) utilization,
allowing states to receive full rebates under either type of utilization
More than half of the gross cost of Medicaid prescription drugs comes back to the federal
government and the states through rebates.
37
Since the Omnibus Budget Reconciliation Act of
1990, manufacturers have been required to provide rebates on prescription drugs as a condition
of state Medicaid coverage for their products. For single source/innovator multiple source (brand
name) drugs, rebate amounts are based on the greater of a percentage of the Average
Manufacturer Price (AMP) or the difference between AMP and the “best price” available to
other purchasers.
38
Additional rebates that apply when the cost of a branded drug increases
faster than inflation now account for about half of total rebate amounts on these drugs.
39
The
35
Department of Health and Human Services [HHS]. 2017 Actuarial Report on the Financial Outlook for Medicaid.
2018. Available at https://www.cms.gov/Research-Statistics-Data-and-
Systems/Research/ActuarialStudies/Downloads/MedicaidReport2017.pdf
36
Centers for Medicare & Medicaid Services [CMS]. Medicaid Managed Care Enrollment and Program
Characteristics, 2016 Spring 2018. Available at https://www.medicaid.gov/medicaid/managed-
care/downloads/enrollment/2016-medicaid-managed-care-enrollment-report.pdf
37
MACPAC. MACStats: Medicaid and CHIP Data Book. EXHIBIT 28. Medicaid Gross Spending and Rebates for
Drugs by Delivery System, FY 2017 (millions).December 2018. Available from: https://www.macpac.gov/wp-
content/uploads/2015/11/EXHIBIT-28.-Medicaid-Gross-Spending-and-Rebates-for-Drugs-by-Delivery-System-FY-
2017.pdf
38
“Best price” is defined at section 1927(c)(1)(C) of the Social Security Act, and 42 CFR 447.505. Exclusions from
the prices used in this calculation include the prices charged to Medicare Part D Plans and to the Veterans Health
Administration.
39
U.S. Department of Health and Human Services, Office of Inspector General. Medicaid rebates for brand-name
drugs exceeded Part D rebates by a substantial margin. Publication number OEI-03-00650. April 2015. Available at
http://oig.hhs.gov/oei/reports/oei-03-13-00650.pdf.
28
Bipartisan Budget Act of 2015 (Public Law 114-74) amended the Social Security Act to provide
for the payment of additional inflation-based rebates for non-innovator multiple source drugs
(generic drugs).
The ACA made several important changes to Medicaid prescription drug rebates. The minimum
rebate percentage for single source/innovator multiple source (brand name) drugs was raised
from 15.1 percent to 23.1 percent of AMP for most drugs, with a lower rate of 17.1 percent for
blood clotting factors and drugs approved by the FDA exclusively for pediatric indications. The
minimum rebate percentage for non-innovator (generic) drugs was increased from 11.0 to 13.0
percent of AMP. A line extension of a single source drug or an innovator multiple source drug
that is an oral dosage was made subject to an additional penalty that discourages manufacturers
from making trivial changes to avoid inflation rebates.
40
The ACA also provided for a maximum
rebate amount (or cap) with respect to each dosage form and strength of a brand drug for a rebate
period (basic plus additional inflation-based) at 100 percent of AMP; this maximum rebate
amount or cap applies to rebates for generic drugs (basic plus additional inflation-based) as
well.
41
Medicaid Spending and Spending Trends
Overall spending and spending trends
Medicaid gross spending on prescription drugs in CY 2017 totaled $67.6 billion (Table 4-1).
However, more than half of that spending came back to the federal government and the states as
rebates. Table 4-1 shows estimates of Medicaid prescription drug gross and net spending. In
2014, the combination of new, expensive drugs for hepatitis C and other conditions, price
increases in existing drugs, a relatively low number of patent expirations, and increased
enrollment due to Medicaid expansion under the ACA increased gross prescription drug
spending by 21.6 percent to $47.3 billion. From 2006 to 2017, Medicaid gross spending on
prescription drugs grew from $13.0 billion to $67.6 billion with an average annual growth rate of
16.2 percent. The Medicaid Drug Rebate Program, however, substantially reduced spending in
all years, and pushed net spending below half of gross spending in 2016 and 2017. Due to the
expansion of rebates, the average annual growth for net spending, 5.1 percent was much lower
than the gross growth rate over this period.
40
Line extension is defined in statute at section 1927(c)(2)(C) of the Social Security Act to mean, with respect to a
drug, a new formulation of the drug, such as an extended release formulation, but does not include an abuse-
deterrent formulation of the drug (as determined by the Secretary), regardless of whether such abuse-deterrent
formulation is an extended release formulation.
41
The President’s FY 2020 budget proposes elimination of the cap. See Department of Health and Human Services,
Budget in Brief (https://www.hhs.gov/sites/default/files/fy-2020-budget-in-brief.pdf), p. 15.
29
Table 4-1 Medicaid Prescription Drug Gross and Net Spending, 2006-2017
Year
Total
Gross
Medicaid
Spending
($B)
Annual
Growth
Total
Net
Medicaid
Spending
($B)
Annual
Growth
2006
13.0
-
19.1
-
2007
16.7
28.5%
18.3
-4.1%
2008
24.7
47.5%
19.2
4.9%
2009
26.0
5.6%
20.3
5.8%
2010
33.0
26.8%
20.4
0.4%
2011
37.7
14.2%
21.0
2.6%
2012
37.8
0.2%
21.4
2.3%
2013
38.9
3.0%
22.1
2.9%
2014
47.3
21.6%
27.3
23.9%
2015
57.8
22.2%
30.5
11.6%
2016
64.5
11.5%
32.0
5.0%
2017
67.6
4.9%
33.0
3.1%
Average Annual 2006-17
16.2%
5.1%
Sources: Gross Spending: 2006-2012 Lewin analysis of Medicaid State Drug Utilization public-use data, 2013-2017
Centers for Medicaid and Medicaid Services analysis of Medicaid State Drug Utilization public-use data. Medicaid
data for years before 2010 does not include spending by Managed Care Organizations and may be subject to data
errors. Net Spending: National Health Expenditure Accounts (https://www.cms.gov/Research-Statistics-Data-and-
Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/Tables.zip), Table 16.
Spending concentration for top ten drugs
The top 10 highest cost drugs have historically accounted for approximately 20 percent of
Medicaid spending on all drugs, although the contraction of spending in the top 10 has decreased
over the last few years as certain brand drugs have been moved to the generic market. Table 4-2
presents the top 10 drugs in term of Medicaid drug program spending over the last ten years. For
brevity, we present only 2008, 2011, 2014, and 2017 here, with tables for all years included in
Appendix Table A-7.
In 2008, the top 10 highest-cost drugs accounted for $5.5 billion in Medicaid spending, or 22
percent of $24.7 billion in Medicaid spending for all drugs. Comparatively, in 2017, the top 10
highest-cost drugs accounted for $9.2 billion in Medicaid spending, or 14 percent of $67.6
billion in Medicaid spending for all drugs. At $1.3 billion in Medicaid spending, Humira Pen,
used to treat rheumatoid arthritis and other chronic conditions, accounted for more spending than
any other drug in 2017. Medicaid spending for the Humira Pen was $2,152.22 per unit in 2017.
A unit refers to the lowest dispensable amount (e.g. one pill or a standardized volume for
liquids). Prior to 2017, the psychotropic drug Abilify was one of the highest-cost drugs in
Medicaid, with $2.5 billion in spending in 2014, but generic equivalents for Abilify became
available in mid-2015. Medicaid spending on Abilify decreased with the availability of a lower-
cost generic version of the drug.
30
Table 4-3 shows the top 10 drugs by gross unit cost. The competing macular degeneration drugs
Eylea and Lucentis topped the list in 2014, but the emphysema drug Aralast was highest in 2017,
with an average price over $36,000. Spending and pricing data for all years are included in
Appendix Table A-8. Because Medicaid volume for the highest unit cost drugs is typically low,
the top 10 accounted for less than 1 percent of total Medicaid gross spending in all the years
shown.
As shown in Table 4-4, the top 10 most frequently prescribed drugs in Medicaid (as calculated
by number of claims), are relatively inexpensive and typically account for less than 5 percent of
total Medicaid drug spending. In 2017, spending on the top 10 most frequently prescribed drugs
was $1.5 billion, or 2% of $67.6 billion in total Medicaid spending for all drugs. Hydrocodone-
Acetaminophen and Amoxicillin are typically among the most prescribed drugs in Medicaid.
Spending and pricing data for all years are included in Appendix Table A-9.
31
Table 4-2 Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Total Spending
2008
2011
Rank
Generic Drug
Name
Spending
per Unit
Total Spending
Generic Drug
Name
Spending
per Unit
Total Spending
1
Quetiapine
Fumarate
$5.59
$962,610,903
Aripiprazole
$16.92
$1,667,289,509
2
Aripiprazole
$12.98
$864,665,984
Quetiapine
Fumarate
$8.47
$1,618,779,336
3
Olanzapine
$14.00
$655,125,344
Olanzapine
$20.49
$983,556,014
4
Risperidone
$5.54
$624,080,668
Montelukast
Sodium
$4.65
$892,903,221
5
Montelukast
Sodium
$3.46
$452,731,574
Methylphenidate
HCL
$4.09
$709,352,216
6
Palivizumab
$1,603.26
$440,111,778
Fluticasone/
Salmeterol
$4.14
$640,832,463
7
Lansoprazole
$4.80
$396,513,136
Esomeprazole Mag
Trihydrate
$5.82
$573,215,157
8
Fluticasone/
Salmeterol
$3.38
$372,878,837
Emtricitabine/
Tenofovir
$35.81
$455,304,078
9
Esomeprazole Mag
Trihydrate
$5.00
$351,395,745
Albuterol Sulfate
$3.10
$453,110,973
10
Topiramate
$3.79
$335,536,619
Efavirenz/Emtricib
/Tenofovir
$55.06
$443,793,760
Medicaid Spending, Top 10
$5,455,650,587
$8,438,136,726
Medicaid Spending, All Drugs
$24,655,613,645
$37,679,593,904
2014
2017
Rank
Generic Drug
Name
Spending
per Unit
Total Spending
Generic Drug
Name
Spending
per Unit
Total Spending
1
Abilify
$27.91
$2,464,660,026
Humira Pen
$2,152.22
$1,263,696,300
2
Sovaldi
$1,023.55
$1,386,209,781
Harvoni
$1,084.68
$1,211,321,698
3
Atorvastatin
Calcium
$8.31
$1,139,715,919
Latuda
$37.35
$1,096,733,005
4
Vyvanse
$6.75
$659,026,202
Vyvanse
$8.87
$971,454,153
5
Truvada
$43.53
$616,663,462
Epclusa
$874.83
$943,299,246
6
Methylphenidate Er
$4.96
$581,063,019
Genvoya
$90.40
$814,372,297
7
Atripla
$68.56
$579,363,530
Invega Sustenna
$1,530.28
$743,459,699
8
Lantus
$21.66
$573,919,545
Methylphenidate
Er
$6.69
$734,238,237
9
Advair Diskus
$4.98
$546,197,570
Lyrica
$6.54
$721,237,876
10
Lantus Solostar
$22.55
$460,267,881
Zepatier
$647.27
$705,462,489
Medicaid Spending, Top 10
$9,007,086,936
$9,205,275,001
Medicaid Spending, All Drugs
$47,308,056,863
$67,585,558,174
Source: 2006-2012 Lewin analysis of Medicaid State Drug Utilization public-use data, 2013-2017 Centers for
Medicaid and Medicaid Services analysis of Medicaid State Drug Utilization public-use data.

32
Table 4-3 Top 10 Most Frequently Prescribed Drugs, Medicaid, Ranked by Unit Cost
2014
2017
Rank
Generic Drug Name
Spending
per Unit
Total Spending
Generic Drug
Name
Spending
per Unit
Total Spending
1
Lucentis
$22,890.95
$42,675,634
Aralast
$36,417.29
$164,897
2
Eylea
$18,281.66
$18,866,688
Eylea
$33,809.93
$155,473,882
3
Supprelin La
$17,858.48
$12,554,513
Supprelin La
$28,283.06
$20,986,031
4
Ilaris
$16,106.46
$17,651,242
Lucentis
$24,721.29
$39,847,591
5
Stelara
$14,515.75
$ 52,042,820
Spinraza
$23,477.05
$119,583,270
6
Jetrea
$11,938.28
$ 211,307
Retisert
$19,168.74
$274,535
7
Somatuline Depot
$11,768.56
$2,221,257
Lemtrada
$18,289.10
$17,467,005
8
Marqibo
$6,895.33
$151,697
Xofigo
$18,227.44
$2,320,827
9
Jevtana
$6,450.22
$ 3,252,343
Stelara
$17,235.71
$221,874,939
10
H.P. Acthar
$6,258.93
$126,837,119
Krystexxa
$16,421.79
$1,299,374
Medicaid Spending, Top 10
$276,464,623
$579,292,350
Medicaid Spending, All Drugs
$47,308,056,863
$67,585,558,174
Source: Centers for Medicaid and Medicaid Services analysis of Medicaid State Drug Utilization public-use data.
33
Table 4-4 Top 10 Most Frequently Prescribed Drugs, Medicaid, Ranked by Number of Claims
2008
2011
Rank
Generic Drug Name
Spending
per Unit
Total Spending
Generic Drug
Name
Spending
per Unit
Total Spending
1
Hydrocodone
bit/Acetaminophen
$0.23
$112,492,086
Hydrocodone
bit/Acetaminophen
$0.20
$185,851,039
2
Amoxicillin
$0.09
$64,329,996
Amoxicillin
$0.09
$127,704,574
3
Ibuprofen
$0.09
$49,200,240
Ibuprofen
$0.09
$82,215,957
4
Azithromycin
$2.40
$145,355,482
Albuterol Sulfate
$3.10
$453,110,973
5
Alprazolam
$0.13
$39,489,546
Azithromycin
$1.47
$182,600,085
6
Clonazepam
$0.20
$51,623,548
Alprazolam
$0.12
$59,014,244
7
Lorazepam
$0.27
$57,573,711
Loratadine
$0.16
$54,014,819
8
Montelukast sodium
$3.46
$452,731,574
Clonazepam
$0.13
$53,746,692
9
Aspirin
$0.09
$14,808,907
Lisinopril
$0.19
$43,334,762
10
Loratadine
$0.24
$44,987,853
Montelukast sodium
$4.65
$892,903,221
Medicaid Spending, Top 10
$1,032,592,942
$2,134,496,367
Medicaid Spending, All Drugs
$24,655,613,645
$37,679,593,904
2014
2017
Rank
Generic Drug Name
Spending
per Unit
Total Spending
Generic Drug
Name
Spending
per Unit
Total Spending
1
Hydrocodone-
Acetaminophen
$0.29
$232,638,855
Amoxicillin
$0.12
$104,708,104
2
Amoxicillin
$0.13
$98,843,746
Ibuprofen
$0.11
$89,449,829
3
Ibuprofen
$0.10
$74,011,233
Gabapentin
$0.16
$202,816,058
4
Lisinopril
$0.14
$43,124,965
Lisinopril
$0.12
$51,994,254
5
Omeprazole
$0.34
$107,844,436
Hydrocodone-
Acetaminophen
$0.31
$176,562,712
6
Azithromycin
$1.58
$121,677,230
Atorvastatin
Calcium
$0.31
$120,312,292
7
Gabapentin
$0.23
$164,292,251
Omeprazole
$0.24
$98,121,378
8
Cetirizine Hcl
$0.15
$56,034,828
Ventolin Hfa
$2.92
$543,229,325
9
Loratadine
$0.16
$38,240,930
Cetirizine Hcl
$0.16
$77,908,465
10
Levothyroxine
Sodium
$0.37
$83,000,884
Metformin Hcl
$0.08
$52,936,615
Medicaid Spending, Top 10
$1,019,709,357
$1,518,039,032
Medicaid Spending, All Drugs
$47,308,056,863
$67,585,558,174
Source: 2006-2012 Lewin analysis of Medicaid State Drug Utilization public-use data, 2013-2017 Centers for
Medicaid and Medicaid Services analysis of Medicaid State Drug Utilization public-use data.

34
Data and Methods
Gross spending amounts and utilization volumes were summarized for individual brand drugs.
Gross spending amounts and utilization volumes for multi-source generic drugs (generic drugs
manufactured by more than one company) were combined. Net spending amounts are CMS
estimates from the National Health Expenditure Accounts, as released in 2018. Annual
prescription drug costs as shown are not adjusted for inflation.
Medicaid drug data represent national-level drug utilization data for covered outpatient drugs
paid for by State Medicaid agencies and include both FFS and MCO drug spending.
42
These data
include state and national-level reports listing the number of prescription fills and amounts paid
by states by NDC. Data were summarized by drug by linking NDCs to a commercially available
database and aggregated to the drug brand name and generic name.
The following Medicaid drugs were excluded from the 2013 to 2017 analysis: over-the-counter
drugs in the Medicaid State Drug Utilization data as well as NDCs with fewer than 50 claims in
the current (2017) or previous year (2016). In addition, NDCs with large variations in reported
units from year to year were reviewed on a case-by-case basis and data anomalies were excluded.
Medicaid drug spending contains both the federal and state reimbursement and is inclusive of
any applicable dispensing fees.
43
In addition, this total is not reduced or affected by Medicaid
rebates paid to the states.
The top 10 highest cost drugs, top 10 most frequently prescribed drugs, and top 10 drugs by unit
cost present average spending per dosage unit. Units refer to the drug unit in the lowest
dispensable amount. Since drugs are available in multiple strengths and dosage forms, the
average spending per dosage unit at the brand name and generic name level is weighted to
account for variation in claims volume for specific brand name, generic name, strength, dosage
form, routes of administration, and manufacturer levels. The overall brand name/generic name
claim weighted spending per unit is calculated by first summarizing each drug to specific
strength, form, route of administration, and manufacture levels. For each unique level, spending
is divided by the number of units and multiplied by its proportion of total claims, so that claims
volume becomes the weight. The claim-weighted average spending per dosage unit at the overall
brand name/generic name level is then calculated by summarizing across the strength, form,
route, and manufacturer levels. We use number of claims to identify the “most frequently
prescribed drugs” as more directly related to the Congressional request than number of units.
Number of scripts (including prescriptions that were never filled) is not available from the
Medicaid drug data discussed above.
42
The Medicaid drug spending dashboard is based on non-public data, but the public Medicaid State Drug
Utilization data are available at https://www.medicaid.gov/medicaid/prescription-drugs/state-drug-utilization-
data/index.html.
43
Medicaid drug spending is based on the “Total Amount Reimbursed” field in the publicly available data.

35
SECTION 5: DRUGS BENEFITING FROM GOVERNMENT
GRANTS OR RESEARCH SUBSIDIES
Congress requested that HHS identify prescription drug products recently approved by the Food
and Drug Administration (FDA) whose development “benefited significantly from government
grants or research subsidies in either the pre-clinical or clinical stages of development.” There is
some empirical evidence which suggests that in virtually all cases, new drug products approved
for sale in recent years involved research and development activity that was based at least in part
on advances in basic medical science that were made possible by public funding.
The mission of the National Institutes of Health (NIH) is to improve human health by conducting
and supporting research in the causes, diagnosis, prevention, and cure of diseases. Basic science
research funded by NIH identifies mechanisms of disease and describes biological processes,
highlighting potential drug targets that offer the promise of valuable new strategies for therapy.
Knowledge gleaned from such research is often described as a “public good” available to all
parties for use. Private markets are known to underproduce public goods such as basic scientific
knowledge because private investments in their development yield economic benefits that cannot
be held solely for private use, so such investments are not profitable; that dilemma provides the
rationale for public funding of basic science.
In a typical case of new commercial drug development, private investments in research on the
safety and efficacy of specific drug compounds are based directly or indirectly on insights into
basic science knowledge made possible by public funding. Private firms have strong incentives
to conduct applied research into the development of new drug therapies because patent
protection confers time-limited exclusive sales rights, allowing drugmakers to earn potentially-
high rates of return on investments in new product development. Those high rates of return are
necessary to induce investments in pharmaceutical R&D given the high capital costs of such
investments and the fact that such investments are made in the face of scientific uncertainty as to
a compound’s clinical value. Of the many compounds that are studied by innovative drugmakers,
the overwhelming majority fail to reach FDA approval and marketing by the sponsor.. Evenwhen
a drug is approved , and marketed, the drugmaker sees no revenue for years after the initial
investment decision, so there are high opportunity costs for investment capital during the
research stage.
A recent analysis addresses the question of how public funding has contributed to the
development of new prescription drug products in recent years.
44
The authors examine every one
of the 210 new molecular entities (NMEs)—essentially, new drugs that contain active
ingredients not previously approved—which were approved for marketing by the FDA from
44
Ekaterina Galkina Cleary, Jennifer M. Beierlein, Navleen Surjit Khanuja, Laura M. McNamee, and Fred D.
Ledley, “Contribution of NIH funding to new drug approvals 2010–2016”,
www.pnas.org/cgi/doi/10.1073/pnas.1715368115.

36
2010 to 2016. In an extensive analysis of published medical literature related to those drugs as
well as a thorough review of government data on NIH-funded research projects, the authors find
that “NIH funding contributed to every one of the NMEs approved from 2010–2016 and was
focused primarily on the drug targets rather than on the NMEs themselves” and that the
contribution of public funding “primarily involved funding for research related to molecular
targets for new drugs and likely represents basic research.” These findings are consistent with the
idea that publicly-funded basic science enables subsequent private research efforts which focus
on applications of basic science with viable commercial prospects.
In some cases, the development of a newly-approved drug might benefit from public research
funding in ways that go beyond basic science. In other words, some new drugs likely have more-
extensive public funding in their development history than others do, and determining the extent
of the contribution of publicly-funded research presents complex challenges. It appears safe to
conclude, however, that all or nearly all new drugs have at least some history of public funding
in their development, usually in the form of basic science.
The Committee requested a list of all drugs approved for sale in the past five years that involved
public funding. Below (Table 5-1) is a list of all drugs approved during the five-year period for
which data are currently available, 2013 through 2017. This list does not correspond exactly to
the set of drugs examined in the study mentioned above, but because that study examined all new
NMEs from 2010 to 2016 that said, there will be considerable overlap in the two sets of drugs.
Because all NMEs examined in that study had at least some history of public funding, it appears
highly likely that a similar conclusion would be drawn in a full analysis of more-recent drug
approvals. Table 5-1 also presents 2017 total and per unit spending in Medicare Part D and
Medicaid where available for each prescription drug. Because Medicare Part B drug claims are
aggregated to the HCPCS level, which may include multiple drugs, we are unable to include
Medicare Part B spending for individual drugs.
Table 5-1 Prescription Drugs Approved for Sale by the Food and Drug Administration from
2013 to 2017, and 2017 Spending by Program Where Applicable
Medicare Part D
Medicaid
Brand Name
Total Spending
in 2017
Spending
per Unit in
2017
Total Spending
in 2017
Spending
per Unit in
2017
Year of FDA
Approval
Actemra
$86,400,039
$906.83
$48,017,654
$407.56
2013
Addyi
$77,832
$27.04
2015
Adempas
$241,035,244
$106.26
$31,569,719
$105.88
2013
Adlyxin
$24,669
$91.64
$76,034
$93.08
2016
Akynzeo
$756,007
$544.51
$537,990
$531.37
2014
Alecensa
$47,575,891
$56.89
$10,434,411
$56.73
2015
Aliqopa
**
**
2017
Alunbrig
$3,389,101
$81.74
$1,136,002
$80.65
2017
Anoro Ellipta
$384,993,452
$5.91
$37,371,071
$5.87
2013
Anthim
2016
Aptiom
$59,766,627
$28.36
$40,899,769
$27.35
2013
Aristada
$68,821,741
$726.04
$66,279,961
$692.26
2015
Austedo
$17,550,907
$71.55
$2,028,124
$69.57
2017

37
Medicare Part D
Medicaid
Brand Name
Total Spending
in 2017
Spending
per Unit in
2017
Total Spending
in 2017
Spending
per Unit in
2017
Year of FDA
Approval
Avycaz
$2,664,813
$12.94
$1,742,468
$334.46
2015
Axumin
2016
Bavencio
$74,642
$162.27
$71,948
$117.95
2017
Baxdela
2017
Beleodaq
$412,260
$1,717.75
$626,679
$1,808.81
2014
Belsomra
$60,805,250
$10.25
$11,919,689
$10.18
2014
Benznidazole
2017
Besponsa
$55,539
$1,988.29
2017
Bevyxxa
2017
Blincyto
$1,821,295
$853.67
$8,387,395
$2,982.89
2014
Breo Ellipta
$812,599,446
$5.39
$148,483,935
$5.33
2013
Bridion
$2,190,176
$177.65
2015
Brineura
2017
Brintellix
$550,472
$10.76
$471,539
$10.03
2013
Briviact
$24,739,138
$16.70
$20,725,853
$15.38
2016
Calquence
$705,323
$239.91
$71,093
$236.98
2017
Cerdelga
$15,314,837
$429.32
$4,322,241
$385.91
2014
Cholbam
$388,599
$863.55
$11,994,900
$497.67
2015
Cinqair
$378,335
$86.58
$920,851
$77.68
2016
Corlanor
$11,571,724
$7.05
$4,499,295
$6.99
2015
Cosentyx*
$220,359,536
$2,187.78
$110,977,552
$2,124.10
2015
Cotellic
$3,844,912
$105.39
$1,888,687
$102.43
2015
Cresemba
$16,905,039
$80.16
$7,312,790
$80.96
2015
Cyramza
$2,368,992
$113.09
$19,016,530
$111.32
2014
Daklinza
$137,214,398
$761.14
$18,353,791
$730.63
2015
Dalvance
$2,246,625
$109.23
$3,298,764
$588.67
2014
Darzalex
$7,540,100
$97.13
$18,464,378
$90.12
2015
Defitelio
2016
Dotarem
2013
Duavee
$1,680,209
$5.21
$371,192
$5.32
2013
Dupixent
$26,650,730
$719.14
$14,313,170
$702.69
2017
Emflaza
$1,256,323
$192.01
$8,531,153
$167.53
2017
Empliciti
$3,305,028
$2,226.89
$3,453,119
$696.66
2015
Entresto
$227,622,190
$6.97
$29,009,236
$6.96
2015
Entyvio
$16,196,446
$1,282.99
$57,372,196
$4,314.59
2014
Epclusa
$941,351,453
$897.35
$943,299,246
$874.83
2016
Esbriet
$477,230,088
$36.34
$14,076,495
$34.97
2014
Eucrisa
$3,634,363
$9.68
$9,247,846
$9.55
2016
Exondys 51
$3,231,919
$839.84
$24,164,485
$878.73
2016
Farxiga
$193,250,737
$14.20
$33,515,739
$14.16
2014
Farydak
$5,798,773
$1,256.47
$545,332
$1,247.83
2015
Fasenra
**
**
$52,329
$4,757.22
2017
Gazyva
$1,106,000
$151.92
$3,930,261
$144.47
2013
Genvoya
$689,941,011
$92.88
$814,372,297
$90.40
2015
Giapreza
2017
Gilotrif
$86,748,908
$265.82
$12,856,461
$261.17
2013

38
Medicare Part D
Medicaid
Brand Name
Total Spending
in 2017
Spending
per Unit in
2017
Total Spending
in 2017
Spending
per Unit in
2017
Year of FDA
Approval
Harvoni
$2,555,839,934
$1,119.62
$1,211,321,698
$1,084.68
2014
Hemlibra
2017
Hetlioz
$65,211,444
$459.85
$11,579,569
$450.67
2014
Ibrance
$1,399,517,851
$534.68
$245,368,530
$522.25
2015
Idhifa
$11,692,885
$841.90
$480,611
$801.02
2017
Imbruvica
$1,368,727,295
$125.74
$63,978,556
$122.79
2013
Imfinzi
$265,553
$353.36
$391,938
$210.55
2017
Impavido
$484,603
$572.82
2014
Ingrezza
$83,396,864
$188.23
$14,189,177
$181.77
2017
Invokana
$717,362,001
$13.97
$188,379,479
$14.05
2013
Jardiance
$321,839,321
$14.07
$107,278,880
$14.20
2014
Jublia
$34,526,305
$141.47
$4,089,011
$137.37
2014
Kadcyla
$2,202,844
$3,319.95
$26,953,379
$1,495.48
2013
Kanuma
$4,460,821
$1,148.22
2015
Kengreal
$3,427
$63.44
2015
Kerydin
$13,288,567
$143.91
$986,819
$141.46
2014
Kevzara
$3,161,323
$1,331.57
$498,150
$1,314.41
2017
Keytruda
$14,585,744
$1,158.70
$91,215,243
$1,010.15
2014
Kisqali*
$28,028,426
$187.07
$5,365,840
$181.57
2017
Kybella
2015
Kynamro
$3,228,484
$7,543.19
$791,688
$7,330.45
2013
Lartruvo
$907,506
$49.19
$3,571,383
$60.57
2016
Lenvima
$79,194,380
$282.00
$12,858,211
$2,333.81
2015
Lonsurf
$88,408,625
$181.29
$20,183,636
$182.72
2015
Lumason
2014
Luzu
$723,546
$7.50
$163,167
$7.33
2013
Lymphoseek
2013
Lynparza
$45,911,717
$47.09
$10,737,846
$45.25
2014
Macrilen
2017
Mavyret
$71,065,533
$158.30
$113,895,225
$348.72
2017
Mekinist
$50,847,497
$296.26
$22,623,999
$280.01
2013
Mepsevii
2017
Movantik
$75,658,178
$10.56
$18,997,726
$10.41
2014
Myalept
$11,515,682
$3,991.57
$17,656,249
$3,845.84
2014
Natpara
$36,971,388
$4,565.51
$16,838,925
$4,624.66
2015
Nerlynx
$4,096,875
$59.57
$1,988,772
$59.72
2017
Nesina
$4,485,458
$11.81
$701,140
$12.38
2013
Netspot
2016
Neuraceq
2014
Ninlaro
$212,933,728
$3,188.19
$10,711,088
$3,149.17
2015
Northera
$169,715,303
$49.90
$8,009,720
$45.23
2014
Nucala
$18,862,489
$2,775.12
$17,115,079
$2,240.99
2015
Nuplazid
$107,725,863
$38.29
$1,871,758
$37.45
2016
Ocaliva
$38,666,557
$201.92
$7,377,148
$199.58
2016
Ocrevus
$24,098,189
$1,375.39
$11,222,699
$386.36
2017
Odomzo
$6,310,591
$339.61
$719,842
$333.26
2015

39
Medicare Part D
Medicaid
Brand Name
Total Spending
in 2017
Spending
per Unit in
2017
Total Spending
in 2017
Spending
per Unit in
2017
Year of FDA
Approval
Ofev
$494,127,312
$148.10
$15,465,997
$146.87
2014
Olysio
$5,838,192
$801.73
$2,043,069
$768.07
2013
Opdivo
$28,619,177
$263.10
$206,274,929
$256.36
2014
Opsumit
$400,507,430
$288.39
$65,587,260
$281.88
2013
Orbactiv
$687,031
$41.06
$1,617,463
$441.91
2014
Orkambi
$83,330,029
$185.36
$297,773,454
$181.62
2015
Osphena
$12,547
$6.08
$415,292
$6.02
2013
Otezla
$243,149,801
$49.57
$81,355,941
$48.30
2014
Ozempic
2017
Parsabiv
2017
Plegridy*
$81,390,738
$6,353.99
$18,163,470
$3,316.23
2014
Pomalyst
$639,636,457
$711.31
$35,511,974
$691.35
2013
Portrazza
$66,922
$83.65
$881,034
$135.58
2015
Praluent*
$168,199,351
$572.01
$5,362,029
$556.06
2015
Praxbind
$5,511
$51.51
2015
Prevymis
$22,064
$197.00
2017
Radicava
$8,801,715
$5.55
$637,544
$5.85
2017
Rapivab
2014
Repatha*
$149,192,429
$569.87
$10,077,857
$549.22
2015
Rexulti
$154,854,512
$33.93
$128,082,228
$32.86
2015
Rhopressa
2017
Rubraca
$17,003,163
$116.90
$3,701,960
$117.40
2016
Rydapt
$9,817,343
$136.03
$4,149,975
$133.61
2017
Savaysa
$11,775,581
$10.40
$926,475
$10.45
2015
Siliq
$123,026
$1,188.66
$35,778
$1,192.60
2017
Simponi Aria*
$129,030,017
$405.81
$56,037,375
$336.45
2013
Sivextro
$5,228,331
$323.04
$1,217,779
$299.05
2014
Solosec
2017
Sovaldi
$211,121,755
$1,013.14
$36,998,037
$976.07
2013
Spinraza
$1,154,258
$25,650.18
$119,583,270
$23,477.05
2016
Steglatro
2017
Strensiq
$56,537,768
$5,955.45
$38,702,500
$4,030.50
2015
Striverdi Respimat
$2,751,942
$45.51
$821,392
$44.77
2014
Sylvant
$72,030
$3,601.50
$515,545
$1,176.55
2014
Symproic
$123,285
$10.54
$28,694
$10.58
2017
Tafinlar
$45,644,597
$78.00
$17,179,644
$77.14
2013
Tagrisso
$189,639,073
$486.90
$26,195,457
$475.02
2015
Taltz*
$72,197,880
$4,786.28
$26,175,641
$4,660.38
2016
Tanzeum
$29,166,096
$125.31
$37,829,320
$126.75
2014
Tecentriq
$4,135,067
$447.52
$9,307,801
$97.35
2016
Tecfidera
$1,024,106,779
$116.81
$307,295,038
$395.11
2013
Tivicay
$518,567,843
$51.87
$412,871,260
$50.42
2013
Tremfya
$9,424,317
$9,766.13
$3,059,127
$9,529.99
2017
Tresiba*
$438,621,820
$59.22
$75,764,368
$58.85
2015
Trulance
$4,360,061
$11.78
$1,398,081
$11.71
2017
Trulicity
$699,368,134
$329.75
$95,090,166
$326.93
2014

40
Medicare Part D
Medicaid
Brand Name
Total Spending
in 2017
Spending
per Unit in
2017
Total Spending
in 2017
Spending
per Unit in
2017
Year of FDA
Approval
Tymlos
$4,903,353
$1,060.81
$211,071
$1,025.02
2017
Unituxin
2015
Uptravi
$216,625,694
$222.21
$54,168,963
$224.56
2015
Vabomere
2017
Varubi
$4,054,107
$281.73
$858,429
$268.24
2015
Veltassa
$38,802,627
$23.11
$3,936,528
$22.95
2015
Venclexta*
$45,993,352
$81.85
$3,269,134
$84.17
2016
Verzenio
$6,131,338
$199.67
$789,734
$198.09
2017
Viberzi
$52,540,250
$17.42
$14,203,963
$17.23
2015
Viekira Pak*
$24,361,652
$332.47
$120,328,382
$951.48
2014
Vimizim
$11,021,290
$226.03
$51,750,474
$237.53
2014
Vizamyl
2013
Vosevi
$74,489,556
$886.12
$24,056,794
$880.98
2017
Vraylar
$98,960,685
$36.65
$88,857,469
$35.53
2015
Vyzulta
$17,484
$69.93
$1,049
$69.96
2017
Xadago
$1,419,720
$22.76
$33,261
$22.70
2017
Xepi
2017
Xermelo
$6,617,349
$63.54
$604,116
$61.87
2017
Xiidra
$74,508,228
$7.47
$14,516,011
$28.20
2016
Xofigo
$2,320,827
$18,227.44
2013
Xtoro
2014
Xuriden
2015
Yondelis
$153,287
$2,875.94
$1,180,431
$2,282.48
2015
Zejula
$33,844,004
$168.91
$5,041,899
$166.09
2017
Zepatier
$520,138,312
$660.44
$705,462,489
$647.27
2016
Zerbaxa
$2,461,143
$4.30
$1,193,668
$72.17
2014
Zinbryta
$19,662,817
$7,367.11
$5,798,931
$7,123.99
2016
Zinplava
$112,316
$67.95
$20,963
$82.75
2016
Zontivity
$2,734,005
$9.59
$233,591
$9.40
2014
Zurampic
$723,921
$12.06
$220,875
$12.07
2015
Zydelig
$50,968,069
$168.68
$2,743,861
$164.00
2014
Zykadia
$14,779,325
$105.62
$2,820,819
$100.40
2014
Source: The list of approved drugs was obtained at
https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologic
ApprovalReports/NDAandBLAApprovalReports/ucm373420.htm
Spending data is from an analysis of CMS Medicare and Medicaid State Drug Utilization data. Blank fields indicate
no associated volume or spending in 2017. Some of these drugs may be orphan or pediatric drugs.
* Drugs with an asterisk next to the name have more than one product listed separately in the dashboard data for
Medicare Part B, Medicare Part B, or Medicaid. In such cases, Total Spending represents the sum of those products.
Spending per Dosage Unit represents the reported figure for the product with the largest number of service units in
2017.
** Drugs with 1-11 service units have drug information redacted to protect patient confidentiality

41
APPENDIX: TOP 10 DRUGS BY TOTAL COST, SPENDING PER UNIT, AND
PRESCRIPTION FREQUENCY IN MEDICARE PART B, MEDICARE PART D, AND
MEDICAID
Table A-1 Top 10 High-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
Year
Rank
HCPCS
Description
Total Payment
($M)
Beneficiaries
(1000s)
Spending per
Beneficiary ($)
Services
(1000s)
Spending per
Service ($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2008
1
J9310
Rituximab cancer treatment
1,151.1
63
18,271.43
299
3,849.83
2,227
516.88
918.7
2
J9035
Bevacizumab injection
944.7
149
6,340.27
581
1,625.99
16,457
57.40
761.3
3
J1745
Infliximab injection
814.8
59
13,810.17
339
2,403.54
14,589
55.85
647.8
4
J2505
Injection, pegfilgrastim 6mg
798.8
101
7,908.91
361
2,212.74
362
2,206.63
634.3
5
J2778
Ranibizumab injection
735.8
87
8,457.47
364
2,021.43
1,853
397.09
588.1
6
J0881
Darbepoetin alfa, non-esrd
676.7
203
3,333.50
1,142
592.56
232,618
2.91
534.5
7
J9263
Oxaliplatin
457.5
26
17,596.15
149
3,070.47
48,582
9.42
365.9
8
J0885
Epoetin alfa, non-esrd
462.3
185
2,498.92
1,522
303.75
49,918
9.26
362.3
9
J9170
Docetaxel
396.7
46
8,623.91
240
1,652.92
1,210
327.85
316.8
10
J9201
Gemcitabine HCl
330.8
46
7,191.30
313
1,056.87
2,498
132.43
263.7
Medicare Part B Spending, Top 10
6,769.2
5,393.4
Medicare Part B Spending, All Drugs
13,614.3
10,843.8

42
Table A-1 continued Top 10 High-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
Year
Rank
HCPCS
Description
Total Payment
($M)
Beneficiaries
(1000s)
Spending per
Beneficiary ($)
Services
(1000s)
Spending per
Service ($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2009
1
J9310
Rituximab injection
1,195.5
64
18,679.69
293
4,080.20
2,203
542.67
954.5
2
J9035
Bevacizumab injection
1,086.7
167
6,507.19
679
1,600.44
19,024
57.12
875.5
3
J2778
Ranibizumab injection
899.0
99
9,080.81
435
2,066.67
2,236
402.06
718.6
4
J1745
Infliximab injection
854.4
59
14,481.36
338
2,527.81
14,943
57.18
678.7
5
J2505
Injection, pegfilgrastim 6mg
793.1
99
8,011.11
355
2,234.08
1,356
584.88
628.5
6
J0881
Darbepoetin alfa, non-esrd
583.6
164
3,558.54
966
604.14
193,897
3.01
460.5
7
J9263
Oxaliplatin
467.5
27
17,314.81
152
3,075.66
49,594
9.43
373.7
8
J0885
Epoetin alfa, non-esrd
463.9
170
2,728.82
1,453
319.27
48,219
9.62
364.0
9
J9170
Docetaxel injection
409.8
46
8,908.70
234
1,751.28
1,200
341.50
327.3
10
J9305
Pemetrexed injection
363.9
20
18,195.00
81
4,492.59
7,505
48.49
292.6
Medicare Part B Spending, Top 10
7,117.4
5,673.9
Medicare Part B Spending, All Drugs
14,488.5
11,547.5

43
Table A-1 continued Top 10 High-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
Year
Rank
HCPCS
Description
Total Payment
($M)
Beneficiaries
(1000s)
Spending per
Beneficiary ($)
Services
(1000s)
Spending per
Service ($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2010
1
J9310
Rituximab injection
1,253.1
65
19,278.46
290
4,321.03
2,171
577.20
1,000.9
2
J2778
Ranibizumab injection
1,171.8
115
10,189.57
557
2,103.77
2,900
404.07
936.4
3
J9035
Bevacizumab injection
1,105.6
162
6,824.69
699
1,581.69
19,142
57.76
890.7
4
J1745
Infliximab injection
899.8
59
15,250.85
339
2,654.28
15,232
59.07
713.9
5
J2505
Injection, pegfilgrastim 6mg
876.2
100
8,762.00
357
2,454.34
358
2,447.49
694.3
6
J0881
Darbepoetin alfa, non-esrd
500.4
138
3,626.09
833
600.72
169,876
2.95
393.5
7
J9305
Pemetrexed injection
429.8
22
19,536.36
92
4,671.74
8,439
50.93
346.0
8
J0885
Epoetin alfa, non-esrd
421.9
150
2,812.67
1,297
325.29
43,247
9.76
330.6
9
J9171
Docetaxel injection
392.4
44
8,918.18
221
1,775.57
21,902
17.92
312.8
10
J9355
Trastuzumab injection
365.4
14
26,100.00
177
2,064.41
5,510
66.32
291.5
Medicare Part B Spending, Top 10
7,416.4
5,910.6
Medicare Part B Spending, All Drugs
15,478.6
12,336.5

44
Table A-1 continued Top 10 High-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
Year
Rank
HCPCS
Description
Total Payment
($M)
Beneficiaries
(1000s)
Spending per
Beneficiary ($)
Services
(1000s)
Spending per
Service ($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2011
1
J2778
Ranibizumab injection
1,428.9
133
10,743.61
670
2,132.69
3,525
405.36
1,141.9
2
J9310
Rituximab injection
1,373.0
68
20,191.18
298
4,607.38
2,245
611.58
1,098.3
3
J9035
Bevacizumab injection
1,012.5
174
5,818.97
756
1,339.29
16,750
60.45
817.4
4
J2505
Injection, pegfilgrastim 6mg
1,020.5
106
9,627.36
386
2,643.78
386
2,643.78
808.4
5
J1745
Infliximab injection
960.5
60
16,008.33
344
2,792.15
15,620
61.49
761.6
6
J9263
Oxaliplatin
498.3
30
16,610.00
164
3,038.41
52,882
9.42
398.0
7
J9305
Pemetrexed injection
464.4
22
21,109.09
97
4,787.63
8,751
53.07
374.0
8
J0881
Darbepoetin alfa, non-esrd
435.4
112
3,887.50
687
633.77
138,222
3.15
341.9
9
J9355
Trastuzumab injection
410.7
15
27,380.00
182
2,256.59
5,817
70.60
327.2
10
J9171
Docetaxel injection
395.9
45
8,797.78
210
1,885.24
21,168
18.70
315.8
Medicare Part B Spending, Top 10
8,000.1
6,384.5
Medicare Part B Spending, All Drugs
17,191.6
13,733.3

45
Table A-1 continued Top 10 High-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
Year
Rank
HCPCS
Description
Total Payment
($M)
Beneficiaries
(1000s)
Spending per
Beneficiary ($)
Services
(1000s)
Spending per
Service ($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2012
1
J9310
Rituximab injection
1,466.2
70
20,945.71
302
4,854.97
2,364
620.22
1,175.0
2
J2778
Ranibizumab injection
1,268.0
136
9,323.53
605
2,095.87
3,166
400.51
1,013.4
3
J2505
Injection, pegfilgrastim 6mg
1,118.7
107
10,455.14
395
2,832.15
396
2,825.00
886.0
4
J9035
Bevacizumab injection
1,027.5
183
5,614.75
789
1,302.28
16,713
61.48
831.2
5
J1745
Infliximab injection
1,040.5
61
17,057.38
352
2,955.97
16,201
64.22
824.7
6
J9263
Oxaliplatin
532.7
31
17,183.87
172
3,097.09
55,077
9.67
425.6
7
J9305
Pemetrexed injection
521.9
23
22,691.30
103
5,066.99
9,386
55.60
421.1
8
J0897
Denosumab injection
503.8
164
3,071.95
386
1,305.18
34,654
14.54
400.4
9
J9355
Trastuzumab injection
478.6
17
28,152.94
192
2,492.71
6,441
74.31
381.5
10
J9041
Bortezomib injection
436.4
20
21,820.00
311
1,403.22
10,145
43.02
347.5
Medicare Part B Spending, Top 10
8,394.3
6,706.4
Medicare Part B Spending, All Drugs
19,001.0
15,201.6

46
Table A-1 continued Top 10 High-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
Year
Rank
HCPCS
Description
Total Payment
($M)
Beneficiaries
(1000s)
Spending per
Beneficiary ($)
Services
(1000s)
Spending per
Service ($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2013
1
J9310
Rituximab injection
1,554.8
72
21,594.44
304
5,114.47
2,321
669.88
1,245.7
2
J2778
Ranibizumab injection
1,354.1
144
9,403.47
678
1,997.20
3,443
393.29
1,078.9
3
J2505
Injection, pegfilgrastim 6mg
1,159.5
105
11,042.86
386
3,003.89
387
2,996.12
915.1
4
J1745
Infliximab injection
1,139.7
62
18,382.26
359
3,174.65
16,779
67.92
900.8
5
J0178
Aflibercept injection
1,079.3
109
9,901.83
520
2,075.58
1,117
966.25
859.7
6
J9035
Bevacizumab injection
1,060.2
188
5,639.36
795
1,333.58
16,689
63.53
855.6
7
J0897
Denosumab injection
655.9
237
2,767.51
537
1,221.42
46,311
14.16
517.8
8
J9305
Pemetrexed injection
564.6
23
24,547.83
107
5,276.64
9,664
58.42
455.2
9
J9355
Trastuzumab injection
518.5
18
28,805.56
190
2,728.95
6,670
77.74
413.2
10
J9041
Bortezomib injection
465.3
21
22,157.14
321
1,449.53
10,448
44.53
368.8
Medicare Part B Spending, Top 10
9,551.9
7,610.8
Medicare Part B Spending, All Drugs
20,331.5
16,231.8

47
Table A-1 continued Top 10 High-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
Year
Rank
HCPCS
Description
Total Payment
($M)
Beneficiaries
(1000s)
Spending per
Beneficiary ($)
Services
(1000s)
Spending per
Service ($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2014
1
J9310
Rituximab injection
1,552.5
70
22,178.57
292
5,316.78
2,239
693.39
1,244.6
2
J2778
Ranibizumab injection
1,336.0
142
9,408.45
676
1,976.33
3,420
390.64
1,063.4
3
J0178
Aflibercept injection
1,302.0
133
9,789.47
627
2,076.56
1,350
964.44
1,036.1
4
J2505
Injection, pegfilgrastim 6mg
1,235.7
102
12,114.71
373
3,312.87
373
3,312.87
974.4
5
J1745
Infliximab injection
1,223.5
62
19,733.87
360
3,398.61
17,060
71.72
965.6
6
J9035
Bevacizumab injection
1,091.4
217
5,029.49
907
1,203.31
16,747
65.17
880.3
7
J0897
Denosumab injection
799.3
306
2,612.09
670
1,192.99
55,891
14.30
629.8
8
J9355
Trastuzumab injection
581.0
19
30,578.95
198
2,934.34
7,192
80.78
464.0
9
J9305
Pemetrexed injection
575.4
24
23,975.00
107
5,377.57
9,670
59.50
463.6
10
J9041
Bortezomib injection
489.3
21
23,300.00
328
1,491.77
10,745
45.54
387.3
Medicare Part B Spending, Top 10
10,186.1
8,109.1
Medicare Part B Spending, All Drugs
21,597.8
17,240.2

48
Table A-1 continued Top 10 High-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
Year
Rank
HCPCS
Description
Total Payment
($M)
Beneficiaries
(1000s)
Spending per
Beneficiary ($)
Services
(1000s)
Spending per
Service ($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2015
1
J0178
Aflibercept injection
1,823.5
181
10,074.59
870
2,095.98
1,890
964.81
1,451.2
2
J9310
Rituximab injection
1,615.8
70
23,082.86
286
5,649.65
2,213
730.14
1,297.7
3
J2505
Injection, pegfilgrastim 6mg
1,326.4
100
13,264.00
369
3,594.58
369
3,594.58
1,047.4
4
J1745
Infliximab injection
1,302.7
61
21,355.74
354
3,679.94
17,064
76.34
1,026.8
5
J9035
Bevacizumab injection
1,155.0
209
5,526.32
906
1,274.83
16,985
68.00
931.9
6
J2778
Ranibizumab injection
1,153.9
120
9,615.83
575
2,006.78
2,980
387.21
918.5
7
J0897
Denosumab injection
956.8
371
2,578.98
791
1,209.61
64,348
14.87
753.1
8
J9355
Trastuzumab injection
669.5
20
33,475.00
207
3,234.30
7,857
85.21
536.1
9
J9305
Pemetrexed injection
566.2
22
25,736.36
104
5,444.23
9,362
60.48
456.1
10
J9041
Bortezomib injection
526.2
22
23,918.18
345
1,525.22
11,347
46.37
416.2
Medicare Part B Spending, Top 10
11,096.0
8,835.0
Medicare Part B Spending, All Drugs
23,813.3
19,007.1

49
Table A-1 continued Top 10 High-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
Year
Rank
HCPCS
Description
Total Payment
($M)
Beneficiaries
(1000s)
Spending per
Beneficiary ($)
Services
(1000s)
Spending per
Service ($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2016
1
J0178
Aflibercept injection
2,224.1
211
10,540.76
1,052
2,114.16
2,303
965.74
1,769.4
2
J9310
Rituximab injection
1,723.1
72
23,931.94
286
6,024.83
2,235
770.96
1,388.1
3
J2505
Injection, pegfilgrastim 6mg
1,433.3
99
14,477.78
367
3,905.45
367
3,905.45
1,137.9
4
J1745
Infliximab injection
1,402.9
60
23,381.67
354
3,962.99
17,241
81.37
1,105.9
5
J9299
Injection, nivolumab
1,260.3
28
45,010.71
217
5,807.83
49,748
25.33
1,010.3
6
J9035
Bevacizumab injection
1,141.9
208
5,489.90
904
1,263.16
16,205
70.47
923.2
7
J0897
Denosumab injection
1,133.4
436
2,599.54
904
1,253.76
71,943
15.75
890.4
8
J2778
Ranibizumab injection
1,046.8
107
9,783.18
526
1,990.11
2,765
378.59
833.1
9
J9355
Trastuzumab injection
730.6
21
34,790.48
211
3,462.56
8,137
89.79
585.7
10
J0129
Abatacept injection
601.0
23
26,130.43
198
3,035.35
14,727
40.81
474.5
Medicare Part B Spending, Top 10
12,697.4
10,118.5
Medicare Part B Spending, All Drugs
27,266.7
21,789.6

50
Table A-1 continued Top 10 High-Cost Prescribed Drugs, Medicare Part B, Ranked by Total Spending
Year
Rank
HCPCS
Description
Total Payment
($M)
Beneficiaries
(1000s)
Spending per
Beneficiary ($)
Services
(1000s)
Spending per
Service ($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2017
1
J0178
Aflibercept injection
2,473.4
230
10,753.91
1,166
2,121.27
2,573
961.29
1,967.3
2
J9310
Rituximab injection
1,815.3
72
25,212.50
282
6,437.23
2,216
819.18
1,467.6
3
J9299
Injection, nivolumab
1,516.5
30
50,550.00
252
6,017.86
58,432
25.95
1,207.8
4
J2505
Injection, pegfilgrastim 6mg
1,461.7
94
15,550.00
349
4,188.25
349
4,188.25
1,164.3
5
J1745
Infliximab not biosimil 10mg
1,413.9
59
23,964.41
336
4,208.04
16,633
85.01
1,113.0
6
J0897
Denosumab injection
1,296.6
491
2,640.73
995
1,303.12
77,429
16.75
1,016.7
7
J9035
Bevacizumab injection
1,100.2
220
5,000.91
935
1,176.68
15,017
73.26
887.5
8
J9271
Inj pembrolizumab
1,062.2
22
48,281.82
119
8,926.05
23,017
46.15
885.9
9
J2778
Ranibizumab injection
1,039.1
105
9,896.19
525
1,979.24
2,790
372.44
826.9
10
J9355
Trastuzumab injection
814.1
21
38,766.67
208
3,913.94
8,581
94.87
654.3
Medicare Part B Spending, Top 10
13,993.0
11,191.3
Medicare Part B Spending, All Drugs
30,294.0
24,260.5
Source: Analysis of carrier, durable medical, and outpatient claims data 2006-2017 by Acumen for ASPE. Data include Part B covered drugs administered in
physicians' offices and furnished by suppliers, covered drugs in hospital outpatient departments; and reflect only Part B drugs paid under the average sales price
plus 6 percent (ASP). The Healthcare Common Procedure Coding System (HCPCS) codes and prices for carrier and DM were obtained from the CMS ASP file,
those for OP come from the CMS Addendum B file. Lines with denied payments or Medicare as secondary payer were dropped. Spending per Beneficiary,
Spending per Service, and Spending per Unit include beneficiary cost-sharing and include the sequester.

51
Table A-2 Top 10 Highest-Cost Prescribed Drugs, Medicare Part B, Ranked by
Spending per Unit
Year
Rank
Description
Spending per
Unit ($)
Total
Payment ($)
2011
1
Sipuleucel-t, minimum of 50 million
autologous cd54+ cells activated with pap-gm-
csf, including leukapheresis and all other
preparatory procedures, per infusion
32,857.47
89,930,907
2
Fluocinolone acetonide, intravitreal implant
16,253.07
1,397,764
3
Injection, porfimer sodium, 75 mg
5,041.82
857,110
4
Leuprolide acetate implant, 65 mg
4,378.09
341,491
5
Ganciclovir, 4.5 mg, long-acting implant
3,485.88
400,876
6
Histrelin implant (vantas), 50 mg
2,945.12
17,096,411
7
Injection, pegaspargase, per single dose vial
2,680.69
343,128
8
Injection, pegfilgrastim, 6 mg
2,615.20
972,009,437
9
Injection, denileukin diftitox, 300 micrograms
1,585.58
7,020,970
10
Injection, reteplase, 18.1 mg
1,425.91
517,605
Year
Rank
Description
Spending per
Unit ($)
Total
Payment ($)
2012
1
Sipuleucel-t, minimum of 50 million
autologous cd54+ cells activated with pap-gm-
csf, including leukapheresis and all other
preparatory procedures, per infusion
32,775.67
218,482,586
2
Injection, porfimer sodium, 75 mg
18,961.14
2,768,327
3
Fluocinolone acetonide, intravitreal implant
18,838.95
2,241,835
4
Injection, pegaspargase, per single dose vial
4,857.72
563,495
5
Ganciclovir, 4.5 mg, long-acting implant
4,679.71
196,548
6
Leuprolide acetate implant, 65 mg
4,639.39
259,806
7
Histrelin implant (vantas), 50 mg
3,055.20
12,272,745
8
Injection, pegfilgrastim, 6 mg
2,788.06
1,060,046,676
9
Injection, basiliximab, 20 mg
2,193.03
767,562
10
Injection, crotalidae polyvalent immune fab
(ovine), up to 1 gram
2,163.68
1,514,578

52
Table A-2 continued Top 10 Highest-Cost Prescribed Drugs, Medicare Part B, Ranked by
Spending per Unit
Year
Rank
Description
Spending per
Unit ($)
Total
Payment ($)
2013
1
Sipuleucel-t, minimum of 50 million
autologous cd54+ cells activated with pap-gm-
csf, including leukapheresis and all other
preparatory procedures, per infusion
32,301.81
178,822,808
2
Injection, porfimer sodium, 75 mg
19,061.72
2,611,456
3
Fluocinolone acetonide, intravitreal implant
18,766.14
2,364,534
4
Ganciclovir, 4.5 mg, long-acting implant
5,970.86
298,543
5
Injection, pegaspargase, per single dose vial
5,872.50
669,465
6
Leuprolide acetate implant, 65 mg
4,364.58
183,312
7
Injection, pegfilgrastim, 6 mg
2,960.29
1,098,433,150
8
Histrelin implant (vantas), 50 mg
2,923.37
8,115,285
9
Injection, basiliximab, 20 mg
2,397.81
721,741
10
Injection, reteplase, 18.1 mg
2,243.78
40,388
Year
Rank
Description
Spending per
Unit ($)
Total
Payment ($)
2014
1
Sipuleucel-t, minimum of 50 million
autologous cd54+ cells activated with pap-gm-
csf, including leukapheresis and all other
preparatory procedures, per infusion
33,866.12
173,767,079
2
Injection, porfimer sodium, 75 mg
19,291.79
2,778,018
3
Fluocinolone acetonide, intravitreal implant
19,003.57
1,748,329
4
Injection, pegaspargase, per single dose vial
5,952.34
1,148,802
5
Leuprolide acetate implant, 65 mg
3,666.89
47,670
6
Injection, pegfilgrastim, 6 mg
3,270.46
1,174,026,333
7
Histrelin implant (vantas), 50 mg
2,916.24
5,858,729
8
Injection, basiliximab, 20 mg
2,527.25
710,157
9
Injection, crotalidae polyvalent immune fab
(ovine), up to 1 gram
2,410.05
1,689,442
10
Ganciclovir, 4.5 mg, long-acting implant
2,254.84
54,116
53
Table A-2 continued Top 10 Highest-Cost Prescribed Drugs, Medicare Part B, Ranked by
Spending per Unit
Year
Rank
Description
Spending per
Unit ($)
Total
Payment ($)
2015
1
Sipuleucel-t, minimum of 50 million
autologous cd54+ cells activated with pap-gm-
csf, including leukapheresis and all other
preparatory procedures, per infusion
35,205.98
170,854,604
2
Injection, porfimer sodium, 75 mg
19,691.17
2,225,102
3
Fluocinolone acetonide, intravitreal implant
18,543.62
2,225,234
4
Injection, pegaspargase, per single dose vial
6,103.05
860,529
5
Injection, pegfilgrastim, 6 mg
3,550.86
1,259,962,339
6
Histrelin implant (vantas), 50 mg
2,935.87
4,427,293
7
Injection, basiliximab, 20 mg
2,744.71
930,456
8
Injection, aldesleukin, per single use vial
2,489.43
1,453,825
9
Injection, crotalidae polyvalent immune fab
(ovine), up to 1 gram
2,480.59
1,781,064
10
Injection, digoxin immune fab (ovine), per vial
2,300.00
1,060,302
Year
Rank
Description
Spending per
Unit ($)
Total
Payment ($)
2016
1
Sipuleucel-t, minimum of 50 million
autologous cd54+ cells activated with pap-gm-
csf, including leukapheresis and all other
preparatory procedures, per infusion
36,980.27
179,058,484
2
Injection, pegaspargase, per single dose vial
10,333.47
3,007,040
3
Injection, pegfilgrastim, 6 mg
3,868.65
1,374,291,300
4
Injection, carmustine, 100 mg
3,281.57
1,476,708
5
Injection, basiliximab, 20 mg
3,042.49
1,025,318
6
Histrelin implant (vantas), 50 mg
3,014.13
3,662,168
7
Injection, digoxin immune fab (ovine), per vial
2,864.88
1,054,277
8
Injection, crotalidae polyvalent immune fab
(ovine), up to 1 gram
2,614.49
1,113,773
9
Injection, vincristine sulfate liposome, 1 mg
2,304.81
1,601,842
10
Injection, melphalan hydrochloride, 50 mg
1,741.54
4,555,858

54
Table A-2 continued Top 10 Highest-Cost Prescribed Drugs, Medicare Part B, Ranked by
Spending per Unit
Year
Rank
Description
Spending per
Unit ($)
Total
Payment ($)
2017
1
Sipuleucel-t, minimum of 50 million
autologous cd54+ cells activated with pap-gm-
csf, including leukapheresis and all other
preparatory procedures, per infusion
38,716.24
202,485,909
2
Injection, pegaspargase, per single dose vial
9,666.53
2,281,300
3
Injection, pegfilgrastim, 6 mg
4,142.60
1,400,100,685
4
Injection, basiliximab, 20 mg
3,309.41
1,102,034
5
Injection, carmustine, 100 mg
3,278.80
1,891,865
6
Histrelin implant (vantas), 50 mg
3,127.67
3,002,560
7
Injection, digoxin immune fab (ovine), per vial
2,933.29
882,920
8
Injection, crotalidae polyvalent immune fab
(ovine), up to 1 gram
2,766.58
1,269,859
9
Injection, vincristine sulfate liposome, 1 mg
2,325.16
1,064,924
10
Injection, pentostatin, 10 mg
1,925.04
843,166
Note: Medicare Part B Spending per Unit data only available for years 2011-2017.
Source: Analysis of carrier, durable medical, and outpatient claims data 2006-2017. Data include Part B covered
drugs administered in physicians' offices and furnished by suppliers, covered drugs in hospital outpatient
departments; and reflect only Part B drugs paid under the average sales price plus 6 percent (ASP). HCPCS codes
and prices for carrier and DM were obtained from the CMS ASP file, those for OP come from the CMS Addendum
B file. Lines with denied payments or Medicare as secondary payer were dropped. Total Spending and Spending per
Unit include beneficiary cost-sharing and include the sequester.

55
Table A-3 Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number of Services
Year
Rank
HCPCS
Description
Total
Payment
($M)
Beneficiaries
(1000s)
Spending
per
Beneficiary
($)
Services
(1000s)
Spending
per
Service
($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2008
1
J3420
Vitamin b12 injection
0.9
638
1.41
2,824
0.32
3,226
0.28
0.6
2
J1100
Dexamethasone sodium phos
1.9
824
2.31
2,466
0.77
21,530
0.09
1.5
3
J2405
Ondansetron hcl injection
7.8
1,520
5.13
2,281
3.42
17,940
0.43
5.4
4
J3301
Triamcinolone acetonide inj
13.3
1,404
9.47
2,250
5.91
9,213
1.44
10.0
5
J7620
Albuterol ipratrop non-comp
157.8
435
362.76
1,790
88.16
240,892
0.66
121.6
6
J7050
Normal saline solution infus
0.8
389
2.06
1,630
0.49
2,734
0.29
0.6
7
J1030
Methylprednisolone 40 MG inj
10.1
1,013
9.97
1,591
6.35
2,294
4.40
7.7
8
J0885
Epoetin alfa, non-esrd
462.3
185
2498.92
1,522
303.75
49,918
9.26
362.3
9
J7613
Albuterol non-comp unit
15.7
457
34.35
1,455
10.79
366,913
0.04
12.5
10
J1040
Methylprednisolone 80 MG inj
14.2
840
16.90
1,340
10.60
1,663
8.54
10.8
Medicare Part B Spending, Top 10
684.8
533.0
Medicare Part B Spending, All Drugs
13,614.3
10,843.8

56
Table A-3 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number of Services
Year
Rank
HCPCS
Description
Total
Payment
($M)
Beneficiaries
(1000s)
Spending
per
Beneficiary
($)
Services
(1000s)
Spending
per
Service
($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2009
1
J3420
Vitamin b12 injection
0.8
673
1.19
3,014
0.27
3,299
0.24
0.6
2
J2405
Ondansetron hcl injection
8.2
1,841
4.45
2,792
2.94
20,737
0.40
5.6
3
J1100
Dexamethasone sodium phos
2.0
849
2.36
2,484
0.81
21,572
0.09
1.5
4
J3301
Triamcinolone acet inj NOS
14.7
1,453
10.12
2,345
6.27
9,619
1.53
11.2
5
J7613
Albuterol non-comp unit
22.6
510
44.31
1,909
11.84
479,730
0.05
17.0
6
J7620
Albuterol ipratrop non-comp
51.7
407
127.03
1,730
29.88
205,555
0.25
39.0
7
J1030
Methylprednisolone 40 MG inj
9.4
1,053
8.93
1,661
5.66
2,395
3.92
7.2
8
J7050
Normal saline solution infus
0.7
382
1.83
1,547
0.45
2,532
0.28
0.5
9
J0885
Epoetin alfa, non-esrd
463.9
170
2728.82
1,453
319.27
48,219
9.62
364.0
10
J1040
Methylprednisolone 80 MG inj
12.8
878
14.58
1,403
9.12
1,701
7.52
9.7
Medicare Part B Spending, Top 10
586.8
456.3
Medicare Part B Spending, All Drugs
14,488.5
11,547.5

57
Table A-3 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number of Services
Year
Rank
HCPCS
Description
Total
Payment
($M)
Beneficiaries
(1000s)
Spending
per
Beneficiary
($)
Services
(1000s)
Spending
per
Service
($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2010
1
J3420
Vitamin b12 injection
0.8
654
1.22
2,930
0.27
3,008
0.27
0.6
2
J1100
Dexamethasone sodium phos
2.0
894
2.24
2,536
0.79
21,768
0.09
1.5
3
J3301
Triamcinolone acet inj NOS
15.5
1,501
10.33
2,426
6.39
10,007
1.55
11.8
4
J7613
Albuterol non-comp unit
27.0
519
52.02
1,863
14.49
467,175
0.06
20.5
5
J7620
Albuterol ipratrop non-comp
44.3
404
109.65
1,790
24.75
202,350
0.22
33.4
6
J1030
Methylprednisolone 40 MG inj
9.0
1,079
8.34
1,702
5.29
2,394
3.76
6.9
7
J1040
Methylprednisolone 80 MG inj
12.2
907
13.45
1,449
8.42
1,742
7.00
9.3
8
J7050
Normal saline solution infus
0.6
341
1.76
1,368
0.44
2,155
0.28
0.5
9
J0885
Epoetin alfa, non-esrd
421.9
150
2812.67
1,297
325.29
43,247
9.76
330.6
10
Q9967
LOCM 300-399mg/ml
iodine,1ml
16.5
857
19.25
1,139
14.49
94,413
0.17
13.0
Medicare Part B Spending, Top 10
549.8
428.1
Medicare Part B Spending, All Drugs
15,478.6
12,336.5

58
Table A-3 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number of Services
Year
Rank
HCPCS
Description
Total
Payment
($M)
Beneficiaries
(1000s)
Spending
per
Beneficiary
($)
Services
(1000s)
Spending
per
Service
($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2011
1
J3420
Vitamin b12 injection
1.0
638
1.57
2,753
0.36
2,759
0.36
0.7
2
J3301
Triamcinolone acet inj NOS
17.1
1,590
10.75
2,568
6.66
10,559
1.62
13.0
3
J1100
Dexamethasone sodium phos
1.8
855
2.11
2,388
0.75
20,543
0.09
1.4
4
J7613
Albuterol non-comp unit
28.2
537
52.51
1,886
14.95
456,741
0.06
21.2
5
J7620
Albuterol ipratrop non-comp
45.0
403
111.66
1,774
25.37
191,322
0.24
34.5
6
J1030
Methylprednisolone 40 MG inj
6.7
1,108
6.05
1,741
3.85
2,424
2.76
5.1
7
J1040
Methylprednisolone 80 MG inj
11.9
927
12.84
1,477
8.06
1,721
6.91
9.0
8
J7050
Normal saline solution infus
0.5
298
1.68
1,207
0.41
1,901
0.26
0.4
9
Q9967
LOCM 300-399mg/ml
iodine,1ml
15.3
842
18.17
1,129
13.55
91,794
0.17
12.0
10
J0885
Epoetin alfa, non-esrd
354.8
129
2750.39
1,086
326.70
35,638
9.96
278.1
Medicare Part B Spending, Top 10
482.3
375.4
Medicare Part B Spending, All Drugs
17,191.6
13,733.3

59
Table A-3 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number of Services
Year
Rank
HCPCS
Description
Total
Payment
($M)
Beneficiaries
(1000s)
Spending
per
Beneficiary
($)
Services
(1000s)
Spending
per
Service
($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2012
1
J3420
Vitamin b12 injection
1.5
662
2.27
2,842
0.53
2,848
0.53
1.1
2
J3301
Triamcinolone acet inj NOS
17.9
1,560
11.47
2,514
7.12
10,659
1.68
13.9
3
J1100
Dexamethasone sodium phos
2.4
915
2.62
2,456
0.98
20,347
0.12
1.9
4
J7613
Albuterol non-comp unit
26.7
558
47.85
1,902
14.04
449,362
0.06
19.9
5
J1030
Methylprednisolone 40 MG inj
8.6
1,127
7.63
1,774
4.85
2,469
3.48
6.6
6
J7620
Albuterol ipratrop non-comp
45.2
412
109.71
1,746
25.89
182,771
0.25
33.9
7
J1040
Methylprednisolone 80 MG inj
11.7
938
12.47
1,491
7.85
1,742
6.72
8.9
8
J2785
Regadenoson injection
231.9
1,111
208.73
1,128
205.59
4,357
53.22
183.7
9
Q9967
LOCM 300-399mg/ml
iodine,1ml
11.9
819
14.53
1,122
10.61
87,385
0.14
9.4
10
J0702
Betamethasone acet&sod phosp
13.9
687
20.23
1,074
12.94
2,526
5.50
10.7
Medicare Part B Spending, Top 10
371.7
290.0
Medicare Part B Spending, All Drugs
19,001.0
15,201.6

60
Table A-3 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number of Services
Year
Rank
HCPCS
Description
Total
Payment
($M)
Beneficiaries
(1000s)
Spending
per
Beneficiary
($)
Services
(1000s)
Spending
per
Service
($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2013
1
J3420
Vitamin b12 injection
2.9
635
4.57
2,611
1.11
2,616
1.11
2.2
2
J3301
Triamcinolone acet inj NOS
19.3
1,611
11.98
2,609
7.40
11,041
1.75
14.6
3
J1100
Dexamethasone sodium phos
2.1
953
2.20
2,460
0.85
19,725
0.11
1.7
4
J7613
Albuterol non-comp unit
21.6
581
37.18
1,871
11.54
429,856
0.05
16.1
5
J1030
Methylprednisolone 40 MG inj
7.2
1,153
6.24
1,816
3.96
2,542
2.83
5.5
6
J7620
Albuterol ipratrop non-comp
32.6
423
77.07
1,673
19.49
169,941
0.19
24.3
7
J1040
Methylprednisolone 80 MG inj
9.5
946
10.04
1,500
6.33
1,745
5.44
7.2
8
J2785
Regadenoson injection
248.6
1,181
210.50
1,199
207.34
4,638
53.60
196.2
9
Q9967
LOCM 300-399mg/ml
iodine,1ml
14.0
820
17.07
1,146
12.22
86,550
0.16
11.1
10
J0702
Betamethasone acet&sod phosp
14.2
707
20.08
1,099
12.92
2,559
5.55
10.9
Medicare Part B Spending, Top 10
372.0
289.8
Medicare Part B Spending, All Drugs
20,331.5
16,231.8

61
Table A-3 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number of Services
Year
Rank
HCPCS
Description
Total
Payment
($M)
Beneficiaries
(1000s)
Spending
per
Beneficiary
($)
Services
(1000s)
Spending
per
Service
($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2014
1
J3301
Triamcinolone acet inj nos
19.5
1,641
11.88
2,642
7.38
11,168
1.75
14.8
2
J1100
Dexamethasone sodium phos
2.6
982
2.65
2,461
1.06
19,556
0.13
2.0
3
J3420
Vitamin b12 injection
4.7
581
8.09
2,327
2.02
2,331
2.02
3.4
4
J1030
Methylprednisolone 40 mg inj
7.8
1,192
6.54
1,880
4.15
2,656
2.94
6.0
5
J7613
Albuterol non-comp unit
18.1
530
34.15
1,647
10.99
354,647
0.05
13.4
6
J7620
Albuterol ipratrop non-comp
26.9
409
65.77
1,508
17.84
151,477
0.18
20.0
7
J1040
Methylprednisolone 80 mg inj
9.7
946
10.25
1,491
6.51
1,732
5.60
7.4
8
Q9967
Locm 300-399mg/ml
iodine,1ml
15.8
827
19.11
1,160
13.62
86,806
0.18
12.4
9
J0702
Betamethasone acet&sod phosp
14.6
721
20.25
1,110
13.15
2,611
5.59
11.1
10
J0696
Ceftriaxone sodium injection
2.6
598
4.35
1,019
2.55
3,857
0.67
2.0
Medicare Part B Spending, Top 10
122.3
92.5
Medicare Part B Spending, All Drugs
21,597.8
17,240.2

62
Table A-3 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number of Services
Year
Rank
HCPCS
Description
Total
Payment
($M)
Beneficiaries
(1000s)
Spending
per
Beneficiary
($)
Services
(1000s)
Spending
per
Service
($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2015
1
J3301
Triamcinolone acet inj nos
20.8
1,724
12.06
2,783
7.47
11,915
1.75
15.8
2
J1100
Dexamethasone sodium phos
2.7
1,032
2.62
2,483
1.09
19,403
0.14
2.1
3
J3420
Vitamin b12 injection
6.8
572
11.89
2,367
2.87
2,369
2.87
5.0
4
J1030
Methylprednisolone 40 mg inj
9.8
1,233
7.95
1,943
5.04
2,774
3.53
7.5
5
J7613
Albuterol non-comp unit
20.6
517
39.85
1,556
13.24
321,972
0.06
15.4
6
J1040
Methylprednisolone 80 mg inj
11.5
960
11.98
1,511
7.61
1,759
6.54
8.7
7
J7620
Albuterol ipratrop non-comp
23.6
416
56.73
1,457
16.20
144,047
0.16
17.5
8
Q9967
Locm 300-399mg/ml
iodine,1ml
13.2
837
15.77
1,165
11.33
87,560
0.15
10.3
9
J0702
Betamethasone acet&sod phosp
15.9
750
21.20
1,156
13.75
2,744
5.79
12.1
10
J0696
Ceftriaxone sodium injection
2.8
634
4.42
1,072
2.61
4,071
0.69
2.1
Medicare Part B Spending, Top 10
127.7
96.5
Medicare Part B Spending, All Drugs
23,813.3
19,007.1

63
Table A-3 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number of Services
Year
Rank
HCPCS
Description
Total
Payment
($M)
Beneficiaries
(1000s)
Spending
per
Beneficiary
($)
Services
(1000s)
Spending
per
Service
($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2016
1
J3301
Triamcinolone acet inj nos
22.5
1,819
12.37
2,950
7.63
12,824
1.75
16.9
2
J1100
Dexamethasone sodium phos
2.6
1,091
2.38
2,536
1.03
19,762
0.13
2.0
3
J3420
Vitamin b12 injection
9.1
584
15.58
2,448
3.72
2,454
3.71
6.8
4
J1030
Methylprednisolone 40 mg inj
13.2
1,254
10.53
1,969
6.70
2,840
4.65
10.0
5
J7613
Albuterol non-comp unit
14.3
510
28.04
1,508
9.48
304,221
0.05
10.5
6
J1040
Methylprednisolone 80 mg inj
15.6
960
16.25
1,507
10.35
1,763
8.85
11.7
7
J7620
Albuterol ipratrop non-comp
22.5
426
52.82
1,439
15.64
139,231
0.16
16.7
8
Q9967
Locm 300-399mg/ml
iodine,1ml
11.1
864
12.85
1,209
9.18
90,733
0.12
8.7
9
J0702
Betamethasone acet&sod phosp
17.0
777
21.88
1,204
14.12
2,922
5.82
12.9
10
J0696
Ceftriaxone sodium injection
2.5
642
3.89
1,091
2.29
4,165
0.60
1.8
Medicare Part B Spending, Top 10
130.4
98.0
Medicare Part B Spending, All Drugs
27,266.7
21,789.6

64
Table A-3 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part B, Ranked by Total Number of Services
Year
Rank
HCPCS
Description
Total
Payment
($M)
Beneficiaries
(1000s)
Spending
per
Beneficiary
($)
Services
(1000s)
Spending
per
Service
($)
Units
(1000s)
Spending
per Unit
($)
Medicare
Payment net
of Cost-
Sharing ($M)
2017
1
J3301
Triamcinolone acet inj nos
24.1
1,873
12.87
3,033
7.95
13,373
1.80
18.0
2
J1100
Dexamethasone sodium phos
2.3
1,145
2.01
2,538
0.91
19,677
0.12
1.7
3
J3420
Vitamin b12 injection
6.8
571
11.91
2,399
2.83
2,400
2.83
4.8
4
J1030
Methylprednisolone 40 mg inj
16.2
1,258
12.88
1,966
8.24
2,840
5.70
12.2
5
J7613
Albuterol non-comp unit
13.6
528
25.76
1,481
9.18
288,582
0.05
9.9
6
J1040
Methylprednisolone 80 mg inj
18.8
939
20.02
1,474
12.75
1,728
10.88
14.0
7
J7620
Albuterol ipratrop non-comp
19.9
459
43.36
1,451
13.71
136,709
0.15
14.5
8
Q9967
Locm 300-399mg/ml
iodine,1ml
11.2
872
12.84
1,209
9.26
91,796
0.12
8.7
9
J0702
Betamethasone acet&sod phosp
20.1
781
25.74
1,209
16.63
2,982
6.74
15.2
10
J0178
Aflibercept injection
2,473.4
230
10753.91
1,166
2121.27
2,573
961.29
1,967.3
Medicare Part B Spending, Top 10
2,606.4
2,066.3
Medicare Part B Spending, All Drugs
30,294.0
24,260.5
Source: Analysis of carrier, durable medical, and outpatient claims data 2006-2017 by Acumen for ASPE. Data include Part B covered drugs administered in
physicians' offices and furnished by suppliers, covered drugs in hospital outpatient departments; and reflect only Part B drugs paid under the average sales price
plus 6 percent (ASP). The Healthcare Common Procedure Coding System (HCPCS) codes and prices for carrier and DM were obtained from the CMS ASP file,
those for OP come from the CMS Addendum B file. Lines with denied payments or Medicare as secondary payer were dropped. Spending per Beneficiary,
Spending per Service, and Spending per Unit include beneficiary cost-sharing and include the sequester.

65
Table A-4 Top 10 High-Cost Prescribed Drugs, Medicare Part D, Ranked by Total Spending
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC per
Claim
($)
GDC per
Unit ($)
GDC per
User ($)
Total
Estimated
Government
(net of Cost-
Sharing)
2008
1
Lipitor
2,397,810,581
18,446,176
812,966,497
3,124,832
129.99
2.95
767.34
1,349,438,705
2
Plavix
2,305,109,427
14,268,262
601,948,554
2,156,115
161.56
3.83
1,069.10
1,319,750,590
3
Nexium
1,487,032,499
7,910,170
314,143,679
1,480,139
187.99
4.73
1,004.66
978,699,313
4
Seroquel
1,462,192,107
6,038,847
306,095,001
767,140
242.13
4.78
1,906.03
1,152,462,605
5
Aricept
1,326,127,575
7,102,698
256,076,304
1,041,464
186.71
5.18
1,273.33
803,585,924
6
Zyprexa
1,229,036,652
2,814,458
97,134,631
339,610
436.69
12.65
3,618.96
1,004,409,138
7
Advair Diskus
1,213,291,695
5,507,722
413,199,209
1,278,659
220.29
2.94
948.88
774,547,166
8
Actos
1,062,971,313
5,174,585
220,536,473
846,079
205.42
4.82
1,256.35
663,050,193
9
Prevacid
947,177,275
5,176,233
206,602,227
949,056
182.99
4.58
998.02
780,493,358
10
Abilify
837,069,575
1,841,706
59,250,742
279,425
454.51
14.13
2,995.69
696,702,527
Medicare Part D Spending, Top 10
14,267,818,700
Medicare Part D Spending, All Drugs
68,223,634,359

66
Table A-4 continued Top 10 High-Cost Prescribed Drugs, Medicare Part D, Ranked by Total Spending
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2009
1
Plavix
2,721,310,465
15,091,663
577,272,241
2,308,937
180.32
4.71
1,178.60
1,527,909,326
2
Lipitor
2,288,382,394
16,124,575
653,606,534
2,797,115
141.92
3.50
818.12
1,236,373,392
3
Nexium
1,676,837,923
8,185,878
316,028,844
1,538,118
204.85
5.31
1,090.19
1,097,548,465
4
Seroquel
1,646,967,166
6,114,594
335,895,271
778,728
269.35
4.90
2,114.95
1,276,249,228
5
Aricept
1,585,272,216
7,537,448
247,633,819
1,107,884
210.32
6.40
1,430.90
939,773,260
6
Advair Diskus
1,394,191,141
5,919,710
427,927,470
1,360,522
235.52
3.26
1,024.75
871,329,957
7
Zyprexa
1,341,353,367
2,730,627
117,748,028
328,810
491.23
11.39
4,079.42
1,085,095,774
8
Actos
1,188,283,678
5,105,629
213,588,728
839,028
232.74
5.56
1,416.26
729,316,458
9
Abilify
1,079,226,741
2,209,956
73,310,515
337,848
488.35
14.72
3,194.42
879,179,487
10
Flomax
958,921,116
6,533,776
288,158,779
1,226,255
146.76
3.33
781.99
500,814,755
Medicare Part D Spending, Top 10
15,880,746,208
Medicare Part D Spending, All Drugs
73,519,974,148

67
Table A-4 continued Top 10 High-Cost Prescribed Drugs, Medicare Part D, Ranked by Total Spending
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC per
Claim
($)
GDC per
Unit ($)
GDC per
User ($)
Total
Estimated
Government
(net of Cost-
Sharing)
2010
1
Plavix
3,105,178,149
15,235,338
573,566,385
2,371,198
203.81
5.41
1,309.54
1,772,649,718
2
Lipitor
2,280,928,513
14,347,583
586,401,338
2,457,157
158.98
3.89
928.28
1,244,905,964
3
Nexium
1,858,708,061
8,488,232
332,456,521
1,555,953
218.97
5.59
1,194.58
1,270,385,767
4
Seroquel
1,817,003,253
5,989,979
282,975,890
771,668
303.34
6.42
2,354.64
1,415,035,158
5
Aricept
1,796,634,659
7,206,450
238,494,833
1,096,902
249.31
7.53
1,637.92
1,083,048,836
6
Advair Diskus
1,517,451,761
6,113,502
423,206,311
1,402,405
248.21
3.59
1,082.04
959,574,589
7
Zyprexa
1,487,036,867
2,688,409
91,173,519
325,262
553.13
16.31
4,571.81
1,215,283,825
8
Actos
1,270,431,244
5,019,208
199,166,341
853,424
253.11
6.38
1,488.63
788,749,236
9
Abilify
1,226,393,347
2,296,670
71,850,284
338,610
533.99
17.07
3,621.85
1,003,896,670
10
Crestor
1,095,786,398
7,020,891
273,762,886
1,304,029
156.08
4.00
840.31
617,814,149
Medicare Part D Spending, Top 10
17,455,552,250
Medicare Part D Spending, All Drugs
77,418,365,994

68
Table A-4 continued Top 10 High-Cost Prescribed Drugs, Medicare Part D, Ranked by Total Spending
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2011
1
Plavix
3,656,699,410
15,209,882
591,894,651
2,395,374
240.42
6.18
1,526.57
2,047,817,281
2
Lipitor
2,672,879,391
13,773,982
584,820,877
2,607,477
194.05
4.57
1,025.08
1,441,606,472
3
Seroquel
2,045,283,552
5,937,351
280,754,054
762,673
344.48
7.28
2,681.73
1,568,902,343
4
Nexium
1,970,100,370
8,223,817
334,287,800
1,516,235
239.56
5.89
1,299.34
1,334,765,962
5
Advair Diskus
1,664,854,955
6,168,133
436,745,338
1,402,384
269.91
3.81
1,187.16
1,028,444,936
6
Zyprexa
1,625,313,684
2,408,081
81,766,333
312,584
674.94
19.88
5,199.61
1,317,403,357
7
Abilify
1,469,648,771
2,447,937
77,464,364
360,656
600.36
18.97
4,074.93
1,186,107,582
8
Crestor
1,416,291,996
7,826,713
318,709,283
1,496,029
180.96
4.44
946.70
782,545,280
9
Actos
1,294,116,294
4,484,509
184,544,494
778,684
288.57
7.01
1,661.93
779,666,784
10
Spiriva
1,288,422,807
4,815,356
172,359,461
981,349
267.57
7.48
1,312.91
765,469,240
Medicare Part D Spending, Top 10
19,103,611,230
Medicare Part D Spending, All Drugs
84,639,248,565

69
Table A-4 continued Top 10 High-Cost Prescribed Drugs, Medicare Part D, Ranked by Total Spending
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC per
Claim
($)
GDC per
Unit ($)
GDC per
User ($)
Total
Estimated
Government
(net of Cost-
Sharing)
2012
1
Nexium
2,124,218,164
7,911,790
337,691,577
1,434,163
268.49
6.29
1,481.16
1,388,577,944
2
Advair Diskus
1,886,299,997
6,288,946
452,552,646
1,424,280
299.94
4.17
1,324.39
1,131,579,989
3
Crestor
1,789,810,336
8,624,468
361,021,613
1,598,135
207.53
4.96
1,119.94
971,693,605
4
Abilify
1,758,037,036
2,571,956
82,133,718
376,576
683.54
21.40
4,668.48
1,388,120,259
5
Plavix
1,690,659,194
6,468,845
258,051,370
2,032,708
261.35
6.55
831.73
968,418,803
6
Spiriva
1,603,172,111
5,275,894
193,071,238
1,065,523
303.87
8.30
1,504.59
928,329,554
7
Cymbalta
1,453,805,691
5,776,278
243,795,227
912,752
251.69
5.96
1,592.77
924,397,167
8
Atorvastatin Calcium
1,347,821,093
16,152,770
734,283,287
3,655,888
83.44
1.84
368.67
752,733,860
9
Namenda
1,327,400,166
5,425,391
345,808,953
757,593
244.66
3.84
1,752.13
750,941,044
10
Januvia
1,112,999,393
3,766,131
149,648,728
666,558
295.53
7.44
1,669.77
645,275,326
Medicare Part D Spending, Top 10
16,094,223,182
Medicare Part D Spending, All Drugs
89,524,597,876

70
Table A-4 continued Top 10 High-Cost Prescribed Drugs, Medicare Part D, Ranked by Total Spending
Year
Rank
Brand Name
Gross Drug Cost
Claims
Units
Dispensed
Beneficiaries
GDC per
Claim
($)
GDC per
Unit ($)
GDC per
User ($)
Total
Estimated
Government
(net of Cost-
Sharing)
2013
1
Nexium
2,526,329,043
8,192,355
360,017,674
1,483,991
308.38
7.02
1,702.39
1,516,813,929
2
Advair Diskus
2,263,078,809
6,605,655
492,979,426
1,527,214
342.60
4.59
1,481.83
1,246,651,185
3
Crestor
2,216,220,164
9,066,550
401,735,047
1,732,744
244.44
5.52
1,279.02
1,098,929,360
4
Abilify
2,107,112,690
2,886,903
86,132,660
396,757
729.89
24.46
5,310.84
1,593,920,589
5
Cymbalta
1,961,193,714
6,887,719
279,891,880
1,032,763
284.74
7.01
1,898.98
1,147,155,273
6
Spiriva
1,958,639,360
5,735,304
218,670,547
1,181,612
341.51
8.96
1,657.60
1,043,670,202
7
Namenda
1,564,805,787
6,878,538
347,984,961
798,719
227.49
4.50
1,959.14
812,379,862
8
Januvia
1,460,557,480
4,358,892
174,161,373
761,772
335.08
8.39
1,917.32
781,403,946
9
Lantus Solostar
1,372,036,942
3,863,962
80,270,373
862,886
355.09
17.09
1,590.06
719,320,445
10
Revlimid
1,349,922,413
153,778
3,227,508
24,636
8,778.38
418.26
54,794.71
853,549,484
Medicare Part D Spending, Top 10
18,779,896,401
Medicare Part D Spending, All Drugs
103,334,267,690

71
Table A-4 continued Top 10 High-Cost Prescribed Drugs, Medicare Part D, Ranked by Total Spending
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC per
Claim
($)
GDC per
Unit ($)
GDC per
User ($)
Total
Estimated
Government
(net of Cost-
Sharing)
2014
1
Sovaldi
3,102,238,669
109,386
3,050,759
33,013
28,360.47
1,016.87
93,970.21
2,311,510,097
2
Nexium
2,658,298,959
7,533,546
340,109,618
1,405,344
352.86
7.82
1,891.56
1,568,122,484
3
Crestor
2,541,222,052
9,065,383
418,795,544
1,751,569
280.32
6.07
1,450.83
1,205,292,977
4
Abilify
2,524,876,962
2,961,393
88,079,850
404,785
852.60
28.67
6,237.58
1,878,444,406
5
Advair Diskus
2,273,757,268
6,087,461
459,844,720
1,419,693
373.51
4.94
1,601.58
1,185,536,668
6
Spiriva
2,156,225,605
5,847,089
228,758,323
1,211,107
368.77
9.43
1,780.38
1,105,438,708
7
Lantus Solostar
2,014,697,663
4,437,229
92,674,101
972,529
454.04
21.74
2,071.61
1,011,638,558
8
Januvia
1,773,791,068
4,493,183
183,418,827
789,395
394.77
9.67
2,247.03
902,974,243
9
Lantus
1,724,176,517
4,281,217
80,133,256
786,572
402.73
21.52
2,192.01
935,291,308
10
Revlimid
1,670,480,534
178,256
3,705,059
27,137
9,371.24
450.86
61,557.30
972,203,098
Medicare Part D Spending, Top 10
22,439,765,297
Medicare Part D Spending, All Drugs
121,001,364,760

72
Table A-4 continued Top 10 High-Cost Prescribed Drugs, Medicare Part D, Ranked by Total Spending
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC per
Claim
($)
GDC per
Unit ($)
GDC per
User ($)
Total
Estimated
Government
(net of Cost-
Sharing)
2015
1
Harvoni
7,031,577,074
225,872
6,238,770
75,734
31,130.80
1,127.08
92,845.71
4,771,342,376
2
Crestor
2,883,152,025
8,712,209
416,827,231
1,733,013
330.93
6.92
1,663.66
1,270,232,886
3
Lantus Solostar
2,483,345,299
4,861,704
100,569,084
1,041,383
510.80
24.69
2,384.66
1,175,390,020
4
Advair Diskus
2,270,170,360
5,649,525
430,440,516
1,321,521
401.83
5.27
1,717.85
1,084,451,912
5
Spiriva
2,191,689,316
5,447,163
212,261,764
1,142,233
402.35
10.33
1,918.78
1,042,474,509
6
Januvia
2,131,939,796
4,623,562
193,043,850
828,797
461.10
11.04
2,572.33
1,011,428,998
7
Revlimid
2,077,659,819
204,888
4,240,785
30,455
10,140.47
489.92
68,220.65
1,121,468,296
8
Nexium
2,012,910,273
5,470,939
251,472,740
1,127,926
367.93
8.00
1,784.61
1,184,335,248
9
Lantus
1,876,332,368
3,993,873
75,074,893
733,952
469.80
24.99
2,556.48
961,395,647
10
Lyrica
1,765,662,458
4,731,568
346,639,553
825,518
373.17
5.09
2,138.85
963,667,184
Medicare Part D Spending, Top 10
26,724,438,787
Medicare Part D Spending, All Drugs
136,841,893,327

73
Table A-4 continued Top 10 High-Cost Prescribed Drugs, Medicare Part D, Ranked by Total Spending
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC per
Claim
($)
GDC per
Unit ($)
GDC per
User ($)
Total
Estimated
Government
(net of Cost-
Sharing)
2016
1
Harvoni
4,399,701,570
141,708
3,933,297
52,801
31,047.66
1,118.58
83,326.10
2,957,983,690
2
Revlimid
2,661,602,600
239,093
4,896,215
35,373
11,132.08
543.60
75,243.90
1,398,050,043
3
Lantus Solostar
2,526,426,478
5,029,203
102,691,210
1,075,429
502.35
24.60
2,349.23
1,161,422,334
4
Januvia
2,440,387,636
4,743,201
203,654,169
864,600
514.50
11.98
2,822.56
1,119,693,056
5
Crestor
2,323,124,806
6,013,491
285,722,221
1,560,325
386.32
8.13
1,488.87
1,066,537,566
6
Advair Diskus
2,320,125,120
5,195,116
401,111,250
1,196,197
446.60
5.78
1,939.58
1,070,421,686
7
Lyrica
2,097,128,770
4,939,772
364,403,474
852,328
424.54
5.75
2,460.47
1,123,526,220
8
Xarelto
1,954,991,423
4,403,860
167,518,130
807,951
443.93
11.67
2,419.69
729,566,986
9
Eliquis
1,926,315,154
4,456,265
328,547,796
827,075
432.27
5.86
2,329.07
690,088,508
10
Spiriva
1,819,080,770
4,153,674
164,588,242
903,637
437.95
11.05
2,013.07
817,848,163
Medicare Part D Spending, Top 10
24,468,884,327
Medicare Part D Spending, All Drugs
145,439,626,252

74
Table A-4 continued Top 10 High-Cost Prescribed Drugs, Medicare Part D, Ranked by Total Spending
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC per
Claim
($)
GDC per
Unit ($)
GDC per
User ($)
Total
Estimated
Government
(net of Cost-
Sharing)
2017
1
Revlimid
3,312,773,264
259,693
5,283,681
37,457
12,756.50
626.98
88,442.03
1,654,079,040
2
Eliquis
3,078,896,052
6,352,153
480,038,487
1,143,117
484.70
6.41
2,693.42
1,032,933,807
3
Januvia
2,786,061,883
4,833,933
216,563,622
893,614
576.36
12.86
3,117.75
1,220,938,158
4
Lantus Solostar
2,632,358,172
5,236,014
106,072,375
1,112,417
502.74
24.82
2,366.34
1,151,976,902
5
Xarelto
2,611,788,536
5,246,063
204,631,912
952,928
497.86
12.76
2,740.80
949,587,410
6
Harvoni
2,555,839,934
81,898
2,282,783
32,397
31,207.60
1,119.62
78,891.25
1,611,125,318
7
Lyrica
2,516,912,784
5,071,920
377,949,770
876,088
496.24
6.66
2,872.90
1,275,493,366
8
Advair Diskus
2,374,829,262
4,881,672
381,843,204
1,135,883
486.48
6.22
2,090.73
1,040,538,861
9
Humira Pen
2,015,734,946
370,804
901,506
51,832
5,436.12
2,235.96
38,889.78
1,124,269,617
10
Spiriva
1,662,019,377
3,358,319
136,812,844
717,857
494.90
12.15
2,315.25
702,724,704
Medicare Part D Spending, Top 10
25,547,214,209
Medicare Part D Spending, All Drugs
154,229,569,493
Source: Analysis of Medicare claims data (carrier, outpatient, and Part D event) by Acumen for ASPE.

75
Table A-5 Top 10 Highest-Cost Drugs, Medicare Part D, Ranked by Spending per
Unit
Year
Rank
Brand Name
GDC per Unit ($)
Gross Drug Cost ($)
2008
1
Retisert
19,784.33
59,353
2
Somatuline Depot
7,648.06
341,103
3
Animas 2020
5,663.20
5,663
4
Arcalyst
5,198.06
1,070,801
5
Viadur
4,905.50
73,582
6
Vantas
4,738.90
464,412
7
H.P. Acthar
4,547.26
6,939,125
8
Fabrazyme
3,443.73
1,422,259
9
Neulasta
3,399.84
19,494,056
10
Herceptin
2,728.76
2,326,592
Year
Rank
Brand Name
GDC per Unit ($)
Gross Drug Cost ($)
2009
1
Lucentis
41,126.75
164,507
2
Ilaris
16,280.16
32,560
3
Macugen
11,549.38
25,986
4
Stelara
9,541.40
1,521,853
5
Somatuline Depot
7,875.82
1,056,935
6
Mozobil
5,564.27
687,744
7
Arcalyst
5,235.69
2,209,461
8
Neulasta
5,009.98
22,476,021
9
H.P. Acthar
4,863.39
9,118,851
10
Vantas
4,725.97
264,654
Year
Rank
Brand Name
GDC per Unit ($)
Gross Drug Cost ($)
2010
1
Lucentis
22,702.37
340,535
2
Ilaris
16,322.31
440,702
3
Macugen
11,403.58
28,737
4
Stelara
9,739.63
16,966,439
5
Somatuline Depot
7,898.25
2,137,268
6
Mozobil
5,647.74
637,066
7
Jevtana
5,441.85
334,674
8
Neulasta
5,200.94
23,452,906
9
Arcalyst
5,196.49
2,722,963
10
H.P. Acthar
4,838.15
19,304,226

76
Table A-5 continued Top 10 Highest-Cost Drugs, Medicare Part D, Ranked by Spending per
Unit
Year
Rank
Brand Name
GDC per Unit ($)
Gross Drug Cost ($)
2011
1
Lucentis
38,985.58
1,062,357
2
Eylea
37,720.00
1,886
3
Ilaris
16,278.40
797,641
4
Stelara
10,756.61
31,699,743
5
Somatuline Depot
8,342.85
3,539,038
6
Neulasta
5,952.62
28,322,581
7
Mozobil
5,703.27
1,142,936
8
Sylatron 4-Pack
5,567.04
116,908
9
Jevtana
5,528.20
1,218,969
10
H.P. Acthar
5,303.69
49,456,911
Year
Rank
Brand Name
GDC per Unit ($)
Gross Drug Cost ($)
2012
1
Eylea
38,331.14
948,696
2
Lucentis
36,617.53
1,812,568
3
Supprelin La
16,614.57
16,615
4
Ilaris
16,282.76
1,742,255
5
Stelara
11,838.64
50,290,538
6
Somatuline Depot
8,953.32
5,273,507
7
Neulasta
6,308.60
33,028,023
8
H.P. Acthar
5,977.25
141,451,608
9
Mozobil
5,786.18
1,451,174
10
Jevtana
5,680.17
1,644,409
Year
Rank
Brand Name
GDC per Unit ($)
Gross Drug Cost ($)
2013
1
Eylea
36,383.59
1,619,070
2
Lucentis
34,887.59
2,300,139
3
Gattex
20,118.72
15,169,518
4
Ilaris
16,413.48
2,018,858
5
Stelara
13,330.93
89,503,840
6
Somatuline Depot
9,834.27
7,505,515
7
Neulasta
6,810.18
46,715,657
8
H.P. Acthar
6,158.54
262,581,602
9
Mozobil
5,930.15
1,730,535
10
Jevtana
5,770.35
1,834,972

77
Table A-5 continued Top 10 Highest-Cost Drugs, Medicare Part D, Ranked by Spending per
Unit
Year
Rank
Brand Name
GDC per Unit ($)
Gross Drug Cost ($)
2014
1
Eylea
38,360.36
2,911,551
2
Lucentis
36,442.45
2,653,011
3
Gattex
28,077.72
46,665,171
4
Ilaris
16,381.65
4,095,412
5
Stelara
14,510.12
156,883,419
6
Jetrea
11,850.00
18,960
7
Somatuline Depot
11,421.89
10,216,877
8
Krystexxa
7,744.50
774,450
9
Neulasta
7,384.42
57,763,922
10
H.P. Acthar
6,497.68
391,089,016
Year
Rank
Brand Name
GDC per Unit ($)
Gross Drug Cost ($)
2015
1
Eylea
35,457.43
6,431,977
2
Lucentis
35,086.39
3,089,357
3
Gattex
24,311.15
74,173,306
4
Supprelin La
23,426.95
23,427
5
Lemtrada
16,856.91
4,349,084
6
Ilaris
16,395.66
4,295,664
7
Stelara
16,219.81
205,334,691
8
Krystexxa
14,054.97
224,880
9
Somatuline Depot
12,493.25
13,715,094
10
Jetrea
11,850.00
42,660
Year
Rank
Brand Name
GDC per Unit ($)
Gross Drug Cost ($)
2016
1
Eylea
37,668.61
9,503,791
2
Lucentis
31,859.70
2,798,874
3
Gattex
30,719.39
103,403,928
4
Lemtrada
17,140.23
6,910,940
5
Ilaris
16,446.43
5,032,608
6
Krystexxa
16,273.54
4,995,976
7
Somatuline Depot
13,662.80
18,752,192
8
Stelara
13,181.51
265,146,000
9
Jetrea
11,850.00
14,220
10
Signifor Lar
11,505.09
2,945,303
78
Table A-5 continued Top 10 Highest-Cost Drugs, Medicare Part D, Ranked by Spending per
Unit
Year
Rank
Brand Name
GDC per Unit ($)
Gross Drug Cost ($)
2017
1
Eylea
38,341.55
12,957,526
2
Lucentis
37,542.48
3,202,374
3
Gattex
35,331.78
164,787,435
4
Spinraza
25,650.18
1,154,258
5
Krystexxa
19,152.45
13,559,936
6
Lemtrada
18,062.64
7,738,033
7
Jetrea
16,868.29
16,868
8
Ilaris
16,621.41
8,111,250
9
Somatuline Depot
15,515.58
25,740,343
10
Signifor Lar
11,541.03
2,654,436
Source: Analysis of Medicare claims data (carrier, outpatient, and Prescription Drug Event) by Acumen for ASPE.

79
Table A-6 Top 10 Most Frequently Prescribed Drugs, Medicare Part D, Ranked by Number of Claims
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2008
1
Lisinopril
345,033,179
28,462,483
1,384,923,981
4,672,266
12.12
0.25
73.85
212,887,358
2
Simvastatin
694,107,981
27,433,873
1,210,910,748
4,849,045
25.30
0.57
143.14
446,009,244
3
Furosemide
131,780,371
23,854,831
1,198,221,609
4,094,514
5.52
0.11
32.18
74,199,762
4
Hydrocodone-
Acetaminophen
312,498,142
23,826,679
1,706,051,986
5,434,878
13.12
0.18
57.50
226,672,109
5
Levothyroxine Sodium
207,775,091
21,857,844
878,139,406
3,105,090
9.51
0.24
66.91
118,742,442
6
Amlodipine Besylate
401,506,683
19,669,864
887,503,731
3,193,845
20.41
0.45
125.71
252,557,300
7
Lipitor
2,397,810,581
18,446,176
812,966,497
3,124,832
129.99
2.95
767.34
1,349,438,705
8
Omeprazole
695,597,941
16,717,326
807,699,386
3,280,259
41.61
0.86
212.06
501,866,622
9
Hydrochlorothiazide
94,078,157
16,464,783
771,308,882
3,029,210
5.71
0.12
31.06
50,701,104
10
Atenolol
111,703,979
16,257,632
983,693,838
2,574,951
6.87
0.11
43.38
60,040,517
Medicare Part D Spending, Top 10
5,391,892,105
Medicare Part D Spending, All Drugs
68,223,634,359

80
Table A-6 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part D, Ranked by Number of Claims
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2009
1
Simvastatin
739,059,008
34,591,760
1,507,177,776
6,049,843
21.37
0.49
122.16
456,274,026
2
Lisinopril
342,621,227
30,379,362
1,439,338,602
5,160,809
11.28
0.24
66.39
206,411,392
3
Hydrocodone-
Acetaminophen
352,138,198
26,105,718
1,957,212,654
5,919,000
13.49
0.18
59.49
253,321,954
4
Furosemide
134,203,910
23,854,239
1,155,844,748
4,211,379
5.63
0.12
31.87
74,709,691
5
Levothyroxine Sodium
221,245,773
23,635,170
936,334,037
3,418,522
9.36
0.24
64.72
123,633,095
6
Amlodipine Besylate
387,132,689
22,999,998
973,035,446
3,787,990
16.83
0.40
102.20
230,362,091
7
Omeprazole
825,100,521
21,535,305
1,042,306,325
4,212,038
38.31
0.79
195.89
581,439,555
8
Metoprolol Tartrate
129,371,197
18,572,045
1,265,381,666
3,162,689
6.97
0.10
40.91
77,522,705
9
Metformin Hcl
247,928,104
16,815,327
1,466,211,597
2,790,467
14.74
0.17
88.85
163,227,762
10
Hydrochlorothiazide
94,645,156
16,518,919
721,527,165
3,138,395
5.73
0.13
30.16
49,631,159
Medicare Part D Spending, Top 10
3,473,445,785
Medicare Part D Spending, All Drugs
73,519,974,148

81
Table A-6 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part D, Ranked by Number of Claims
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2010
1
Simvastatin
675,428,276
39,103,885
1,719,786,341
6,848,740
17.27
0.39
98.62
395,759,073
2
Lisinopril
319,684,694
31,820,291
1,529,230,022
5,533,553
10.05
0.21
57.77
184,471,782
3
Hydrocodone-
Acetaminophen
380,274,782
28,106,992
2,077,239,358
6,402,734
13.53
0.18
59.39
270,596,470
4
Amlodipine Besylate
369,003,406
25,763,034
1,105,779,372
4,302,789
14.32
0.33
85.76
206,241,277
5
Levothyroxine Sodium
238,534,443
25,517,106
1,039,522,136
3,736,012
9.35
0.23
63.85
129,477,775
6
Omeprazole
774,710,860
25,015,779
1,203,076,099
4,819,188
30.97
0.64
160.76
535,130,604
7
Furosemide
134,475,703
23,836,584
1,170,099,919
4,297,705
5.64
0.11
31.29
73,471,916
8
Metoprolol Tartrate
119,054,604
18,307,777
1,286,033,586
3,105,281
6.50
0.09
38.34
69,510,124
9
Metformin Hcl
220,036,557
17,887,047
1,581,764,097
3,056,379
12.30
0.14
71.99
141,515,237
10
Hydrochlorothiazide
93,037,111
16,505,621
744,455,405
3,209,348
5.64
0.12
28.99
45,743,869
Medicare Part D Spending, Top 10
3,324,240,436
Medicare Part D Spending, All Drugs
77,418,365,994

82
Table A-6 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part D, Ranked by Number of Claims
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2011
1
Simvastatin
594,308,786
40,947,753
1,893,225,089
7,410,716
14.51
0.31
80.20
321,402,216
2
Lisinopril
322,301,752
32,756,394
1,656,392,986
5,883,803
9.84
0.19
54.78
178,264,025
3
Hydrocodone-
Acetaminophen
430,121,896
31,363,719
2,353,900,477
7,091,044
13.71
0.18
60.66
305,490,837
4
Amlodipine Besylate
383,051,253
27,946,424
1,263,380,357
4,798,211
13.71
0.30
79.83
210,220,596
5
Omeprazole
674,651,002
27,327,024
1,350,133,346
5,328,317
24.69
0.50
126.62
439,681,288
6
Levothyroxine Sodium
256,520,252
27,308,831
1,174,724,349
4,132,613
9.39
0.22
62.07
132,976,493
7
Furosemide
133,472,355
23,915,709
1,217,118,146
4,439,200
5.58
0.11
30.07
71,849,462
8
Metformin Hcl
214,445,823
18,853,644
1,740,552,440
3,323,588
11.37
0.12
64.52
133,844,371
9
Metoprolol Tartrate
126,066,900
18,486,839
1,373,459,423
3,245,116
6.82
0.09
38.85
71,778,272
10
Hydrochlorothiazide
95,245,932
16,893,836
800,829,135
3,401,785
5.64
0.12
28.00
44,112,190
Medicare Part D Spending, Top 10
3,230,185,951
Medicare Part D Spending, All Drugs
84,639,248,565

83
Table A-6 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part D, Ranked by Number of Claims
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2012
1
Simvastatin
491,935,459
37,795,740
1,834,651,660
7,036,474
13.02
0.27
69.91
264,666,528
2
Lisinopril
292,683,599
34,012,396
1,829,754,052
6,276,766
8.61
0.16
46.63
159,839,012
3
Hydrocodone-
Acetaminophen
462,029,176
32,935,666
2,514,651,919
7,463,025
14.03
0.18
61.91
322,540,612
4
Amlodipine Besylate
311,355,670
30,511,012
1,458,302,520
5,393,157
10.20
0.21
57.73
161,173,503
5
Levothyroxine Sodium
286,973,233
30,273,540
1,446,101,520
4,917,601
9.48
0.20
58.36
140,672,726
6
Omeprazole
591,537,032
29,740,834
1,535,244,351
5,776,695
19.89
0.39
102.40
362,493,693
7
Furosemide
128,685,671
24,196,918
1,287,834,193
4,603,545
5.32
0.10
27.95
69,277,692
8
Metformin Hcl
209,919,385
19,993,847
1,919,371,720
3,625,576
10.50
0.11
57.90
128,292,608
9
Metoprolol Tartrate
133,800,561
19,548,179
1,546,729,995
3,547,946
6.84
0.09
37.71
76,244,196
10
Hydrochlorothiazide
98,048,635
17,097,618
848,605,473
3,513,708
5.73
0.12
27.90
44,579,142
Medicare Part D Spending, Top 10
3,006,968,420
Medicare Part D Spending, All Drugs
89,524,597,876

84
Table A-6 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part D, Ranked by Number of Claims
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2013
1
Lisinopril
307,023,086
36,880,032
2,038,993,274
7,006,455
8.32
0.15
43.82
165,132,109
2
Simvastatin
433,683,805
36,746,623
1,895,722,816
7,030,150
11.80
0.23
61.69
215,334,706
3
Levothyroxine Sodium
396,082,754
35,175,473
1,703,818,462
5,738,370
11.26
0.23
69.02
197,552,635
4
Hydrocodone-
Acetaminophen
567,716,086
34,757,836
2,665,245,306
8,086,390
16.33
0.21
70.21
397,778,705
5
Amlodipine Besylate
343,309,667
34,597,626
1,718,554,639
6,244,392
9.92
0.20
54.98
174,600,712
6
Omeprazole
641,680,721
32,217,559
1,723,529,693
6,378,142
19.92
0.37
100.61
390,294,673
7
Atorvastatin Calcium
910,773,818
26,672,648
1,321,516,281
5,345,618
34.15
0.69
170.38
467,007,915
8
Furosemide
144,859,527
26,440,872
1,368,750,164
5,002,906
5.48
0.11
28.96
81,770,831
9
Metformin Hcl
226,616,424
22,041,145
2,221,651,225
4,134,234
10.28
0.10
54.81
134,001,853
10
Metoprolol Tartrate
162,010,105
21,032,668
1,734,532,000
3,899,899
7.70
0.09
41.54
91,613,179
Medicare Part D Spending, Top 10
4,133,755,992
Medicare Part D Spending, All Drugs
103,334,267,690

85
Table A-6 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part D, Ranked by Number of Claims
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2014
1
Lisinopril
281,423,341
38,255,921
2,193,230,596
7,454,682
7.36
0.13
37.75
147,879,610
2
Levothyroxine Sodium
631,467,084
37,693,854
1,887,046,786
6,245,423
16.75
0.33
101.11
310,562,769
3
Amlodipine Besylate
303,562,645
36,323,451
1,880,188,070
6,749,857
8.36
0.16
44.97
150,729,392
4
Simvastatin
346,503,211
34,075,701
1,855,648,813
6,768,036
10.17
0.19
51.20
167,199,379
5
Hydrocodone-
Acetaminophen
676,232,255
33,444,013
2,573,595,306
8,005,680
20.22
0.26
84.47
477,865,084
6
Omeprazole
527,551,494
32,981,253
1,811,634,786
6,703,777
16.00
0.29
78.69
312,947,476
7
Atorvastatin Calcium
747,160,164
32,583,821
1,690,100,159
6,739,232
22.93
0.44
110.87
361,926,836
8
Furosemide
135,605,453
27,117,711
1,409,321,185
5,176,237
5.00
0.10
26.20
76,295,388
9
Metformin Hcl
203,817,147
23,461,778
2,444,099,802
4,509,843
8.69
0.08
45.19
117,724,449
10
Gabapentin
491,171,326
22,109,457
2,495,659,495
4,292,286
22.22
0.20
114.43
339,447,643
Medicare Part D Spending, Top 10
4,344,494,119
Medicare Part D Spending, All Drugs
121,001,364,760

86
Table A-6 continued Top 10 Most Frequently Prescribed Drugs, Medicare Part D, Ranked by Number of Claims
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2015
1
Levothyroxine Sodium
777,766,567
39,439,604
2,036,719,775
6,640,827
19.72
0.38
117.12
362,907,764
2
Lisinopril
252,964,497
39,119,711
2,318,174,238
7,766,977
6.47
0.11
32.57
127,546,282
3
Atorvastatin Calcium
711,994,465
38,601,142
2,078,721,827
8,070,332
18.44
0.34
88.22
346,025,754
4
Amlodipine Besylate
264,050,633
38,207,915
2,041,318,602
7,262,710
6.91
0.13
36.36
125,528,065
5
Omeprazole
446,602,260
33,470,470
1,883,411,677
6,941,057
13.34
0.24
64.34
259,949,018
6
Simvastatin
261,232,895
31,741,219
1,807,426,013
6,479,793
8.23
0.14
40.32
122,671,000
7
Hydrocodone-
Acetaminophen
727,097,347
29,544,091
2,359,202,982
7,482,217
24.61
0.31
97.18
504,564,352
8
Furosemide
137,899,185
27,544,913
1,446,438,171
5,314,942
5.01
0.10
25.95
67,083,903
9
Gabapentin
490,110,316
24,808,141
2,857,603,075
4,813,795
19.76
0.17
101.81
336,482,714
10
Metformin Hcl
178,290,718
24,608,787
2,641,097,030
4,835,118
7.25
0.07
36.87
99,519,224
Medicare Part D Spending, Top 10
4,248,008,883
Medicare Part D Spending, All Drugs
136,841,893,327

87
Table A-6 continued Top 10 Mostly Frequent Prescribed Drugs, Medicare Part D, Ranked by Number of Claims
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2016
1
Atorvastatin Calcium
757,045,111
44,481,095
2,491,418,627
9,407,705
17.02
0.30
80.47
343,684,650
2
Levothyroxine Sodium
806,722,765
41,037,321
2,190,683,270
7,027,034
19.66
0.37
114.80
330,868,103
3
Amlodipine Besylate
269,060,102
39,856,014
2,229,469,504
7,791,184
6.75
0.12
34.53
127,866,604
4
Lisinopril
259,639,984
39,461,800
2,424,552,511
8,008,316
6.58
0.11
32.42
125,385,580
5
Omeprazole
411,897,000
32,882,380
1,911,212,368
6,999,197
12.53
0.22
58.85
232,604,997
6
Simvastatin
247,488,652
29,680,713
1,767,731,328
6,199,770
8.34
0.14
39.92
107,544,771
7
Hydrocodone-
Acetaminophen
727,445,566
28,504,601
2,264,853,371
7,222,639
25.52
0.32
100.72
507,647,217
8
Furosemide
135,618,721
27,858,543
1,489,998,138
5,416,268
4.87
0.09
25.04
59,545,263
9
Gabapentin
488,072,139
27,547,913
3,254,818,622
5,346,793
17.72
0.15
91.28
321,746,793
10
Metformin Hcl
184,171,620
25,462,734
2,847,474,670
5,119,835
7.23
0.06
35.97
98,851,870
Medicare Part D Spending, Top 10
4,287,161,660
Medicare Part D Spending, All Drugs
145,439,626,252

88
Table A-6 continued Top 10 Mostly Frequent Prescribed Drugs, Medicare Part D, Ranked by Number of Claims
Year
Rank
Brand Name
Gross Drug
Cost
Claims
Units
Dispensed
Beneficiaries
GDC
per
Claim
($)
GDC per
Unit ($)
GDC
per User
($)
Total
Estimated
Government
(net of Cost-
Sharing)
2017
1
Atorvastatin Calcium
808,877,969
48,580,471
2,883,654,566
10,662,425
16.65
0.28
75.86
358,090,160
2
Levothyroxine Sodium
836,074,268
41,396,259
2,296,350,539
7,343,728
20.20
0.36
113.85
303,834,268
3
Amlodipine Besylate
267,095,429
40,572,462
2,389,099,937
8,300,738
6.58
0.11
32.18
125,907,022
4
Lisinopril
256,219,436
38,680,266
2,496,970,113
8,168,181
6.62
0.10
31.37
118,152,309
5
Omeprazole
394,327,431
31,251,630
1,890,974,572
6,864,952
12.62
0.21
57.44
221,014,792
6
Gabapentin
494,526,394
29,424,560
3,574,001,907
5,806,515
16.81
0.14
85.17
325,921,964
7
Furosemide
139,683,736
27,504,873
1,508,572,667
5,494,606
5.08
0.09
25.42
58,644,109
8
Simvastatin
219,202,502
27,023,810
1,699,708,464
5,882,757
8.11
0.13
37.26
91,937,232
9
Hydrocodone-
Acetaminophen
498,384,609
26,893,075
2,104,453,623
6,846,138
18.53
0.24
72.80
336,133,437
10
Metformin Hcl
188,246,304
25,415,896
3,006,011,939
5,337,168
7.41
0.06
35.27
98,499,540
Medicare Part D Spending, Top 10
4,102,638,079
Medicare Part D Spending, All Drugs
154,229,569,493
Source: Analysis of Medicare claims data (carrier, outpatient, and Part D event) by Acumen for ASPE.

89
Table A-7 Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Total
Spending
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2008
1
Quetiapine Fumarate
$5.59
$962,610,903
2
Aripiprazole
$12.98
$864,665,984
3
Olanzapine
$14.00
$655,125,344
4
Risperidone
$5.54
$624,080,668
5
Montelukast Sodium
$3.46
$452,731,574
6
Palivizumab
$1,603.26
$440,111,778
7
Lansoprazole
$4.80
$396,513,136
8
Fluticasone/Salmeterol
$3.38
$372,878,837
9
Esomeprazole Mag Trihydrate
$5.00
$351,395,745
10
Topiramate
$3.79
$335,536,619
Medicaid Spending, Top 10
$5,455,650,587
Medicaid Spending, All Drugs
$24,655,613,645
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2009
1
Aripiprazole
$14.05
$1,086,225,218
2
Quetiapine Fumarate
$6.33
$1,081,880,930
3
Olanzapine
$12.51
$689,987,147
4
Montelukast Sodium
$3.73
$509,973,605
5
Esomeprazole Mag Trihydrate
$5.31
$435,692,163
6
Lansoprazole
$5.22
$426,174,979
7
Fluticasone/Salmeterol
$3.58
$413,942,343
8
Palivizumab
$1,689.25
$398,919,752
9
Methylphenidate Hcl
$3.14
$357,362,638
10
Amphet Asp/Amphet/D-Amphet
$5.50
$286,553,078
Medicaid Spending, Top 10
$5,686,711,852
Medicaid Spending, All Drugs
$26,029,704,453

90
Table A-7 continued Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Total Spending
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2010
1
Aripiprazole
$15.29
$1,424,014,486
2
Quetiapine Fumarate
$7.36
$1,411,178,430
3
Olanzapine
$17.07
$848,755,243
4
Montelukast Sodium
$4.07
$731,230,131
5
Esomeprazole Mag Trihydrate
$5.52
$577,427,457
6
Fluticasone/Salmeterol
$3.81
$572,958,809
7
Methylphenidate Hcl
$3.72
$559,315,101
8
Ziprasidone Hcl
$7.65
$376,368,788
9
Palivizumab
$1,771.61
$370,882,218
10
Emtricitabine/Tenofovir
$33.23
$368,035,678
Medicaid Spending, Top 10
$7,240,166,341
Medicaid Spending, All Drugs
$32,999,520,529
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2011
1
Aripiprazole
$16.92
$1,667,289,509
2
Quetiapine Fumarate
$8.47
$1,618,779,336
3
Olanzapine
$20.49
$983,556,014
4
Montelukast Sodium
$4.65
$892,903,221
5
Methylphenidate Hcl
$4.09
$709,352,216
6
Fluticasone/Salmeterol
$4.14
$640,832,463
7
Esomeprazole Mag Trihydrate
$5.82
$573,215,157
8
Emtricitabine/Tenofovir
$35.81
$455,304,078
9
Albuterol Sulfate
$3.10
$453,110,973
10
Efavirenz/Emtricitab/Tenofovir
$55.06
$443,793,760
Medicaid Spending, Top 10
$8,438,136,726
Medicaid Spending, All Drugs
$37,679,593,904

91
Table A-7 continued Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Total Spending
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2012
1
Aripiprazole
$19.15
$1,827,786,498
2
Quetiapine Fumarate
$10.86
$985,626,781
3
Montelukast Sodium
$6.23
$713,394,035
4
Methylphenidate Hcl
$4.63
$703,317,971
5
Fluticasone/Salmeterol
$4.59
$670,347,610
6
Emtricitabine/Tenofovir
$39.39
$549,689,170
7
Albuterol Sulfate
$3.54
$538,800,846
8
Insulin Glargine,Hum.Rec.Anlog
$13.13
$524,024,969
9
Efavirenz/Emtricitab/Tenofovir
$59.21
$497,667,487
10
Lisdexamfetamine Dimesylate
$5.23
$459,895,293
Medicaid Spending, Top 10
$7,470,550,662
Medicaid Spending, All Drugs
$37,771,206,170
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2013
1
Abilify
$23.72
$2,011,387,678
2
Advair Diskus
$4.63
$580,242,453
3
Methylphenidate Er
$5.09
$578,534,690
4
Vyvanse
$6.01
$559,256,432
5
Truvada
$41.69
$522,333,513
6
Cymbalta
$6.97
$498,940,945
7
Atripla
$63.69
$494,445,974
8
Seroquel Xr
$14.44
$414,436,248
9
Synagis
$2,346.53
$387,004,169
10
Adderall Xr
$7.28
$377,512,240
Medicaid Spending, Top 10
$6,424,094,342
Medicaid Spending, All Drugs
$38,896,894,112

92
Table A-7 continued Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Total Spending
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2014
1
Abilify
$27.91
$2,464,660,026
2
Sovaldi
$1,023.55
$1,386,209,781
3
Atorvastatin Calcium
$8.31
$1,139,715,919
4
Vyvanse
$6.75
$659,026,202
5
Truvada
$43.53
$616,663,462
6
Methylphenidate Er
$4.96
$581,063,019
7
Atripla
$68.56
$579,363,530
8
Lantus
$21.66
$573,919,545
9
Advair Diskus
$4.98
$546,197,570
10
Lantus Solostar
$22.55
$460,267,881
Medicaid Spending, Top 10
$9,007,086,936
Medicaid Spending, All Drugs
$47,308,056,863
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2015
1
Harvoni
$1,136.52
$2,195,135,262
2
Abilify
$32.00
$2,033,716,141
3
Lantus
$25.32
$787,864,582
4
Vyvanse
$7.43
$776,733,121
5
Truvada
$46.32
$735,793,787
6
Methylphenidate Er
$5.92
$699,121,315
7
Lantus Solostar
$25.23
$650,028,115
8
Latuda
$28.25
$631,173,466
9
Sovaldi
$978.65
$617,704,050
10
Aripiprazole
$21.31
$605,337,794
Medicaid Spending, Top 10
$9,732,607,632
Medicaid Spending, All Drugs
$57,820,639,442

93
Table A-7 continued Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Total Spending
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2016
1
Harvoni
$1,128.11
$2,210,702,438
2
Abilify
$31.80
$1,079,159,586
3
Humira Pen
$1,918.91
$968,237,888
4
Vyvanse
$8.24
$897,132,794
5
Latuda
$32.73
$864,739,470
6
Lantus
$25.05
$785,372,480
7
Truvada
$49.78
$754,171,178
8
Lantus Solostar
$24.95
$752,034,059
9
Methylphenidate Er
$6.25
$735,566,656
10
Aripiprazole
$11.16
$672,887,842
Medicaid Spending, Top 10
$9,720,004,391
Medicaid Spending, All Drugs
$64,455,170,411
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2017
1
Humira Pen
$2,152.22
$1,263,696,300
2
Harvoni
$1,084.68
$1,211,321,698
3
Latuda
$37.35
$1,096,733,005
4
Vyvanse
$8.87
$971,454,153
5
Epclusa
$874.83
$943,299,246
6
Genvoya
$90.40
$814,372,297
7
Invega Sustenna
$1,530.28
$743,459,699
8
Methylphenidate Er
$6.69
$734,238,237
9
Lyrica
$6.54
$721,237,876
10
Zepatier
$647.27
$705,462,489
Medicaid Spending, Top 10
$9,205,275,001
Medicaid Spending, All Drugs
$67,585,558,174
Source: 2006-2012 Lewin analysis of Medicaid State Drug Utilization public-use data, 2013-
2017 Centers for Medicaid and Medicaid Services analysis of Medicaid State Drug Utilization
public-use data.

94
Table A-8 Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Spending
per Unit
Year
Rank
Generic Drug Name
Spending per Unit
Total Spending
2013
1
Eylea
$28,726.14
$11,329,044
2
Lucentis
$27,154.34
$35,875,600
3
Ilaris
$16,441.73
$6,641,802
4
Supprelin La
$15,778.66
$11,234,408
5
Retisert
$13,283.28
$212,532
6
Stelara
$11,528.58
$29,800,559
7
Somatuline Depot
$9,951.89
$1,655,617
8
Jevtana
$7,152.10
$2,891,192
9
H.P. Acthar
$5,841.24
$83,938,624
10
Mozobil
$5,459.21
$2,049,180
Medicaid Spending, Top 10
$185,628,558
Medicaid Spending, All Drugs
$38,896,894,112
Year
Rank
Generic Drug Name
Spending per Unit
Total Spending
2014
1
Lucentis
$22,890.95
$42,675,634
2
Eylea
$18,281.66
$18,866,688
3
Supprelin La
$17,858.48
$12,554,514
4
Ilaris
$16,106.46
$17,651,242
5
Stelara
$14,515.75
$52,042,820
6
Jetrea
$11,938.28
$211,308
7
Somatuline Depot
$11,768.56
$2,221,257
8
Marqibo
$6,895.33
$151,697
9
Jevtana
$6,450.22
$3,252,344
10
H.P. Acthar
$6,258.93
$126,837,119
Medicaid Spending, Top 10
$276,464,623
Medicaid Spending, All Drugs
$47,308,056,863
Year
Rank
Generic Drug Name
Spending per Unit
Total Spending
2015
1
Eylea
$30,640.15
$57,442,354
2
Lucentis
$27,325.91
$40,882,824
3
Supprelin La
$21,244.49
$14,764,918
4
Lemtrada
$19,045.74
$978,951
5
Calcium Disodium Versenate
$16,182.57
$28,158
6
Stelara
$14,987.46
$88,407,205
7
Jetrea
$14,746.69
$210,199
8
Xofigo
$14,211.63
$448,434
9
Somatuline Depot
$12,515.16
$4,253,274
10
Ilaris
$10,860.14
$31,064,973
Medicaid Spending, Top 10
$238,481,290
Medicaid Spending, All Drugs
$57,820,639,442

95
Table A-8 continued Top 10 High-Cost Prescribed Drugs, Medicaid, Ranked by Spending per
Unit
Year
Rank
Generic Drug Name
Spending per Unit
Total Spending
2016
1
Eylea
$30,501.57
$115,662,948
2
Supprelin La
$25,578.29
$19,260,455
3
L-Methionine
$25,130.00
$302
4
Lucentis
$23,391.50
$38,852,316
5
Stelara
$16,331.53
$142,687,915
6
Lemtrada
$13,020.43
$10,081,942
7
Jetrea
$12,086.76
$160,996
8
Xofigo
$11,823.87
$1,019,111
9
Signifor Lar
$11,560.15
$1,260,056
10
Somatuline Depot
$8,962.15
$8,210,191
Medicaid Spending, Top 10
$337,196,232
Medicaid Spending, All Drugs
$64,455,170,411
Year
Rank
Generic Drug Name
Spending per Unit
Total Spending
2017
1
Aralast
$36,417.29
$164,897
2
Eylea
$33,809.93
$155,473,882
3
Supprelin La
$28,283.06
$20,986,031
4
Lucentis
$24,721.29
$39,847,591
5
Spinraza
$23,477.05
$119,583,270
6
Retisert
$19,168.74
$274,535
7
Lemtrada
$18,289.10
$17,467,005
8
Xofigo
$18,227.44
$2,320,827
9
Stelara
$17,235.71
$221,874,939
10
Krystexxa
$16,421.79
$1,299,374
Medicaid Spending, Top 10
$579,292,351
Medicaid Spending, All Drugs
$67,585,558,174
Source: Centers for Medicaid and Medicaid Services analysis of Medicaid State Drug Utilization
public-use data.

96
Table A-9 Top 10 Most Frequently Prescribed Drugs, Medicaid, Ranked by
Number of Claims
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2008
1
Hydrocodone Bit/Acetaminophen
$0.23
$112,492,086
2
Amoxicillin
$0.09
$64,329,996
3
Ibuprofen
$0.09
$49,200,240
4
Azithromycin
$2.40
$145,355,482
5
Alprazolam
$0.13
$39,489,546
6
Clonazepam
$0.20
$51,623,548
7
Lorazepam
$0.27
$57,573,711
8
Montelukast Sodium
$3.46
$452,731,574
9
Aspirin
$0.09
$14,808,907
10
Loratadine
$0.24
$44,987,853
Medicaid Spending, Top 10
$1,032,592,942
Medicaid Spending, All Drugs
$24,655,613,645
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2009
1
Hydrocodone Bit/Acetaminophen
$0.24
$128,705,221
2
Amoxicillin
$0.10
$74,540,834
3
Ibuprofen
$0.09
$54,549,556
4
Azithromycin
$1.76
$138,836,617
5
Albuterol Sulfate
$3.05
$221,791,801
6
Alprazolam
$0.12
$40,524,359
7
Clonazepam
$0.13
$38,998,901
8
Lorazepam
$0.16
$36,034,159
9
Montelukast Sodium
$3.73
$509,973,605
10
Loratadine
$0.22
$47,178,424
Medicaid Spending, Top 10
$1,291,133,475
Medicaid Spending, All Drugs
$26,029,704,453

97
Table A-9 continued Top 10 Most Frequently Prescribed Drugs, Medicaid, Ranked by Number
of Claims
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2010
1
Hydrocodone Bit/Acetaminophen
$0.22
$170,503,632
2
Amoxicillin
$0.09
$108,328,686
3
Ibuprofen
$0.09
$75,096,982
4
Albuterol Sulfate
$2.87
$356,562,839
5
Azithromycin
$1.53
$169,561,708
6
Loratadine
$0.17
$60,859,947
7
Alprazolam
$0.13
$53,806,189
8
Clonazepam
$0.13
$48,683,997
9
Montelukast Sodium
$4.07
$731,230,131
10
Lisinopril
$0.19
$36,786,525
Medicaid Spending, Top 10
$1,811,420,636
Medicaid Spending, All Drugs
$32,999,520,529
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2011
1
Hydrocodone Bit/Acetaminophen
$0.20
$185,851,039
2
Amoxicillin
$0.09
$127,704,574
3
Ibuprofen
$0.09
$82,215,957
4
Albuterol Sulfate
$3.10
$453,110,973
5
Azithromycin
$1.47
$182,600,085
6
Alprazolam
$0.12
$59,014,244
7
Loratadine
$0.16
$54,014,819
8
Clonazepam
$0.13
$53,746,692
9
Lisinopril
$0.19
$43,334,762
10
Montelukast Sodium
$4.65
$892,903,221
Medicaid Spending, Top 10
$2,134,496,367
Medicaid Spending, All Drugs
$37,679,593,904

98
Table A-9 continued Top 10 Most Frequently Prescribed Drugs, Medicaid, Ranked by Number
of Claims
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2012
1
Hydrocodone Bit/Acetaminophen
$0.21
$185,509,603
2
Amoxicillin
$0.08
$109,425,719
3
Albuterol Sulfate
$3.54
$538,800,846
4
Ibuprofen
$0.08
$71,155,236
5
Azithromycin
$1.29
$160,450,118
6
Omeprazole
$0.42
$116,061,714
7
Alprazolam
$0.11
$55,748,894
8
Loratadine
$0.16
$48,268,866
9
Lisinopril
$0.16
$40,290,457
10
Clonazepam
$0.12
$51,067,101
Medicaid Spending, Top 10
$1,376,778,555
Medicaid Spending, All Drugs
$37,771,206,170
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2013
1
Hydrocodone-Acetaminophen
$0.26
$195,051,575
2
Amoxicillin
$0.12
$98,135,545
3
Ibuprofen
$0.11
$62,215,054
4
Omeprazole
$0.38
$114,616,969
5
Azithromycin
$1.66
$129,482,064
6
Lisinopril
$0.15
$38,977,743
7
Loratadine
$0.18
$40,981,632
8
Cetirizine Hcl
$0.16
$53,416,090
9
Albuterol Sulfate
$0.15
$109,339,045
10
Gabapentin
$0.27
$150,887,536
Medicaid Spending, Top 10
$993,103,255
Medicaid Spending, All Drugs
$38,896,894,112

99
Table A-9 continued Top 10 Most Frequently Prescribed Drugs, Medicaid, Ranked by Number
of Claims
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2014
1
Hydrocodone-Acetaminophen
$0.29
$232,638,855
2
Amoxicillin
$0.13
$98,843,746
3
Ibuprofen
$0.10
$74,011,233
4
Lisinopril
$0.14
$43,124,965
5
Omeprazole
$0.34
$107,844,436
6
Azithromycin
$1.58
$121,677,230
7
Gabapentin
$0.23
$164,292,251
8
Cetirizine Hcl
$0.15
$56,034,828
9
Loratadine
$0.16
$38,240,930
10
Levothyroxine Sodium
$0.37
$83,000,884
Medicaid Spending, Top 10
$1,019,709,357
Medicaid Spending, All Drugs
$47,308,056,863
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2015
1
Hydrocodone-Acetaminophen
$0.37
$282,984,779
2
Amoxicillin
$0.12
$108,507,341
3
Ibuprofen
$0.12
$94,664,783
4
Lisinopril
$0.12
$48,649,631
5
Omeprazole
$0.27
$113,501,036
6
Gabapentin
$0.21
$211,713,845
7
Azithromycin
$1.53
$125,819,100
8
Metformin Hcl
$0.08
$46,714,921
9
Levothyroxine Sodium
$0.43
$119,409,992
10
Cetirizine Hcl
$0.15
$61,716,169
Medicaid Spending, Top 10
$1,213,681,596
Medicaid Spending, All Drugs
$57,820,639,442

100
Table A-9 continued Top 10 Most Frequently Prescribed Drugs, Medicaid, Ranked by Number
of Claims
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2016
1
Amoxicillin
$0.12
$107,146,885
2
Hydrocodone-Acetaminophen
$0.33
$240,061,664
3
Ibuprofen
$0.11
$93,837,018
4
Lisinopril
$0.11
$48,082,851
5
Gabapentin
$0.17
$207,844,223
6
Omeprazole
$0.23
$101,969,084
7
Atorvastatin Calcium
$0.35
$112,539,544
8
Cetirizine Hcl
$0.14
$66,964,155
9
Metformin Hcl
$0.07
$48,221,985
10
Levothyroxine Sodium
$0.44
$132,094,048
Medicaid Spending, Top 10
$1,158,761,456
Medicaid Spending, All Drugs
$64,455,170,411
Year
Rank
Generic Drug Name
Spending per
Unit
Total Spending
2017
1
Amoxicillin
$0.12
$104,708,104
2
Ibuprofen
$0.11
$89,449,829
3
Gabapentin
$0.16
$202,816,058
4
Lisinopril
$0.12
$51,994,254
5
Hydrocodone-Acetaminophen
$0.31
$176,562,712
6
Atorvastatin Calcium
$0.31
$120,312,292
7
Omeprazole
$0.24
$98,121,378
8
Ventolin Hfa
$2.92
$543,229,325
9
Cetirizine Hcl
$0.16
$77,908,465
10
Metformin Hcl
$0.08
$52,936,615
Medicaid Spending, Top 10
$1,518,039,032
Medicaid Spending, All Drugs
$67,585,558,174
Source: 2006-2012 Lewin analysis of Medicaid State Drug Utilization public-use data, 2013-
2017 Centers for Medicaid and Medicaid Services analysis of Medicaid State Drug Utilization
public-use data.
