
HeartStartEvent Review Pro
User Guide
About this edition
HeartStart Event Review Pro version 5.0
Publication date: December 2015
Document part number: 453564556351
The information in this document applies to the product
version indicated above. This information is subject to
change without notice.
Philips Medical Systems shall not be liable for errors
contained herein or for incidental or consequential
damages in connection with the furnishing,
performance, or use of the material.
Copyright © 2015
Philips North America Corp.
No part of this publication may be reproduced,
transmitted, transcribed, stored in a retrieval system, or
translated into any human or computer language in any
form by any means without the consent of the copyright
holder.
Unauthorized copying of this publication may not only
infringe copyright but also reduce the ability of Philips
Medical Systems to provide accurate and up-to-date
information to users and operators alike.
Medical device directive
Event Review Pro complies with the requirements of the
Medical Device Directive 93/42/EEC and carries the
mark accordingly.
Trademarks
HeartStart Event Review Pro, HeartStart FR3, HeartStart
FRx, HeartStart HS1, HeartStart Defibrillator, HeartStart
MRx, HeartStart XL+, Efficia DFM100, and the HeartStart
logo are either trademarks or registered trademarks of
Koninklijke Philips N.V.
The Event Review Pro application uses Bluetooth wireless
technology. The Bluetooth wordmark and logos are either
trademarks or registered trademarks of Bluetooth SIG,
Inc. The Bluetooth wordmark and logos are owned by
Bluetooth SIG, Inc.
Adobe, Acrobat, and Reader are either trademarks or
registered trademarks of Adobe Systems Incorporated in
the United States and/or other countries. Microsoft®
Windows, Windows 7, Windows 8.1, Windows 10,
Windows Explorer, Microsoft .NET Framework, and
Internet Explorer are either trademarks or registered
trademarks of Microsoft® Corporation in the United
States and/or other countries.
Actiontec is either a trademark or a registered trademark
of Actiontec Electronics, Inc. CompactFlash is either a
trademark or a registered trademark of SanDisk
Corporation. IrDA and IR logo are trademarks or
registered trademarks of Infrared Data Association.
Kingston is either a trademark or a registered trademark
of Kingston Technologies Company. Microsoft, Windows,
Internet Explorer, Excel, Word, and Outlook are either
trademarks or registered trademarks of Microsoft
Corporation in the United States and/or other countries.
Q-CPR is either a trademark or a registered trademark of
Laerdal Medical.
Authorized EU representative
Philips Medizin Systeme
Boeblingen GmbH
Hewlett-Packard Strasse 2
71034 Boeblingen, Germany
(+49) 7031 463-2254
Manufacturer
Philips Medical Systems
22100 Bothell Everett Highway
Bothell, WA 98021-8431, USA
EN 11/2/2015

Table of contents
Installing Event Review Pro 8
About the Event Review Pro software 9
System requirements 9
Downloading the software 12
Checking for software updates 13
The installation procedure 13
Activating the software 14
Activate over the Internet 15
Activate by email 15
Activate later 17
Uninstalling the software 17
Product compatibility 18
Getting started 19
Intended use 19
What’s new in this release 19
Getting assistance 20
Conventions used in this guide 21
Starting the application 23
Understanding the Getting Started options 23
Using the navigation pane 24
Using the Administration workspace 25
Understanding the application window 26
Understanding the Case Editor 28
Using the application tables and logs 32
Working with columns 35
Grouping and sorting entries 35
Filtering entries 37
Removing filters 39
Printing table entries 39
Exporting data to Excel 40
Completing fields 40
3

4
Table of contents Event Review Pro User Guide
Resizing panes and workspaces 41
Using a Tablet PC 41
Saving your work 42
Restoring confirmation messages 42
Working with cases 43
Searching for cases 44
Using queries 46
Creating a case (including ECG)with wizards 47
Creating a case 50
AttachingECGs 51
AttachingECGs table 54
Attachingan ECG from a data card 55
Attachingan ECG from a USB port 56
Attaching an ECG using an FR3 Bluetooth transmission 56
Downloadingan ECG using a HeartStart MRx Bluetooth transmission 57
Downloadingan ECG from an infrared connection 58
Attachingan ECG from a file 60
After you add an ECG 60
RemovingECGs from a case 61
Displaying case details 61
Sorting and grouping cases 62
Hiding and displaying grouped cases 63
Importing case files manually 64
Importing case files automatically 65
Saving cases 66
Adjusting the date and time of the defibrillator data 66
Erasing the data source 67
Printing case information 69
Exporting cases 69
Emailing a case 71
Deleting cases 72
Reviewing cases with duplicate ECGs 73
Merging related cases 74
Merging related cases automatically 76
Adding case details 78
Identifying the case 79
Describing the conditions at the scene 84
Documenting events 85
Adding, describing, and removing events 85

5
Table of contents Event Review Pro User Guide
Sorting events 86
Changing the date and time of the event 87
Documenting the patient's outcome 87
Working with ECGs 88
Understanding the Case Editor 88
Using the Events pane 93
Viewing waveforms 97
Working with waveforms 97
Displaying and hiding waveforms 99
Arranging sections of the ECGdisplay 99
Magnifying waveforms 100
Changing the display time 100
Changing the time scale 101
Scrolling through the waveform 102
Customizing waveforms 102
Changing the waveform offset 104
Changing the waveform scale 106
Reviewing waveform information 106
Defining and viewing ECG selections 108
Creating ECG selections 109
Managing ECG selections 109
Naming, finding, and sorting ECG selections 111
Using selection templates 112
Copying ECGs to the Clipboard 113
Exporting waveform data 113
Managing notes on the waveform 114
Zooming in and out of the waveform 116
Customizing the ECG display 117
Reviewing vital trends data 117
Working with 12-lead ECGs 119
Configuring the 12-lead view 121
Reviewing the 12-lead ECG 122
Magnifying 12-lead ECGs 122
Working with CPR data 123
Customizing CPR episodes 123
Creating CPRexclusions 125
Removing CPR exclusions 126
Adding CPR annotations 127
Adding CPR responder annotations 128

6
Table of contents Event Review Pro User Guide
Using key command and mouse shortcuts 129
Reviewing case details 133
Reviewing case events 133
Attaching files to a case 135
Completing a final case review 137
Using the Import Service 138
Managing Import Service inboxes 139
Configuring the Import Service 141
Managing Import Service archives 142
Using the HTTPImport Service 143
Working with the System Log 145
Working with reports 147
Selecting cases for reports 148
Selecting CPR Guidelines for reporting 150
Generating reports 151
Generating a report for an open case 151
Generating a report using the Reports workspace 152
Working in Report Preview 153
Printing reports 154
Exporting reports 154
Working with case reports 155
Working with ECG reports 155
Working with 12-lead reports 156
Working with Q-CPR Report Card reports 158
Information in the Q-CPR Report Card chart 159
Information in the Q-CPR Report Card details 163
Information in the Q-CPR Report Card compression strips 166
Working with Q-CPR Details reports 167
Q-CPR Details report data 169
Working with Q-CPR Response Time reports 173
Information in the Q-CPR Response Times overview 174
Information in the Q-CPR Response Times details 178
Working with zCPR Report Card reports 179
Information in the zCPR Report Card chart 180
Information in the zCPR Report Card compression strips 182
Working with Vital Trends reports 183
Working with Response Times reports 183
Working with Utstein reports 186
Working with Device Self-test History reports 187

7
Table of contents Event Review Pro User Guide
Customer support 189
Working with defibrillators 192
Supported defibrillators 192
Selecting accessories for data transfer 193
Reading FR3 series cards 195
Retrieving FR3 data using Bluetooth transmission 195
Reading HeartStart MRx cards 196
Setting up Bluetooth transmissions for the HeartStart MRx 197
Bluetooth option prerequisites for the HeartStart MRx 198
Pairing and testing the HeartStart MRx Bluetooth option with the computer 198
Sending the HeartStart MRx Bluetooth transmission 199
Reading FR2 series cards 200
Reading HeartStart XL cards 201
Transferring HeartStart XL+ data 201
Transferring Efficia DFM100 data 202
Determining the HS1 and FRx case date and time 202
Using infrared connections for the HS1 and FRx 203
Setting up an infrared adapter 204
Setting up the infrared connection 204
Understanding voice and system messages 206
Emailing device history data 207
Importing device self-test data using the Device Self-test Wizard 209
Managing the database 212
Using the Event Review Pro database on a remote database server 213
Migrating cases from previous versions 214
CPC and OPC 217
Glasgow Coma score 219
Glossary 221
Index 223

1
Installing Event
Review Pro
HeartStart Event Review Pro 5.0 is a major upgrade for both Event
Review Pro and Event Review, and requires a new license. You can
install and run the new version for 60 days while you purchase a license.
Event Review Pro stops working if you do not activate the software
within 60 days of installation.
HeartStart Event Review Pro 5.0 installation removes any existing
4.x versions of Event Review Pro and Event Review from the
computer.
If you have Event Review or Event Review Pro older than version
3.5, You must first upgrade and migrate the data to 3.5, then you
can migrate the data to Event Review Pro 5.0.
• For information about migrating data from earlier versions, see
Migrating cases from previous versions on page.214.
• For information about product compatibilty, see Product
compatibility on page.18.
• For full details about hardware and software requirements, see
System requirements on page.9.
• For help with downloading, see Downloading the software on
page.12.
• For help with installing, see The installation procedure on page.13.
• For help setting up a remote database for use with Event Review
Pro, see Using the Event Review Pro database on a remote database
server on page.213.
8

9
About the Event Review Pro software
Event Review Pro is available in two editions. Each offers viewing and
editing features for cases acquired from defibrillators in specific
situations:
• EMS edition: defibrillators used by first-responders, in resuscitations
outside a hospital
• Hospital edition: defibrillators used for resuscitations within a
hospital
When you install the software, the default option is the EMS edition;
however, you will have the opportunity to select the Hospital edition
instead.
Event Review Pro is available in different license types, determined at
the time of purchase:
• Single-installation license, for use on only one computer
• Site license, for use on multiple computers
System requirements
Event Review Pro operates on a computer running the Microsoft
Windows 7, Windows 8.1 or Windows 10 operating system. Make sure
that any hardware you choose is certified as Microsoft - compatible.
Philips provides only the Event Review Pro software. The software and
hardware listed in the following table are provided by the customer,
unless noted.
This table has the following sections:
• Software requirements
• Hardware requirements
• Accessories
1 - Installing Event Review Pro Event Review Pro User Guide

10
Software requirements table
Component Requirement
Operating
system
Microsoft Windows 7, 32-bit or 64-bit, with SP1
Microsoft Windows 8.1, 32-bit or 64-bit
Microsoft Windows 10, 32-bit or 64-bit
Database Note: Windows SQL 2014 Express is included and delivered
with the product.
For a shared database: Microsoft SQL Server 2008, 2008 R2,
2012, 2012 R2, or 2014
For a remote server running the shared database:
Microsoft Windows Server 2008 R2 with SP1, 2012,
or 2012 R2
Microsoft Windows 7, 32-bit or 64-bit, with SP1
Microsoft Windows 8.1, 32-bit or 64-bit
Microsoft Windows 10, 32-bit or 64-bit
Hardware requirements table
Component Requirements
Processor speed Minimum: 1 GHZ x86 or x64 processor
Recommended: 2 GHZ core duo or higher
Display
resolution
Minimum: 1024 x 768
Recommended: 1600 x 1200 or higher
Video memory Minimum: 64 MB video memory
Recommended: 256 MB video memory
Memory Minimum: 2 GB
Recommended: 4 GB or higher
1 - Installing Event Review Pro Event Review Pro User Guide
11
Component Requirements
Disk space Minimum: 25 GB of available disk space for database
storage and backup
Hard-disk space requirements vary depending on usage and
defibrillator type. Variables affecting disk-space
requirements include the number of cases archived and the
amount of audio information archived. For example, a 15-
minute FR2 ECG with no audio is approximately 100 KB.
The same ECG with audio can exceed 5 MB. An ECG and
audio from a HeartStart MRx defibrillator can be as large as
256 MB.
Internet
connection
Required to activate the application software, to use the
Email feature, and to receive software updates.
Accessories table
Component Requirement
Printer To print reports
Preferred: Color printer
PDF Reader To view HeartStart Event Review Pro user guide
Recommended: Adobe Reader, latest version. For more
information, see the following Web site:
https://get2.adobe.com/reader/
Backup and
restore tool
To help prevent data loss and corruption
Connection to
a time server
To synchronize your computer date and time
Note: If you use a time server, do not alter your system clock
manually.
Email
application
To send cases using email or activate the software by email.
Spreadsheet
application
To display Q-CPR data in charts; data is exported in .xlsx format
only
Sound card
and speaker
To play audio from the defibrillator
1 - Installing Event Review Pro Event Review Pro User Guide
12
Component Requirement
Card reader To read information from a defibrillator, see your Philips
customer representative to purchase the appropriate card
reader.
See Selecting accessories for data transfer on page.193 for a
summary of the data card types.
IrDA support Required to read information from an HS1 or FRx defibrillator
Your computer must support the following technology:
• IrDA functionality
• An infrared transceiver or an infrared adapter
The transceiver will appear as a small, dark red or black window
on the computer and device.
Bluetooth
adapter
To transfer HeartStart MRx and HeartStart FR3 data to your
computer
Bluetooth
stack
To use Bluetooth to transfer MRx or FR3 data from the
defibrillator to your computer
Bluetooth version: 2.0 or higher
You must have a supported adapter for Bluetooth
transmission. Most Bluetooth adapters are suitable. You
can order an adapter from your Philips representative.
Downloading the software
You can download and use Event Review Pro on a trial basis for 60 days
before purchasing and activating the software.
Use the authorization that you receive from your sales representative to
download the software from the Internet. Save the downloaded file to
your computer. You can use the software on a trial basis and activate it
later.
To download the software
1. Navigate to the URL provided on your Proof of Purchase Certificate
or by your sales representative.
2. Click the download link.
3. Save the installation file to your computer.
1 - Installing Event Review Pro Event Review Pro User Guide

13
For information on possible updates to the software, see Checking for
software updates on page.13.
For instructions on installation, see The installation procedure on
page.13.
Checking for software updates
You can check at any time to see if new updates have been released for
Event Review Pro software.
Before you install any software updates on the computer, make
sure that you have Windows Administrator privileges for the
computer, and that the computer is connected to the Internet.
To check for software updates
1. On the Help menu, click Check for Updates.
The software connects to the Web site.
2. Click Show Updates.
The Web site displays a list of available updates. If a new update is
available, follow the rest of this procedure to download and install it.
3. Click Get Update for the Application.
4. Click Download and follow the instructions to save the update.
5. Close the older version of the software.
6. Note the name and location of the saved, downloaded file and follow
the instructions to install it.
The installation procedure
Once you have saved the installation file to your computer (see
Downloading the software on page.12), follow these instructions.
To install Event Review Pro
1. In the folder where the installation file has been saved, right-click
the file and click Run as Administrator.
You may see a User Account Control box that asks you to authorize
this program to make changes. Click Yes.
1 - Installing Event Review Pro Event Review Pro User Guide

14
2. The Install Shield wizard checks for your database and for versions
of the Microsoft .NETFramework. If it needs to install or upgrade
either of these, it does so. Follow any instructions during this
process (which can take some time); if the User Account Control box
asks you to authorize this program to make changes, click Yes.
3. The Install Shield wizard dialog box is now ready to begin
installation of Event Review Pro. Click Install.
4. On the Welcome screen, click Next.
5. On the next page, click I Accept the Terms.... and click Next.
6. The next page displays the current Read Me file, containing any late-
breaking information. Read the text and then click Next.
7. On the next page, click the edition of Event Review Pro that you want
to install, EMS or Hospital edition, and then click Next.
8. In the Custom Setup dialog box, you have the opportunity to save
Event Review Pro in a different location from the default. If you need
to check this new location for available space, click the Space
button. If you want to choose a different location, click Change and
navigate to the new location. When you are finished, or if you want
to accept the default location, click Next.
9. Confirm that you want to continue install; click Install.
10. When the installation is finished, click Finish.
11. An entry for Event Review Pro now appears on the Start menu. See
Starting the application on page.23.
Activating the software
After you install the software, activate the product.
You can use the Internet or email to activate the software at any time
after you install the application. Until you complete the activation, you
will see the Philips HeartStart Activation Wizard window when the
application starts, At the end of 60 days, however, if you have not yet
activated, you cannot run the application on this computer
Once the software is activated, you can view the license number by
clicking the Help menu and clicking About Event Review Pro. In addition,
save the license number in a safe place that you can access in case you
need to re-install the software.
Email activation support is available in English only at:
activation.support@philips.com
1 - Installing Event Review Pro Event Review Pro User Guide

15
Product support via email is available in English only at:
eventreview.support@philips.com
For support telephone numbers and other information about customer
support, see Customer support on page.189.
Activate over the Internet
You can activate the software by the Internet at any point when you first
install the application, preferably within 60 days of installation.
At the end of the 60 days, however, if you have not yet activated, you
cannot run the application on this computer.
The activation wizard uses an encrypted transfer to validate that the
license number you enter has not already been used and to activate
your software. If you are not connected to the Internet, the wizard
alerts you that there is no connection.
For more information, see Customer support on page.189.
To activate the software by the Internet
1. Start the application.
The application opens the HeartStart Activation Wizard.
2. Type the license number that you received.
3. Click I want to activate the software over the Internet.
4. Click Activate.
The wizard validates your entry and displays a congratulations
message.
5. Click OK.
The wizard starts the application.
Activate by email
The email activation involves exchanging emails with Philips Customer
Support.
An email application must be installed on this computer or you must
have access to an email application.
For more information, see Customer support on page.189.
Print this topic for future use.
1 - Installing Event Review Pro Event Review Pro User Guide

16
To activate the software by email
1. Start the application.
The application opens the HeartStart Activation Wizard.
2. Type the license number that you received.
3. Click I want to activate the software by email.
4. Click Next.
5. Click Export Activation Request File.
The software saves a file named Philips HeartStart Event Review Pro
5.0 Activation Request File.bin to your desktop.
6. Create a new email message and, in the To: field, enter
activation.support@philips.com.
7. In the email Subject: field, enter Activation Request.
8. Locate the file previously saved on your desktop: Philips HeartStart
Event Review Pro 5.0 Activation Request File.bin, and attach that file to
the email message.
9. Send the email and wait for a response from Customer Support.
If you cannot send email from your computer, transfer the file
saved on your desktop to a computer that can send email, and send
the message from there.
When you have received your activation code
When you receive your activation code from Customer Support, you are
ready to activate your software.
1. Start the application.
The application opens the HeartStart Activation Wizard.
2. If the license number is not already pre-filled, enter the license
number.
3. Click I want to activate the software by email.
4. Click Next.
5. Enter the activation code you received.
6. Click Activate.
The wizard validates your entry and displays a congratulations
message.
7. Click OK.
1 - Installing Event Review Pro Event Review Pro User Guide

17
The wizard starts the application.
Activate later
If you do not want to activate your copy of the application when you first
start it, you can activate the software at any time when you start the
application. At the end of the 60 days, however, if you have not yet
activated, you cannot run the application on this computer.
During the 60 days, you can use the application and save your work.
Until you activate the software, you will see the HeartStart Activation
Wizard window each time that you start the application.
To use the application without activating the software
1. Start the application.
2. When HeartStart Activation Wizard opens, click Skip.
The wizard starts the application.
Uninstalling the software
If you have an active Internet connection at the time you remove the
software, the removal process contacts the Philips activation server and
releases your license number for installation on another computer. The
removal process does not remove the database.
To uninstall the software
1. Navigate to the Windows Control Panel.
2. From Control Panel, click All Control Panel Items, and then click
Programs and Features.
3. Right-clickthe application name, and then click Uninstall.
Your license must be released before you can install your software on
another computer. In the event that the uninstall process is not able to
automatically release the license, follow these instructions to request
license number release from customer support.
Note that if automatic license release is unable to complete, a
Philips HeartStart Event Review Pro 5.0 License Release Request
File.bin file is saved to the desktop.
1 - Installing Event Review Pro Event Review Pro User Guide

18
Sending a support request for license number release
1. Create a new email message and, in the To: field, enter
activation.support@philips.com.
2. In the email Subject: field, enter License Release Request.
3. Locate the file previously saved on your desktop: Philips HeartStart
Event Review Pro 5.0 License Release Request File.bin, and attach
that file to the email message.
4. Send the email and wait for a response from Customer Support.
Customer support will notify you by email when the license number
is released.
If you cannot send email from your computer, transfer the file
saved on your desktop to a computer that can send email, and send
the message from there.
Product compatibility
HeartStart Event Review Pro 5.0 installation removes any existing
4.x versions of Event Review Pro and Event Review from the
computer.
You can migrate the Event Review Pro 3.5 and Event Review Pro 4.x
data to Event Review Pro 5.0 after installing Event Review Pro 5.0. For
more information, see Migrating cases from previous versions on
page.214.
If you have Event Review or Event Review Pro older than version 3.5,
You must first upgrade and migrate the data to 3.5, then you can
migrate the data to Event Review Pro 5.0. For more information, see
Managing the database on page.212.
1 - Installing Event Review Pro Event Review Pro User Guide

2
Getting started
In Event Review Pro, a "case" consists of all the information relating to a
specific cardiac emergency. This includes information recorded by
supported defibrillators as well as information entered by the device
user. Event Review Pro creates a case by downloading ECG information,
shock decisions, and any recorded audio from Philips defibrillators. You
can add details to the case as well.
Intended use
HeartStart Event Review Pro captures, reports, and manages cardiac
arrest information. Event Review Pro has tools for collecting and
analyzing information, reviewing cases, identifying trends, and
evaluating the emergency response. All information relating to a
specific cardiac emergency is created in a case by downloading rhythm
ECG information, 12-lead ECG information, shock decisions, patient vital
sign data, and recorded audio from Philips automated external
defibrillators (AED), and Philips advanced monitors/defibrillators. Event
Review Pro allows users to annotate case details, including Basic Life
Support (BLS) and Advanced Life Support (ALS) responder observations
and interventions.
What’s new in this release
Event Review Pro 5.0 provides the following new features:
• Support for the Philips HeartStart FR3 defibrillator with Q-CPR.
• Improved Q-CPR reporting with AHA and ERC Guidelines-compliant
report card.
• Customizable CPR Guidelines with ability to define depth and rate
targets for CPR Report Cards.
• zCPR for retrospective review of CPR hands-on / hands-off times and
compression rate using patient impedance.
19

20
• Improved ECG viewing, including overlay of multiple waveforms on
screen and reports.
• Icons for incomplete release and compression events.
• Merging of case data allows for manual or automated integration of
patient cases. This is based upon chosen criteria and supports up to
30 minutes of device time overlap. Compatible with any supported
Philips defibrillators.
• Filtering of imported case data and timed purging of archived data.
Automatic cleanup of incomplete MRx BLDT transmissions.
• Auto-completion of Quick Notes.
• Device self-test management, including a wizard which guides the
user through downloading device self-test data.
• Migration of data from Event Review Pro 4.2/4.3 and Event Review
4.2/4.3.
• Supports AHA and ERC Guidelines 2015.
Any late-breaking news about features is in the Read Me document,
available from the Help menu.
Getting assistance
Event Review Pro offers the following sources of help.
Application tooltips
When you hover the cursor over a toolbar button or event on the ECG
waveform, text appears in a pop-up window. The text identifies the
toolbar button and describes the event.
Wizards
A wizard is a software feature that guides you through common tasks.
Several wizards are built into Event Review Pro.
Wizards are available on the Getting Started navigation pane to guide
you when you download data, import cases, and open cases.
Other wizards are available to guide you when you transfer data from a
device.
Online help
To get help with the current feature, press F1.
2 - Getting started Event Review Pro User Guide

21
To read an overview of a topic, on the Help menu, click Help. Select a
topic from Help Contents. You can use the browse buttons to move from
topic to topic.
User guide
The printable user guide, which includes all the information in the online
Help system, is in Portable Document Format (PDF). You can view it
using a PDF reader such as Adobe Acrobat.
You can view the user guide online, print sections, or print the whole
guide.
For customer support, see Customer support on page.189.
Conventions used in this guide
This document uses the following conventions to help identify
information.
Conventions
Used for Example
Bold Menu options, buttons,
field names, and list box
names that you need to
click.
On the File menu, click Import.
Italic Manual names. Instructions for administrator's
guide.
Screen
message
To distinguish defibrillator
voice prompts and
HeartStart FR3 screen
messages from general
text.
The defibrillator voice prompt
announces Administration
Press the center soft key (soft key
#2). The screen message reads
Ready for Data Transfer
Italics with
leading period
File extension types. The configuration is exported as an
.xml file.
Lower-case
italics
Specific file names. Name the configuration file
sample.xml.
Italics Variables and
placeholders
The default folder is located at
C:\Document and Settings\user
name\My Documents.
2 - Getting started Event Review Pro User Guide

22
A yellow box with a triangular caution icon and exclamation mark
identifies circumstances that can result in data corruption or
information loss.
A blue box with a note icon contains information on how features
are used.
A green box with a light bulb tip icon contains information to
complete a task.
Using online help
Use the online help system to search for information using the full-text
search feature, glossary, or an index.
You can access online help in these ways:
• Press F1 to open online help for the selected feature.
• On the Help menu, click Event Review Pro Help.
2 - Getting started Event Review Pro User Guide

3
Starting the
application
Event Review Pro does not place an icon on the desktop. To start Event
Review Pro, use the shortcut on the Start menu or the All Programs
menu.
To start Event Review Pro
1. In Windows, navigate to the programs menu.
2. Under Philips HeartStart Event Review Pro 5.0, click Event
Review Pro.
To use Event Review Pro, click a navigation button on the left pane of
the application window.
Understanding the Getting Started
options
Use the Getting Started navigation pane to access wizards that help you
perform a simple set of tasks with ECG information.
In the Welcome area, under What would you like to do today?, a set of
links open wizards to help you perform common tasks.
You can complete the following tasks under What would you like to do
today?:
• Download patient data from your defibrillator or its data card
• Import a patient case
• Open an Event Review Patient Case
Click one of the links to start a wizard for that task, and follow the steps
in the wizard.
23

24
Using the navigation pane
The navigation pane on the left of the Event Review Pro display provides
access to the application tasks.
To display a set of tasks, click a navigation button.
Following is a description of the navigation buttons and their
workspaces.
• Patient Cases is the workspace you see by default when the
software first opens. Work in this area when you are searching for
cases. For details, see Working with cases on page.43.
• Reports includes a list of all available reports, plus a toolbar for
printing, navigation, view changing, and export. For details, see
Working with reports on page.147.
• Devices takes you to a work area where you can select a wizard to
download AED self-test date, or view a table of self-test data that
has been downloaded. For details, see Importing device self-test
data using the Device Self-test Wizard on page.209.
• Administration helps the system administrator monitor and
troubleshoot system activity. The Administration workspace also has
settings for customizing reports. For details, see Using the
Administration workspace on page.25.
• Getting Started helps you create a case quickly, import an existing
patient case, and open a case already in your database. For details,
see Understanding the Getting Started options on page.23.
You can hide the navigation pane to increase the size of the
workspace in the right pane. You can display the navigation pane
again when you need it. See the following instructions.
To hide the navigation pane
At the top of the navigation, click the Auto Hide icon.
The navigation pane disappears, and the Navigation Panel tab along the
left edge of the pane appears.
To display the navigation pane temporarily
The following illustration shows the general arrangement of a
navigation tab when a navigation pane is hidden. The arrangment is
the same in all languages.
3 - Starting the application Event Review Pro User Guide

25
1. Point to the tab along the left edge of the pane.
The navigation pane appears temporarily.
2. Point to or tap the right pane.
The navigation pane disappears
3. Point to the tab again to redisplay the navigation pane.
To restore the navigation pane
Click the tab, and then click the Stay Open icon.
Using the Administration workspace
The Administration workspace is intended for use by System
Administrators and IT Specialists.
Click the Administration button in the navigation pane to open the
Administration workspace.
Selections in the workspace include:
• System LogOpen the System Log for viewing. For details, see
Working with the System Log on page.145.
• Import ServiceConfigure the Import Service folder. For details,
see Using the Import Service on page.138.
• Merge RulesConfigure rules for merging cases. For details, see
Merging related cases automatically on page.76.
• MigrationConfigure database migration. For details, see Migrating
cases from previous versions on page.214.
• CPR GuidelinesAccess settings for customizing CPR Guidelines,
used in reporting. For details, see Selecting CPR Guidelines for
reporting on page.150.
3 - Starting the application Event Review Pro User Guide

26
Understanding the
application window
In Event Review Pro, the work areas, the menu options, and the toolbar
buttons change, based on the navigation button and navigation pane
selections that you make.
• The menus list options to access the application features.
• The toolbar buttons provide quick access to frequently used menu
options.
Beneath the menus and toolbar buttons, the navigation pane is on the
left and the workspace is on the right.
• The navigation pane has navigation buttons and a pane above the
buttons.
• A navigation button groups major application tasks by their function.
You can hide or display navigation buttons.
• A navigation pane is above the navigation buttons. The pane lists
navigation tasks.
The workspace on the right pane changes based on the navigation button
and the task that you select. For more information about the navigation,
see Using the navigation pane on page.24.
For example, if you click Patient Cases, and then click All Cases, all the
cases stored in the database appear in the case selection table, as
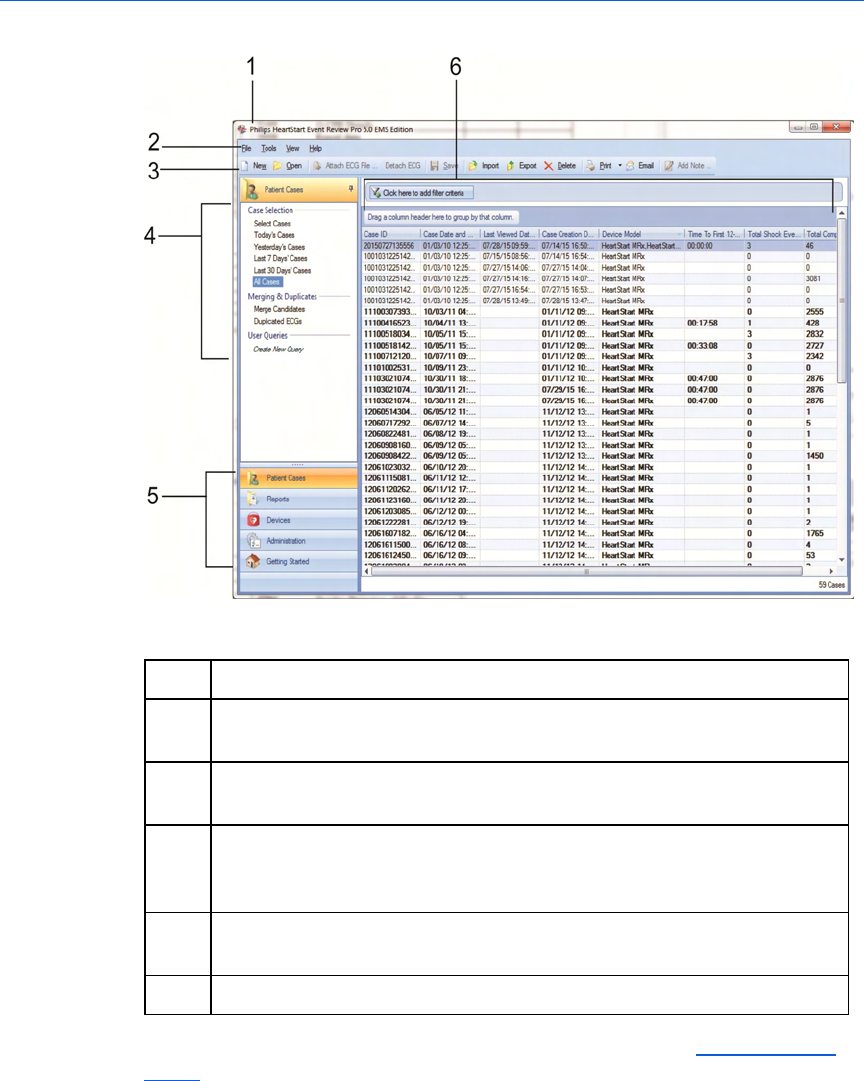
shown in the following illustration.
The case selection table
The following illustration shows the general arrangement of the case
selection table. The general arrangement is the same in all languages.
Refer the table after the illustration for definitions.
3 - Understanding the application window Event Review Pro User Guide
27
Key to illustration numbers for the previous illustration:
1 The title bar displays the product name and software version.
2 The toolbar provides quick access to frequently used actions that you
can perform for the selected workspace, as represented by icons.
3 The menu bar displays the menus suitable for the work area. Use the
menu bar to select the actions that you want to perform.
4 The navigation panelists selections in the workspace. The pane
content changes depending on which navigation button is clicked, and
which workspace is active.
5 Use the navigation buttons in the navigation pane to move among the
workspaces.
6 Case selection table, where you can double-click a row to open a case.
To search for a case, rather than viewing all cases, see Searching for
cases on page.44.
3 - Understanding the application window Event Review Pro User Guide

28
Understanding the Case Editor
When a case opens, it opens on the ECG tab, by default. The ECGtab is
the area where the waveforms are displayed.
If you navigate away from the ECG tab, you can return to it by clicking
it.
The ECGtab is one of a group of tabs on the Case Editor. Each tab in the
Case Editor provides a different work area.
The Case Editor tabs, in order, as they appear from left to right:
• Overview
• Scene
• Timeline
• Outcome (Event Review Pro EMS edition)
• Follow-up
• ECG
• Event Log
• Attachments
• Final Review
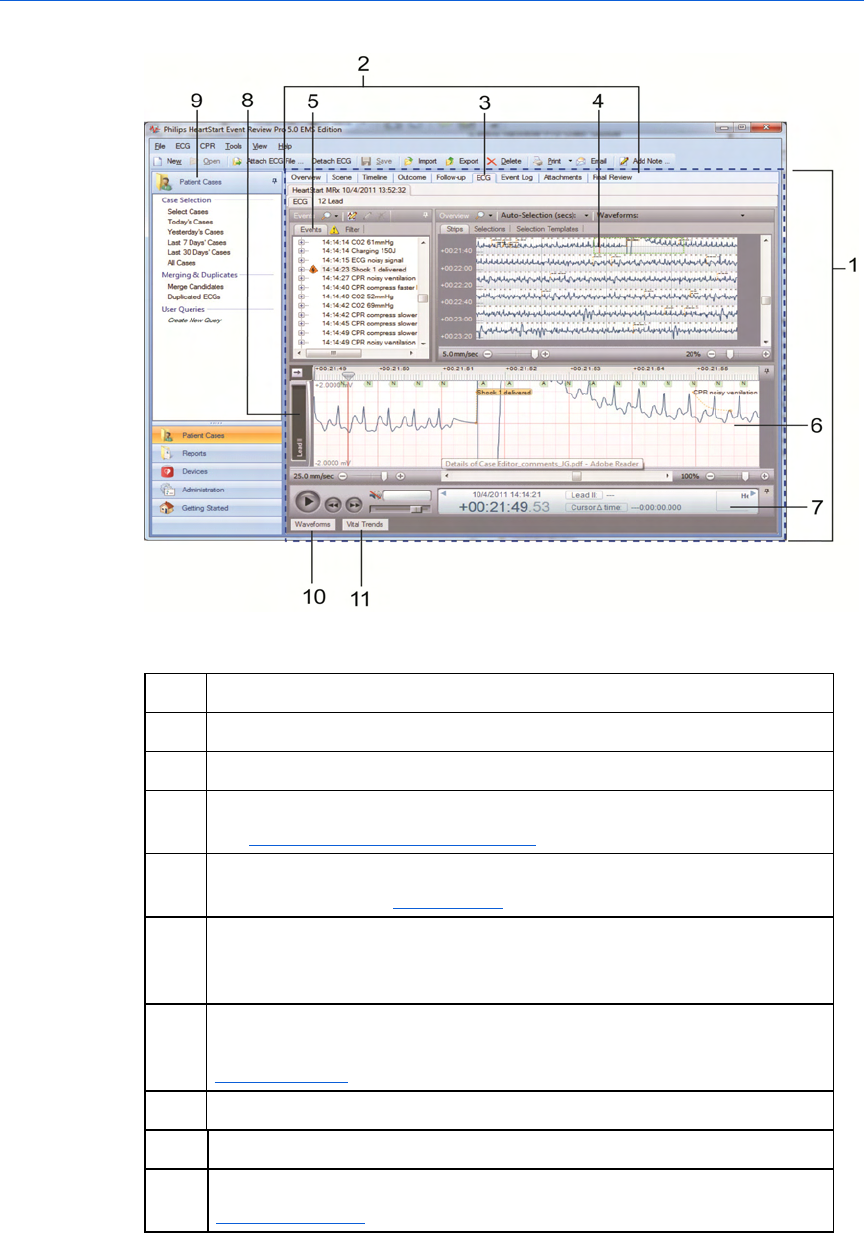
The Case Editor default view
The following illustration shows the general arrangement of the Case
Editor workspaces in the default view. The general arrangement is the
same in all languages. Refer to the table after the illustration for
definitions.
For information on viewing 12-lead ECGs in the Case Editor, see
Working with 12-lead ECGs on page.119.
3 - Understanding the application window Event Review Pro User Guide
29
Key to illustration numbers for the previous illustration:
1 The Case Editor
2 The tabs of the Case Editor, used to open different work areas
3 The ECG tab of the Case Editor, the active tab when a case is opened
4 The ECG overview pane. NOTE: For details about waveform display,
see The Case Editor Waveforms view on page.30.
5 The Events pane, used to view events in the case, ordered
chronologically. See Events pane on page.31.
6 The Rhythm strip pane. Rhythm strip shows the waveforms used for
rhythm identification, recorded by the device sequentially, in one
strip.
7 The Transport pane, used to play, rewind, or fast-forward the
waveform. If audio is present, use to play or mute audio. See
Transport pane on page.32.
8 Label for a displayed waveform, in collapsed view.
9 The Patient Cases navigation pane.
10 The Waveforms button: click to open the Waveforms pane. See
Waveforms pane on page.32.
3 - Understanding the application window Event Review Pro User Guide
30
11 The Vital Trends button: click to open the Vital Trends pane.
Navigating between the work panes in the Case Editor
When a case is first opened, the default view of the Case Editor shows
the ECG tab with the Rhythm strip pane (see item 6 in the previous
table). In the same region of the Case Editor, you can instead choose to
show the Waveforms pane, or the Vital Trends pane. Those work areas,
when not displayed, appear as buttons along the bottom of the Case
Editor. To expand one of these work areas, click the corresponding
button.
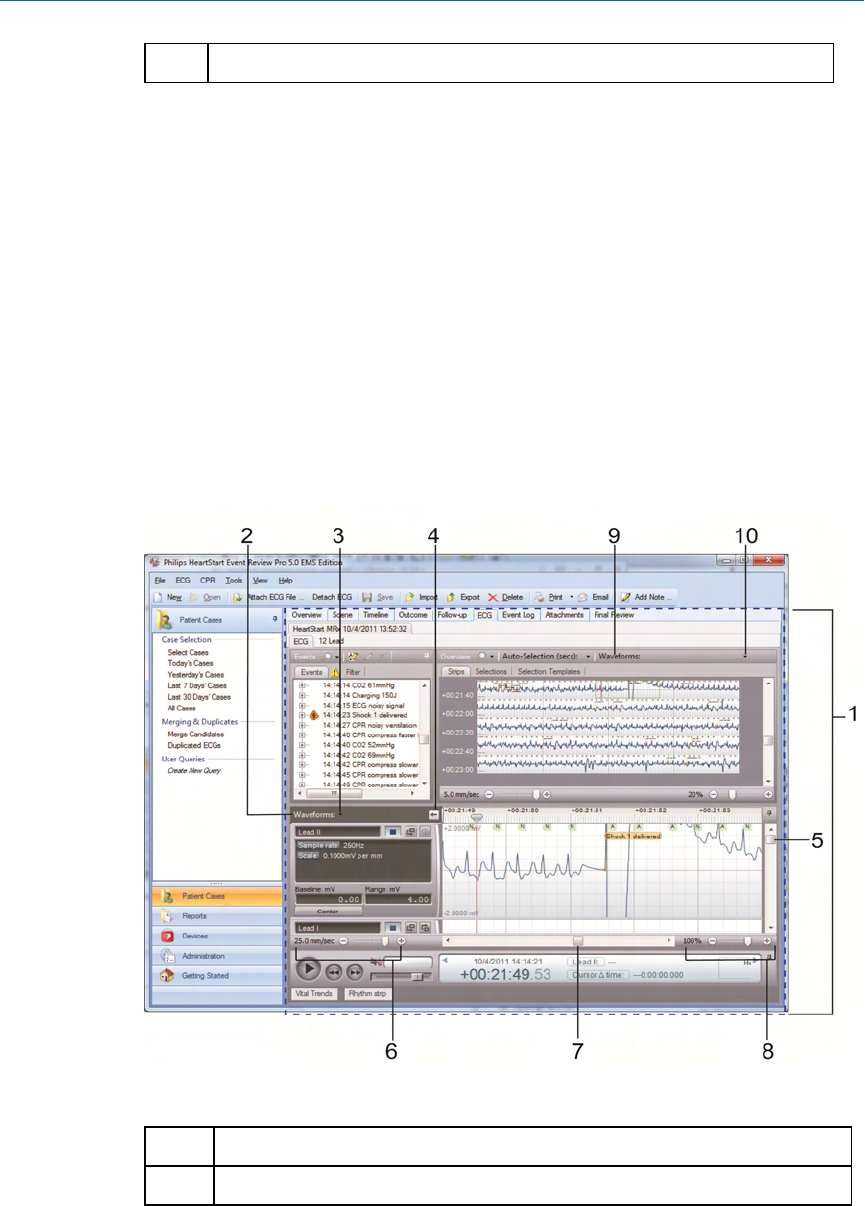
The Case Editor Waveforms view
The following illustration shows the Case Editor, with the expanded
Waveforms pane. The general arrangement is the same in all
languages. Refer the table after the illustration for definitions.
Key to illustration numbers for the previous illustration:
1 The Case Editor, ECG tab.
2 The expanded Waveforms pane.
3 - Understanding the application window Event Review Pro User Guide

31
3 The Waveforms pane drop-down menu: By default, all waveforms in
the case are shown, stacked vertically. Use the menu checkboxes to
hide/show waveforms by type. Changes from the default are
remembered in future sessions and cases.
4 Arrow button, used to expand or collapse the waveform controls.
5 Vertical scroll bar, used to view waveforms above or below the visible
area.
6 Time scale control, used to compress or expand the visible time range
of the waveforms.
7 Horizontal scroll bar, used to scroll the visible waveform left or right.
8 Zoom control, used to scale the waveform as a percentage of the
default 25mm/sec x 40mm.
9 The Waveforms Overview: By default, only the first waveform recorded
in the case is shown in this view. See Overview pane on page.31.
10 The Waveforms Overview drop-down menu: You can choose to overlay
multiple waveforms in this view. Check or uncheck boxes to show/hide
waveforms by type. Changes from the default are remembered in
future sessions and cases.
Events pane
All defibrillator events and notes appear in chronological order on the
Events pane. You can expand events or notes in the Events pane to
display additional details. For more information, see Using the Events
pane on page.93.
Use the Events toolbar to search for specific events or notes. You can
also add a note for an ECG. Use the event filter to control the display of
events and notes on the waveform. The event filter displays all possible
event types and categories. Check or uncheck them to control whether
they appear.
Overview pane
The Overview pane displays the ECG waveform with flagged events and
notes.
You can use the Overview toolbar to:
• Select the waveform displayed on the Overview pane.
• Select multiple waveforms to display them overlayed on one
another.
• Select portions of the ECG for detailed view on the Waveforms pane.
3 - Understanding the application window Event Review Pro User Guide

32
• Change the time scale for the ECG.
Waveforms pane
The Waveforms pane can provide a more detailed view of each
waveform. Event Review Pro can display multiple waveforms in this
pane.
Use the Waveforms pane to do the following:
• Add, edit, or delete notes
• Control the display of waveforms
• Analyze the quality of the CPR administered
Transport pane
The Transport pane at the bottom of the ECG view displays the ECG lead
information, the sweep bar time, and the cursor time relative to the
sweep bar location on the ECG.
Use the Play button to play the entire ECG.
If an audio recording is associated with the ECG, you can use the audio
controls to play the audio while viewing correlated events on the
waveform on the Waveforms pane.
You can also use the controls on the Transport pane to find and select
events on the waveform for review.
For more information, see Reviewing waveform information on
page.106.
Using the application tables and logs
Throughout the application workspaces, information appears in tables
and logs to help you manage your data. This topic introduces some
important tables and logs, and helps you locate them.
You can interact with most tables and logs in the following ways:
• Working with columns on page.35
• Grouping and sorting entries on page.35
• Filtering entries on page.37
• Removing filters on page.39
• Printing table entries on page.39
• Exporting data to Excel on page.40
3 - Understanding the application window Event Review Pro User Guide
33
An example of an important table in the application is the case selection
table. To view it, from Patient Cases, click All Cases. In the case
selection table, each row represents one record in the database.
You can modify your view of the table information by grouping and
sorting the rows. (See the links noted previously, about interacting with
tables.)
For help identifying the regions of the application named in the following
information, see an illustrated overview of the case selection table and
the Case Editor in Understanding the application window on page.26.
The case selection table
In Event Review Pro 4.3 and newer, the Patient Cases workspace
provides selection tools that allow you to narrow your case list to just
the cases you want to see. See Searching for cases on page.44 for more
information.
Alternatively, click All Cases to list all cases in the database. In Event
Review Pro versions earlier than 4.3, All Cases was the default view.
In the case list, the cases show the reference ID, case ID, patient ID,
case date and time, patient name, and other attributes.
In the case selection table, you can do the following tasks:
• Sort and group cases. See Sorting and grouping cases on page.62.
• Hide or display columns.Right-click on the column heading row to
select the columns to display. Check or uncheck the list items to add
or remove columns from your display. Once you customize your
column list, the settings persist for future sessions.
In Event Review Pro 5.0, new column options are added: Includes
STEMI, Pediatric, and Includes zCPR.
• Drag column headers to rearrange the left-to-right order of the
columns displayed.
• Print the table information. See Printing table entries on page.39.
• Export the case selection table to Microsoft® Excel. See Exporting
data to Excel on page.40.
• Open and delete cases. For more information, see Displaying case
details on page.61 and Deleting cases on page.72.
• View and print reports. For more information, see Working with
reports on page.147.
Once you open a case (by double-clicking a case row in the case
selection table), the Case Editor appears.
3 - Understanding the application window Event Review Pro User Guide

34
Case Editor
The Case Editor has tabs across the top, allowing you to view and edit
the case in separate work areas. The ECGtab is opened first, allowing
you to view the ECG and other waveforms. You can click the tabs to go
to the areas.
Click a tab to go to an area. On the tabs, you can view, modify, and
delete the case information. You can use the case tabs in any order.
Event Log
Event Log is one of the tabs in the Case Editor. The log for a selected
case lists all defibrillator events and notes written for that case.
Once you open a case and select the Event Log tab, you can see details
for an event. You can sort, group, and filter the device events and notes
that a responder or reviewer added to the case. You can also print the
events for the open case. See Printing table entries on page.39.
You can export the Event Log to Microsoft® Excel. See Exporting data to
Excel on page.40.
For more information, see Reviewing case events on page.133.
Attachments table
Attachments is one of the tabs in the Case Editor. The Attachments table
lists each file that is attached to the case. It identifies each attachment
by the file name, file type, and date that the file was attached. Use the
Attachments tab to manage the files that you attach to the open case.
Once you open a case and click the Attachments tab, you can add and
delete an attached file. You can also sort, group, and filter the list of
attached files. You can print the list of attachments. See Printing table
entries on page.39. You can export the Attachments table to Microsoft®
Excel. See Exporting data to Excel on page.40.
For more information, see Attaching files to a case on page.135.
System Log
From Administration, click System Log to open the System Log
workspace.
In the System Log workspace, the System Log table identifies all system
activities based on the type, action, description, date and time, and the
user name. You can print the system log. See Printing table entries on
page.39. You can export the System Log table to Microsoft® Excel. See
Exporting data to Excel on page.40.
For more information, see Working with the System Log on page.145.
3 - Understanding the application window Event Review Pro User Guide

35
All Devices table
From Devices, click All Devices to open the All Devices table. AED self-
test data is available in this table. You can print the All Devices table.
See Printing table entries on page.39. You can export the All Devices
table to Microsoft® Excel. See Exporting data to Excel on page.40.
For information about self-test data, see Importing device self-test data
using the Device Self-test Wizard on page.209.
Working with columns
You can customize the columns that appear on the table. You can resize
the width of a column. You can also hide or display the columns on the
table and the order in which they appear.
• To change a column width, use the mouse to click and hold the
column border, and then drag the border to the size you prefer.
• To size a column to fit the width of its current content, use the
mouse to click the column border, and then double-click.
• To change the order in which columns appear, use the mouse to click
and hold the column header, and then drag the column to the left or
right to a new location.
To hide or display the columns on the table
1. Right-click a column header name to display a shortcut menu.
2. Click Columns to display a list of column header names.
3. Click a check box for each column that you want to hide or display on
the table.
Column headers with a check mark display on the table.
Grouping and sorting entries
In any grid, such as the Event Log and the System Log, you can arrange
entries for your convenience.
Displaying and hiding entries
Right-click any column to display a shortcut menu. The shortcut menu
lists all columns available, with check marks next to those that currently
display. Click the check boxes to display or hide any of the columns.
3 - Understanding the application window Event Review Pro User Guide

36
Sorting entries
You can click the column header to sort the list of values in ascending (1
to 9, or a to z) or descending (9 to 1, or z to a) order.
Grouping entries
You can group entries so as to sort them by multiple criteria. For
example, you can select a primary (major)sort by Type and a
secondary (minor)sort by Description, or a primary sort by Device and
a secondary one by Event. Use the gray area above the entries to set up
groups.
If you want to create further minor sorts, you can then drag more
column headings into the gray area to create subgroups.
To group information
1. Click a column header and drag it to the grouping area labeled Drag
a Column Header Here to Group by That Column.
The column name moves to the grouping area, and the table displays
the groups you create.
2. Repeat step 1 as necessary to create more groups. Each additional
column name becomes a minor sort below the major sort that you
created in step 1. To rearrange any of these column names in the
grouping area, drag them to a new position. The table readjusts to
the new order of sorts.
3. Click the column header to sort the order in which the grouped
information appears.
To ungroup information
1. Click a column header (for example, Type or Event) in the grouping
area and drag it below the table header.
If it is the only column header in the grouping area, the table then
reverts to its original organization.
If other column headers are still in the grouping area, the table
readjusts to sort according to the column headers that are still there.
3 - Understanding the application window Event Review Pro User Guide

37
Filtering entries
Use a filter to limit the number of entries that appear. A filter specifies
selection criteria for the entries that appear. Each additional criterion
that you specify for the entries further refines the subset of entries that
appear. You can combine filtering with sorting and grouping.
Use the upper area above the entries to filter the list of entries. To set
up a filter, select a column header and specify a value to use as a
criterion. To further refine the list, set up additional criteria.
For example, you might first filter for entries within a certain date
range. Then, you might filter these for an institution.
To filter entries
You can click the arrow to sort the entries in ascending (1 to 9, or a to z)
or descending (9 to 1, or z to a) order.
1. Click Click Here to Add Filter Criteria.
A row for the criteria condition appears.
2. Click Choose Field and click a column name. For example, if you
are filtering by the Description column, click Description.
3. Click Equal (to open the menu of operators, where Equal is one of
the possible modifiers you can choose), and then click an operator
that qualifies the value for the column. You can specify a specific
value or a range of values, depending on the operator you choose.
For a description of each operator, see Comparison Operators,
below.
For example, to display only entries that exactly match a specified
field value, click Equal.
4. In the next field, click the down arrow and select or type a value for
the column that you selected.
5. (Optional) Click Add Criteria to add another criteria condition.
The list of entries updates and displays another criterion row.
6. Click And and select a logical operator to evaluate the new criterion
in relation to the previous criterion. For a description of each
operator used to evaluate criteria, see Logical Operators, below.
7. Repeat steps 2 through 6 for each additional criterion you want to
add to the filter.
3 - Understanding the application window Event Review Pro User Guide
38
Event Review Pro compares the entries that meet the last condition
to the previous condition. Only the entries that meet the two
conditions appear. The process repeats for each preceding criteria
condition.
Comparison operators table
You can select a comparison operator to filter a number, a date, or text.
For example, to see all entries created after June 1, 2001, click Greater
Than and type 06/01/2001. You can enter a date using the MM/DD/YYYY
format or click the down arrow to choose a date from the calendar. You
can filter for specific dates or for a range of dates. You cannot filter for
specific times. For the purpose of filtering, a time of 12:00 AM is used
for all dates. To select a range of dates, use the Between operator.
Use the comparison operators in the following table with these columns:
User Name, Type, Action, Description, and Date and Time:
Comparison operator Lists the entries whose values for the column
...
Equal ...are the same as the selected value.
Not equal ...are not the same as the selected value.
Greater than ...follow or are larger than the selected value.
Less than ...are preceded or are smaller than the selected
value.
Greater than or equal to ...are the same, follow, or are larger than the selected
value.
Less than or equal to ...are the same, precede, or are smaller than the
selected value.
Between ...are between the first and last selected values. Use
to specify a range of dates, numbers, or text.
Not between ...are before the first selected value or after the last
selected value.
Is null ..do not exist.
Is not null ...exist.
Is empty ...do not appear.
Is not empty ...appear.
Any of ...include the selected items.
None of ...do not include the selected items.
3 - Understanding the application window Event Review Pro User Guide
39
Logical operators table
Select the logical operator from the list of operators that you want to
use to compare two criteria. Event Review Pro outlines the criteria and
connects the conditions with a line.
Logical operator Description
And Displays the entry when the criteria in the first and the
second conditions are both met.
And not Displays the entry when the criteria in the first condition
is met and the criteria in the second condition is not met.
Or Displays the entry when either the criteria in the first
condition or the criteria in the second condition are met.
Or not Displays the entry when the criteria in the first condition
is met or when the criteria in the second condition is not
met.
Removing filters
If you filter the entries and then exit Event Review Pro before removing
the filter, the filter settings persist; they appear the next time you use
the feature.
You can return a single column to its unfiltered state or remove all the
filters.
If you remove a criterion, Event Review Pro removes it and each
criterion that appears below it.
To return a single column to its unfiltered state, click Remove Criteria
for that criteria condition.
To remove all filters, on the first criterion row, click Remove Criteria
.
Printing table entries
You can print a table in portrait or landscape format.
3 - Understanding the application window Event Review Pro User Guide

40
To print table entries
1. On the File menu or toolbar, click Print.
2. Click Table.
3. In the Print window, in the Select Printer list, click the printer that
you want to use.
4. (Optional) Click Preferences.
5. (Optional) In the Print Preferences window, in the Orientation area,
click an orientation.
To print the entire width of the system log table, click Landscape.
6. Click OK to close the window.
7. (Optional) Complete the fields in the Page range area.
8. Click Print or OK.
Exporting data to Excel
Tables and logs in Event Review Pro can be exported to a Microsoft®
Excel spreadsheet. Files are exported in the .xlsx format. Users with
older versions of Excel, such as Excel 2003, can open the .xlsx files
using the Microsoft Office Compatibility Pack. For more information,
contact Microsoft.
To export table data to Excel
1. Right-click in the table.
A menu opens.
2. In the menu, click Export table.
3. Use the settings in the resulting window to export the file.
Completing fields
Use a pointing device, such as a mouse or stylus, to select field values
from a window and to change the way that information on tables and
logs appears.
While working in the Case Editor, when you use the mouse to click or
point to a field, a menu with options for that field might appear.
3 - Understanding the application window Event Review Pro User Guide

41
• If check boxes appear to the left of the options, you can select
multiple options for the field.
• If check boxes do not appear to the left of the options, you can select
only one option for the field.
• If an option is a text box, type or write the information using your
keyboard or stylus.
• If you want to remove the selected option, click Clear.
• If a menu does not appear, type or write the information using the
keyboard or stylus.
Resizing panes and workspaces
You can change the default layout of the application window. The revised
layout persists the next time you use the navigation pane or workspace.
To resize a pane or workspace
1. Use the mouse to click and hold a border on the pane that you want
to resize.
2. Drag the border in the appropriate direction to a new location.
The size of the adjacent panes adjusts.
Using a Tablet PC
You can also work in Event Review Pro using a Tablet PC. Use the stylus
in the same way as you would use a mouse or pointing device. You can
also use the stylus with the Tablet PC Input Panel to enter text.
To complete a text entry field in tablet mode
1. Use the stylus to tap a field.
The tablet displays a floating icon.
2. Tap the icon to display the Tablet PC Input Panel.
3. Click one of the following three modes to enter the information:
• Writing pad
• Character pad
• On-screen keyboard
3 - Understanding the application window Event Review Pro User Guide

42
4. Type the information. For more information, click the Help icon on
the input panel.
5. Click Insert or tap the Enter key on the input panel.
Saving your work
Philips recommends that you save your modified patient details
periodically. To save your work, on the File menu or toolbar, click Save
.
Restoring confirmation messages
A confirmation message does one of two things:
• Informs you that an action might result in a change to a
configuration
• Warns you that an action may cause a permanent change
By default, confirmation messages are enabled.
If you have disabled confirmation messages (by checking the Do Not
Show This Message Again box when such a message appears), you can
re-enable them at any point.
To restore confirmation messages
• On the Tools menu, click Options, Restore Defaults
Confirmations, and then Confirmations.
3 - Understanding the application window Event Review Pro User Guide

4
Working with cases
The word "case" refers to all the information relating to a specific
cardiac or monitoring event. This includes information recorded by
supported defibrillators and information entered by a user. For
information about supported defibrillators, see Supported defibrillators
on page.192.
The first step in working with ECG information (ECG) is to create a case.
You can do this in the following ways:
• Use the case wizard to download data from a defibrillator or its data
card.
• Import a case with an ECG from a .mic, .hic. or .cod file created by
another application, such as HeartStart Data Messenger.
.Mic files are created by the export function of products such as
HeartStart Data Messenger and Event Review Pro.
.Hic files are created by third-party ePCRsoftware using the Philips
Software Development Kit (SDK), and optionally by HeartStart Data
Messenger.
.Cod files are created by the export function of older products such
as Review Express Connect and Event Review Pro 3.5.
• Create a case that does not have ECG information in it yet and add it
later.
Starting in Patient Cases
To create, review, and manage cases, click the Patient Cases
navigation button.
Use the Case Selection options on the Patient Cases navigation area to
control which cases are available for viewing. You can chose from
different date ranges, or use other search options. For more
information, see Searching for cases on page.44.
43

44
Searching for cases
You can use different methods to control which cases appear in the case
selection table. This helps you when you need to quickly find a specific
case for reviewing or reporting. Ways you can search for cases include:
• Case Selection options
• Select Cases
• Using a Query (see the Using queries on page.46)
Case Selection options
The Patient Cases navigation pane contains built-in date ranges. You can
select from the following case dates:
• Today's Cases
• Yesterday's Cases
• Last 7 Days' Cases
• Last 30 Days' Cases
• All Cases
Click any of the above to view a list of matching cases. For a large
database with more than 10,000 cases, clicking All Cases may require
some time. For the fastest response, limit your selections to view fewer
than 2,000 cases.
Using Select Cases
You can search cases by combining the case date range, case properties
and device data. You can search by date ranges only or further define
your search by adding properties for the case and device data.
To search for a case
1. Do one of the following:
• Under Select Cases, click From the last, type any number of
days up to 365, and click Select. The date is calculated from
today’s date and is automatically saved.
• Click From and then choose a date range using the pop-up
calendar to select the dates. This setting is saved only for the
current session.
2. Under the With portion of the screen, in the Case State field, click
any of the following case states:
4 - Working with cases Event Review Pro User Guide

45
• Any
• Unopened
• Not yet reviewed
• Review complete
3. Under the With portion of the screen, type information in any of the
following fields:
• Reference ID
• Institution Name
• Case ID
• Patient ID
• Patient Last Name
Note: Supported wild cards are _ (single-character match) and
% (multiple-character matches). For example, Patient ID = 004_
will match any case in the date range with a Patient ID exactly
four characters long starting with 004, but 004% will match all
cases in the date range with a Patient ID starting with 004
regardless of its length.
4. Under the And have portion of the screen, click any of the following
device data fields:
• ECG
• 12-Lead
• Shocks
• Q-CPR
• Inadequate CPR
• Audio
• EtCO2
• STEMI
• Pediatric Pads
• Device Type
For Device Type, you select from a list of devices. You can select
one or more device types for your search.
Note: All selected Device data properties must exist in the case.
Your selections are saved for future use.
5. Click Select.
If your search criteria are met, then a list of cases appear.
The height of the case list can be adjusted. Adjust it by placing the
mouse over the divided lines, and then clicking and dragging. Once
set, the height is saved for future sessions.
6. Double-click a case to view the case.
Also see these topics about using the case list:
4 - Working with cases Event Review Pro User Guide

46
• Using the application tables and logs on page.32.
• Sorting and grouping cases on page.62.
Using queries
You can create and save custom queries to search for cases using the
advanced case selection editor.
For other ways to search for cases, see Searching for cases on page.44.
To create a custom query
1. On the left side of the Patient Cases screen, under User Queries,
click Create New Query.
2. In Advanced case selection, at the top of the work area, do one of
the following:
• Click From the last, type any number of days up to 365.
• Click From and then choose a date range using the pop-up
calendar to select the dates.
3. From the Choose field menu, click a parameter, such as Patient ID,
or Case creation date and time.
4. To the right of the Choose field menu is the operator menu. Click
an appropriate operator for the selected parameter. The default
operator is Equal; however, it is not always the best option.
When querying for Case creation date and time, consider using one
of the following operators:
• Between
• Greater than
• Greater than or equal to
• Less than
• Less than or equal to
NOTE: When building queries against time fields (such as Recording
length, Time to first shock, and Time to first 12-lead acquired) the
time can be entered either in milliseconds (such as 60000 for 1
minute) or formatted as hh:mm:ss (such as 00:01:00 for 1 minute).
5. To add more criteria to a query, click Add Criteria , and add
multiple criteria with AND/AND NOT/OR/OR NOT statements.
6. To remove an added criteria from a query, click Remove Criteria
.
4 - Working with cases Event Review Pro User Guide

47
7. Click Select to display the case list.
The case list appears below the query, in a separate pane. The
height of the case list can be adjusted. Adjust it by placing the
mouse over the divided lines, and then clicking and dragging. Once
set, the height is saved for future sessions
8. Click the Save as button when the query works as expected.
9. Type a name for the query and click OK.
The named query will appear in the navigation pane for quick
access.
Note: User queries are accessible only by the user who created them.
Each user may create up to 15 queries.
Note: To delete a query, right-click the named query to expose the
Delete menu. Clicking Delete will delete the named query and remove it
from the navigation pane.
Creating a case (including ECG)with
wizards
There are several wizards available in the Getting Started workspace, in
the Welcome area. Use them to guide you through the steps required to
download patient data from a defibrillator or its data card, import a
case, or open a case. For more information, see Understanding the
Getting Started options on page.23.
On the Getting Started navigation pane, the Case wizard is available.
Click Case Wizard to start the wizard. It guides you through the steps.
The Case wizard presents all its options in a sequence of screens. To
bypass the options you do not want, simply un-check those checkboxes
on the screens and click Next. Continue to the last action in the wizard,
Complete the tasks, click Proceed, and then click Exit. It is then that
the case is written to the database. The case opens in the Case Editor
(unless you un-checked the Open case checkbox in the wizard).
The Case wizard workflow
The wizard steps are described here, for your reference:
To start the Case wizard
1. On the navigation pane, click the Getting Started navigation
4 - Working with cases Event Review Pro User Guide

48
button.
2. On the Getting Started navigation pane, click Case Wizard.
3. In the Welcome Page window, click Next to continue.
The Attach ECG page appears.
Attaching an ECG
To attach an ECG from a Philips defibrillator or its data card
1. Use the table in AttachingECGs table on page.54 to identify the
method used to add/download an ECG from your defibrillator.
2. Follow the instructions in AttachingECGs on page.51to attach the
ECG.
You can also use the wizard to help you transfer data. Click an icon
under Data source to start the instructions.
3. Click Next.
The Case Summary page appears.
Entering case summary information
On the Case Summary page, you can handle case information three
ways:
• Add new information
• Accept the information that the defibrillator provides
• Override the information from the defibrillator
Different defibrillators may capture different patient information as
part of recorded ECGdata. If you use data from the HeartStart
MRx, the ECG information might include patient information such as
name, age, and gender.
To complete the Case Summary fields
1. Event Review Pro completes the Case ID field. You can change the
case ID here if you want to.
For defibrillators that generate an incident ID, such as HeartStart
MRx, the case ID corresponds to this incident ID. Each device has its
own system for generating an ID.
2. Fill in the Patient ID, Last Name, and First Name fields.
4 - Working with cases Event Review Pro User Guide

49
3. In Reference ID, type a reference ID for the case that you can use
to identify it later.
For example, "ER Room 3" or "Engine #3, Station 27". This
information may already appear, if the case was imported from an
application or device that provided it.
4. In Institution, type an institution name for the case. For example,
"Valley Hospital" or "District 5 Fire Department." This information
may already appear, if the case was imported from an application or
device that provided it.
5. Click Next.
The Export Case page opens.
Exporting a file
The wizard guides you in exporting a case to a .mic file. The file is then
available to a colleague who uses Event Review Pro.
1. To export a file containing the ECGand case information at the end
of the wizard, check the Export to a File box, and verify the file
name and folder where you want to save it.
The exported file is then available to be imported into your
colleague's Event Review Pro database.
2. If you want to password-protect the exported file, in the Password
box, type the password.
Passwords can have up to 16 characters. They are case-sensitive.
Remember to provide this password to anyone who needs to open
the exported file.
3. If you want to remove confidential patient identifiers such as name,
patient ID, and age (if over 90) from the exported file (called
"redaction"), check the Remove Patient Identity box.
4. Click Next.
The Printing a report page opens.
Printing a report
The wizard guides you in printing a case report.
1. To print a report at the end of the wizard, check the Print a Report
box.
2. Click Next.
4 - Working with cases Event Review Pro User Guide

50
The Email page opens.
Emailing case data
1. If you want to email case data as part of the Case wizard, check the
Email Case box, type or click the appropriate address or
addresses, change the subject if you want, and type a note to
accompany the case if you want.
2. You can also password-protect the file or remove patient identity
information.
3. Click Next.
The Complete the task page opens.
Opening the case
The last pane of the wizard, Complete the tasks, has a checkbox for
Open case.
By default, the case will open after all tasks are completed (the Open
case box is checked), so that you can view the case, add information,
and make annotations.
1. Check or un-check the Open Case box, depending on whether you
want to open the case at the end of the wizard.
2. Click Proceed.
The status of each task for the case being processed appears,
sequentially.
3. When the processing is complete, click Exit.
If you chose to send by email, and if your email system needs
verification before it can send the email, a confirmation message
appears. Click Allow, and the process continues.
Creating a case
You can create a case manually or automatically, with or without
ECGinformation.
To manually create a case that has ECG information
1. Use one of the following methods:
4 - Working with cases Event Review Pro User Guide

51
• Click the Getting Started navigation button, and then click
Case Wizard.
• On the File menu, click Case Wizard.
The Case Wizard window opens. For more information, see Creating
a case (including ECG)with wizards on page.47.
2. Follow the on-screen instructions.
To manually create a case that does not initially contain ECG
information
1. On the File menu or toolbar, click New.
The tabs for the case in the workspace appear.
2. Complete fields to briefly describe or identify the case. For example,
add the site of collapse and location detail.
3. Click Save.
At a later time, you can open the case, attach ECG information, and
complete the tabs.
For information about those tasks, see:
• AttachingECGs on page.51
• Working with ECGs on page.88
• Adding case details on page.78
AttachingECGs
"Attaching" an ECG means reading ECG information from a defibrillator
(downloading it), a defibrillator data card, or a file and adding it to a
case.
You can attach an ECGto a case you are creating, or you can open an
existing case and attach an ECGto it. You can add extra ECGs from other
defibrillators as well. Depending on the defibrillator, you can attach
continuous ECG information and 12-lead information from a data card or
a file, or through a Bluetooth or an infrared transmission.
If you need help identifying the defibrillator or inserting a data card, see
Working with defibrillators on page.192.
4 - Working with cases Event Review Pro User Guide

52
The defibrillator that you use determines the method that you can use to
attach an ECG. The Attach ECG window lists the types of transfer
methods that you can use to attach an ECG or 12-lead report on the left
pane. The window lists data files in the right pane. The data files include
those that were transmitted or are stored on a removable device.
To display the Attach ECG window for an existing case
1. Click the Patient Cases navigation button, and use Case Selection
to display a list of cases. For more information on selecting a case,
see Searching for cases on page.44.
2. On the list of cases, double-click a case to open it.
3. On the toolbar, click Attach ECG File.
Any time you have a case open for reviewing or editing, you can
attach an ECG. On the ECG menu, click Attach, or on the toolbar,
click Attach ECGFile.
To attach an ECG, or ECGs
1. Make sure that the case is open. For more information, see
Displaying case details on page.61.
2. Prepare to use the correct data transfer method for your device. For
details, see Choosing the correct data transfer method for your
device on page.53.
3. On the ECG menu, click Attach, or on the toolbar, click Attach ECG
File.
4. On the right pane of the Attach ECG window, find the ECG file you
want to attach.
The medium for the data source appears in the Source column.
5. Use one of the following methods:
• Click the file for the ECG you want to attach, and then click
Open.
• Double-click the file for the ECG you want to attach.
The attached ECG appears on the ECG tab in the open case.
For information on viewing a case in the Case Editor, see
Understanding the Case Editor on page.88.
4 - Working with cases Event Review Pro User Guide
53
Attaching additional ECGs
If the emergency response for a single patient involved multiple ECGs
on supported defibrillators, you can attach all ECG files to the same
case.
The process for attaching additional ECGs is the same as attaching the
first ECG. The method varies depending on the device from which you
are transferring the ECG. Follow the process in To attach an ECG, or
ECGs on page.52.
Once the additional ECG is attached, you can view it on the ECG tab in
the Case Editor. On the ECG tab, a sub-tab appears for each attached
ECG. The tab text identifies the defibrillator, date, and time transmitted
by the defibrillator. For example: HeartStart MRx 3/10/2007 3:10:44
PM. For information on viewing a case in the Case Editor, see
Understanding the Case Editor on page.88.
Choosing the correct data transfer method for your device
Event Review Pro activates the following defibrillator data transfer
methods automatically.
In the wizard, help is available to guide you. You can also click the
following icons to attach ECG data:
• Bluetooth— opens a wizard with instructions for establishing
either HeartStart MRx or FR3 connections.
If you click MRx, a window opens, allowing you to point Event
Review Pro to the Bluetooth Exchange Folder where the
transmitted Bluetooth files will be saved. For more information,
see Downloadingan ECG using a HeartStart MRx Bluetooth
transmission on page.57.
If you click FR3, a wizard appears with instructions for how to
put the FR3 into Administration mode and ready it for wireless
data transfer. For more information, see Attaching an ECG using
an FR3 Bluetooth transmission on page.56.
• IrDA—opens help on how to transmit data from HS1 and FRx
defibrillators. For more information, see Downloadingan ECG from
an infrared connection on page.58.
4 - Working with cases Event Review Pro User Guide

54
• File System—displays the Open window that you can use to
browse and attach ECG information from the file system on your
computer.
AttachingECGs table
Use the following table to identify the method and instructions used to
read and/or download an ECG from a defibrillator and attach it to a case.
Defibrillator
model
Method used to read
data Instructions
HeartStart FR2
Series
Card reader See Attachingan ECG from a data card
on page.55.
See also Reading FR2 series cards on
page.200.
HeartStart FR3
Series
Card reader See Attachingan ECG from a data card
on page.55.
HeartStart FR3
Series
Bluetooth connection See Attaching an ECG using an FR3
Bluetooth transmission on page.56.
HeartStart XL Card reader See Attachingan ECG from a data card
on page.55.
HeartStart XL+ USBdata drive See Attachingan ECG from a USB port
on page.56 .
Efficia DFM100
defibrillator/monitor
(where available)
USBdata drive See Attachingan ECG from a USB port
on page.56 .
HeartStart MRx
monitor/defibrillator
Card reader See Attachingan ECG from a data card
on page.55.
HeartStart MRx
monitor/defibrillator
HeartStart MRx, version
9.0 or later Bluetooth
connection
See Downloadingan ECG using a
HeartStart MRx Bluetooth transmission
on page.57.
HeartStart MRx
monitor/defibrillator
HeartStart MRx with
BLDT
See Using the HTTPImport Service on
page.143.
Also see the HeartStart MRx
Monitor/Defibrillator Instructions for
use, and the HeartStart Data
Management Solutions
Implementation Guide.
4 - Working with cases Event Review Pro User Guide

55
Defibrillator
model
Method used to read
data Instructions
HeartStart FRx Infrared connection See Downloadingan ECG from an
infrared connection on page.58.
HS1 family of
defibrillators,
including
HeartStart Home
and HeartStart
OnSite defibrillators
Infrared connection See Downloadingan ECG from an
infrared connection on page.58.
Imported case file File system See Attachingan ECG from a file on
page.60.
Event Review Pro documentation uses the HS1 or HS1 family of
defibrillators to refer to HeartStart Home, HeartStart OnSite, and
HeartStart HS1 Defibrillators.
If you need help identifying the defibrillator or using a transfer method,
see Working with defibrillators on page.192.
Attachingan ECG from a data card
The FR2, FR3, XL, and HeartStart MRx defibrillators can use a data card
to transfer the recorded ECG and other data from the defibrillator to
Event Review Pro.
To attach an ECG from a data card
1. Insert the data card into the card reader.
2. Use one of the following methods:
• On the ECG menu, click Attach.
• On the toolbar, click Attach ECG File.
The Attach ECG window opens, listing ECG files grouped by the
source of the file.
3. On the right pane of the Attach ECG window, find the file that is
associated with the ECG informationor 12-lead report. The medium
for the data source appears under the Source column.
4 - Working with cases Event Review Pro User Guide

56
4. (Optional for FR2 Series defibrillators) If the pediatric pads were
used, click the FR2 Pediatric option. This allows Event Review Pro to
properly calculate the pads impedance and the shock energy. If you
have any doubt about which pads the responder used, contact the
responder.
Click the ECG you want to attach. In the Device column, right-click the
FR2 icon and click FR2 Series with infant/child pads. The pink
teddy bear icon for FR2 infant/child pads appears.
5. Use one of the following methods:
• Click the file for the ECG you want to attach, and then click
Open.
• Double-click the file for the ECG you want to attach.
Event Review Pro downloads the file and attaches the ECG to the
case. It appears on the ECGtab.
Attachingan ECG from a USB port
The HeartStart XL+ defibrillators and Efficia DFM100
defibrillator/monitors use a USB data drive to transfer the recorded ECG
from the defibrillator to Event Review Pro.
To import data from the USBdata drive to Event Review Pro
1. Insert the USB data drive into the USBdata port on the computer
running Event Review Pro.
2. Ensure that Windows recognizes the USBdata drive as a connected
device, and that Event Review Prois running.
Windows detects the USBdata drive and begins transferring data. All
data is transferred to Event Review Pro.
3. When data transfer is completed, follow Windows procedures to
safely remove the USBdata drive from your computer.
Attaching an ECG using an FR3 Bluetooth
transmission
You can transfer data from the FR3 to the computer using Bluetooth
wireless transmission. Once the FR3 data is saved in Event Review Pro,
it becomes a case.
4 - Working with cases Event Review Pro User Guide

57
Prepare the FR3 for Bluetooth data transfer as described in Retrieving
FR3 data using Bluetooth transmission on page.195.
This task can also be performed with the help of a wizard. For that
method, from Getting Started, in the Welcome area, click Download
patient data from your defibrillator or its data card.
To attach an ECG using a Bluetooth transmission
1. In Event Review Pro, use one of the following methods:
• From Getting Started (or from Patient Cases, from the menu),
click New to initiate a new case, and then click Attach ECG
File.
• From Patient Cases, open an existing case, and then, from the
menu, click Attach ECGfile.
The Attach ECG window opens.
2. If you need to be reminded of how to prepare the FR3 for wireless
data transfer, in the Attach ECG file window, click the Bluetooth icon
to open the Bluetooth Wizard, and review the steps.
3. Watch the Attach ECG window and the status line, as well as the FR3
screen, for feedback.
4. When data appears in the Attach ECG window, verify that the device
type, serial number, date, and time match your device.
5. Use one of the following methods to attach the ECG to the case:
• In the Attach ECG window, click the file for the ECG you want to
attach, and then click Open.
• Double-click the file for the ECG you want to attach.
Event Review Pro attaches the ECG to the case, and the ECG tab
appears with the attached ECG waveform(s).
6. Remember to save the case.
Downloadingan ECG using a HeartStart MRx
Bluetooth transmission
Depending on the version of your HeartStart MRx monitor /defibrillator,
and its options, you can transfer files from the HeartStart MRx to the
computer using Bluetooth transmission.
4 - Working with cases Event Review Pro User Guide

58
To attach an ECG using a Bluetooth transmission
1. Set up the Bluetooth software and adapter on the computer. For
information, see your defibrillator documentation and Setting up
Bluetooth transmissions for the HeartStart MRx on page.197.
2. Send the Bluetooth transmission. For information, see Sending the
HeartStart MRx Bluetooth transmission on page.199.
3. Use one of the following methods:
• On the ECG menu, click Attach.
• On the toolbar, click Attach ECG File.
4. On the right pane of the Attach ECG window, find the file that is
associated with the ECGor 12-lead.
The medium for the data source appears under the Source column.
5. Use one of the following methods:
• Click the file for the ECG you want to attach, and then click Open.
• Double-click the file for the ECG you want to attach.
Event Review Pro attaches the ECG to the case.
6. If the file does not appear in the right pane, click Bluetooth on the
left pane of the Attach ECG window and use the Bluetooth Wizard to
navigate to the Windows Bluetooth Exchange Folder.
• On the Browse for Folder window, navigate to the Windows
Bluetooth Exchange Folder for the user. The default folder for is
located at C:\Users\user name\My Documents\Bluetooth
Exchange Folder.
• If you specified a different folder when setting up your Bluetooth
stack or Neighborhood, navigate to that folder.
• Event Review Pro scans the location you specify to find the ECG.
It enables the Bluetooth icon to the right of the status bar while it
reads the Bluetooth files.
One or more ECG tabs appear with the attached ECG waveform(s).
Downloadingan ECG from an infrared
connection
The HeartStart HS1 family of defibrillators and the FRx defibrillator use
an IrDA connection to attach an ECG to Event Review Pro.
4 - Working with cases Event Review Pro User Guide

59
To attach an ECG from an infrared connection
To retain the actual event times, do not remove the battery from
HS1 or FRx defibrillators. If you remove the battery before you
download the case information, the files created before you
removed the battery might appear in the list without a date and
time (though they will show elapsed time for events and ECG data
when you open them). For information on HSI and FRx
defibrillators' case date and time, see Determining the HS1 and FRx
case date and time on page.202.
1. Set up the infrared connection between the defibrillator and the
computer. For more information, see Using infrared connections for
the HS1 and FRx on page.203.
2. Use one of the following methods:
• On the ECG menu, click Attach.
• On the toolbar, click Attach ECG File.
3. Put the defibrillator in Administration mode. For more information,
see Setting up the infrared connection on page.204.
4. (Optional) If you need help with setting up the IrDA handshake for
HS1 or FRx defibrillators, use the IrDA Wizard.
On the left pane of the Attach ECG window, click IrDA . On the
IrDA Wizard window, follow the on-screen instructions. The IrDA
icon to the right of the status bar appears enabled while it transfers
the data.
5. When the defibrillator connects, the voice message says Sending (for
the HS1) or Transferring data (for the FRx).
6. On the right pane of the Attach ECG window, find the file that
contains the ECG. This process may take several minutes.
The data source appears in the Source column.
7. Use one of the following methods:
• Click the file for the ECG you want to attach, and then click
Open.
• Double-click the file for the ECG you want to attach.
Event Review Pro downloads the file and attaches the ECG to the
case.
8. A message appears requesting the case start time when the HS1 or
FRx case does not have a start time. Event Review Pro requests the
start time.
4 - Working with cases Event Review Pro User Guide

60
Attachingan ECG from a file
When you attach an ECG or 12-lead information from a file, the file can
be on the hard drive, external media (a data card), or the network.
This might occur when a remote user downloads a case via another
HeartStart application, like Data Messenger, and saves it to a file for
your subsequent use.
To use a wizard to attach a file, from Getting Started, in the
Welcome area, click Download patient data from your
defibrillator or its data card.
To attach an ECG from a file
1. Use one of the following methods:
• On the ECG menu, click Attach ECG.
• On the toolbar, click Attach ECG File.
2. On the left pane of the Attach ECG window, click File System .
The Open window lists file extensions in the Files of Type field.
3. In the Files of Type field, click the file type.
4. Click Device Data for files with the .s01, .index, .cod, .xml, or .mic
extensions—that is, all files except those that include HeartStart
MRx 12-lead reports.
• Click FR2 Series Peds for files that were recorded using FR2
defibrillator pediatric pads.
• Click MRx 12- lead (XML) for 12-lead report files that were
recorded with the HeartStart MRx.
5. Navigate to the file you want and double-click it.
Event Review Pro downloads the file and attaches the ECG to the
case.
After you add an ECG
After you attach the ECG information to a case, use the tabs on the Case
Editor to add details to the case.
The following table lists common tasks and related instructions.
4 - Working with cases Event Review Pro User Guide

61
Task Topic
Add details to a case Adding case details on page.78
Save a case Saving cases on page.66
Print reports Printing reports on page.154
Export a case Exporting cases on page.69
Email a case Emailing a case on page.71
RemovingECGs from a case
Use the Detach option, on the ECGmenu and toolbar, to remove an ECG
from the case.
The ECG menu is available when you open a case and work on the
ECG tab.
To detach an ECG
1. On the case selection table, open a case. For more information, see
Displaying case details on page.61.
The Case Editor opens, to the ECGtab.
2. On Case Editor, click the ECG tab.
3. On the ECG tab, click the ECG ID tab for the ECG you want to
detach.
4. Use one of the following methods:
• On the ECG menu, click Detach.
• On the toolbar, click Detach ECG.
A confirmation message might appear. For more information, see
Restoring confirmation messages on page.42.
5. Click Yes to remove the ECG from the case.
Displaying case details
The case selection table is opened from the Patient Cases, using the
Case Selection menu. Cases in the database are listed in the case
selection table. From there, you can open the cases for viewing.
4 - Working with cases Event Review Pro User Guide

62
In the case selection table, the following details can appear in columns
for each case: the reference ID, case ID, patient ID, date and time,
patient name, and institution.
You can customize how the case selection table information
appears. For more information, see Sorting and grouping cases on
page.62, Working with columns on page.35, and Grouping and
sorting entries on page.35.
Once you open a case, it appears in the Case Editor. You click any tab to
view and modify the detailed information.
To use a wizard to open a case, from Getting Started, in the
Welcome area, click Open an Event Review Patient Case.
To open a saved case
1. Click the Patient Cases navigation button.
2. If you want, limit the display of cases by clicking one of the built-in
date ranges (such as "Last 7 Day's Cases") from the navigation
pane, or search for a particular group of cases. See Searching for
cases on page.44 for information.
3. Use one of the following methods:
• On the case selection table, double-click the case you want to
open.
• On the File menu or toolbar, click Open.
The case appears in the Case Editor.
Sorting and grouping cases
You can change the way information displays on the case selection
table. You can sort cases and group them based on a shared parameter
value.
You can filter or select cases that meet the criteria you select. You can
also hide or display groups of cases.
For information on how to add columns to and delete columns from the
display, see Working with columns on page.35.
Also see Using the application tables and logs on page.32.
4 - Working with cases Event Review Pro User Guide

63
For information on how to group and sort information in columns, see
Grouping and sorting entries on page.35.
For information on working with grouped cases, see Hiding and
displaying grouped cases on page.63.
For information on how to filter the information that appears in the list
of cases, see Filtering entries on page.37.
Hiding and displaying grouped cases
You can hide or display cases that have been grouped in the case
selection table. Grouping is described in Grouping and sorting entries on
page.35.
When cases are grouped, you can expand and collapse all the groups.
You can also hide or display cases at the level of a specific group. Doing
this can help you manage viewing in a long list of cases.
To hide or display all cases
1. Click Patient Cases and then All Cases, to open the case selection
table. For more information, see Displaying case details on page.61.
2. Right-click the case selection table.
A menu of options opens.
3. Click your preferred option:
• Expand all
• Collapse all
All the groups in the case selection table are affected.
To refresh, hide, or display specific groups of cases
• To refresh all the cases, on the View menu or shortcut menu,
click Refresh.
• To hide the cases in a group (collapse that group), to the left of
the group, click Collapse .
• To hide all the cases in groups (Collapse the cases in every
group), on the View menu or shortcut menu, click Collapse All.
• To display a group (expand the cases in a group), to the left of
the group, click Expand .
• To display all the groups (expand the cases in every group), on
the View menu or shortcut menu, click Expand All.
4 - Working with cases Event Review Pro User Guide

64
Importing case files manually
You can import one or more case files that were exported by another
HeartStart software application such as Data Messenger.
For each file you import, Event Review Pro creates a case in the Event
Review Pro database, and adds the information from the file to the case.
You can use the file in the same way as any other case you created. The
case can then be viewed, modified, printed, exported or emailed.
You can import using the Import option (available on the File menu and
toolbar) or using drag-and-drop methods.
When you import a case file, Event Review Pro imports all the
details that were in the case, such as patient demographics,
responder and reviewer notes, and physician notes. When you use
the Attach ECG feature, Event Review Pro attaches only the ECG to
the case.
To import files using drag and drop
1. On the navigation pane, click the Patient Cases navigation button.
2. Open the folder where your .mic., .hic, or .cod files are stored, and
drag one or more files onto the open Event Review Pro application
window.
3. Event Review Pro imports the cases, saves them to the database,
and lists them on the case selection table.
If any of the cases failed to import successfully (because they
required a password, for example), look for the outcome for each in
the System Log. See Working with the System Log on page.145.
To import files using the Import option
1. On the navigation pane, click the Patient Cases navigation button.
2. On the File menu or toolbar, click Import.
The Import Case window opens.
3. Navigate to the folder that contains the files you want to import.
The list of files includes only those with a .cod, .hic, and .mic
extension.
4 - Working with cases Event Review Pro User Guide

65
A .hic file is a defibrillator device file that is forwarded by an
electronic patient care record (ePCR) application or HeartStart Data
Messenger.
4. If you want to import only one file, click it. If you want to import
multiple files, press CTRL and click each one. Click Open.
5. If any of the files were password-protected, type the file password
in the Password field before you attempt to open the file. Passwords
can have up to 16 characters and are case-sensitive. If some of the
files are associated with a different password, they will not import,
and you will need to import them separately with their own
password.
6. Event Review Pro imports the cases, saves them to the database,
and lists them on the case selection table.
If you were working with an open case when you imported more
than one case, the last case in the list that you imported opens. If
you were working in the cases selection table, the same table is
open after the import.
If any of the cases failed to import successfully (because they had a
different password from the others, for example), the outcome for
each appears in the System Log. See Working with the System Log
on page.145.
Importing case files automatically
Use the Import Service on the Administration navigation pane to set up
inboxes to import cases automatically. When the Import Service is
running, Event Review Pro monitors the inboxes for case files with .hic ,
.mic , and .cod extensions, as well as HeartStart MRx BLDT and
Bluetooth files (with .tgz, .tsy, and .don extensions).
If an imported case contains a duplicate ECG, the case appears on the
Duplicated ECGs table. See Reviewing cases with duplicate ECGs on
page.73.
For more information, including configuration options, see Using the
Import Service on page.138.
4 - Working with cases Event Review Pro User Guide

66
Saving cases
When you save a case, Event Review Pro adds it to the database. All the
information is available for reports. Use the case selection table to
manage the case.
To save an open case, on the File menu or toolbar, click Save. The case
is now in the Event Review Pro database.
Adjusting the date and time of the
defibrillator data
It is possible for the internal clock on your defibrillator to drift slightly
over time. In addition, if someone removed the battery from an HS1 or
FRx defibrillator before downloading the patient data, case and event
clock time could be lost, leaving only event elapsed time. In these
cases, you may want to adjust the times of the downloaded case and its
individual events.
The date and time formats used in this software and in the reports
is controlled by the Windows Region and Language control panel.
Use that control panel to adjust the date and time format. For
example, use it to choose 24 hour time.
Before you transfer information from a defibrillator, confirm that your
computer clock is accurate. Also, confirm that the battery was not pulled
from the HS1 or FRx defibrillator since the cardiac emergency. For
information on HS1 and FRx defibrillators case date and time, see
Determining the HS1 and FRx case date and time on page.202.
To adjust the defibrillator date and time based on the computer
date and time
1. Open the case from the case selection table.
The case opens on the ECG tab of the Case Editor. For more
information, see Displaying case details on page.61.
2. On the Case Editor, click the Overview tab, and navigate to the
Defibrillators area.
Confirm that the date and time for the defibrillator are correct in the
Defibrillators area.
4 - Working with cases Event Review Pro User Guide

67
If you transfer the case from a defibrillator through a Bluetooth
transmission, Event Review Pro automatically adjusts the case
event date on the received data based on the computer clock.
3. After you attach an ECG, you can change the date and time for the
appropriate case in the Adjusted “On” Time column.
The date and time automatically adjusts for each event in that case.
The ECG tab displays the adjustment that you enter.
4. On the File menu or toolbar, click Save.
Erasing the data source
After you attach an ECG and save the case to the database, consider
erasing the data from the source. This ensures that the device has full
capacity for the next use.
You can erase ECG information from the data cards used by the
HeartStartMRxmonitor/defibrillators, the FR2, FR3 series, and
HeartStart XL defibrillators.
You can erase ECG information from the USBdata drive used by
HeartStart XL+ defibrillators and EfficiaDFM100monitor/defibrillators.
You can erase ECG information from an HS1 or FRx defibrillator.
You can erase files that were transmitted via Bluetooth from an
HeartStart MRx monitor/defibrillator or from an FR3 defibrillator.
Make sure that you transfer all the information to the database or
an archive before erasing the information. Once the data is erased,
it is lost and cannot be recovered.
To erase a data card
1. Insert the data card into the card reader.
2. On the Tools menu, click Erase Device or Data Card.
The Erase Device or Data Card window opens.
3. Click the card or device name.
4. Click Erase.
A confirmation message might appear. For more information, see
Restoring confirmation messages on page.42.
5. If so, click Yes in the message box.
4 - Working with cases Event Review Pro User Guide

68
To erase data from an HS1 or FRx Defibrillator
1. Set up the defibrillator to communicate with Event Review Pro. For
more information, see Using infrared connections for the HS1 and
FRx on page.203.
2. Put the defibrillator in Data Transfer mode.
3. On the Tools menu, click Erase Device or Data Card.
The Erase Device or Data Card window opens.
4. Click the device name.
5. Click Erase.
A confirmation message might appear.
6. If so, click Yes in the message box.
To erase data from the Bluetooth Exchange folder (HeartStart
MRx only)
1. On the Tools menu, click Erase Device or Data Card.
The Erase Device or Data Card window opens.
2. If it is visible, click Bluetooth Exchange Folder.
3. Click Erase.
A confirmation message might appear.
4. If so, click Yes.
To erase data from an FR3 defibrillator using Bluetooth
1. Once the unit is on and you have heard the voice prompt, place the
FR3 in Administration mode, press the option button, and select
Wireless Data Transfer.
2. In Event Review Pro, on the Tools menu, click Erase Device or
Data Card.
The Erase Device or Data Card window opens.
3. In the Bluetooth section, click the FR3 icon whose serial number
matches the device that you want to erase.
4. Click Erase.
A confirmation message might appear.
5. If so, click Yes.
4 - Working with cases Event Review Pro User Guide

69
The clock on the defibrillator is now synched with the clock on the
computer. This synchronization occurs even if you have no data to
erase.
To erase data from a USBdata drive for the HeartStart XL+
defibrillator
1. Use the HeartStart XL+ defibrillator to erase data from the USB data
drive. For more information, see the HeartStart XL+ Instructions for
Use.
2. Use the Efficia DFM100 defibrillator/monitor to erase data from the
USB data drive. For more information, see the Efficia DFM100
Defibrillator/Monitor Instructions for Use.
Printing case information
You can print case information about an open case and about a case that
is stored in the database. For more information, see Working with
reports on page.147.
You can also print the tables that appear on the Case Records table,
Event Log tab, and Attachments tab. The information that prints is about
the open case. For more information see Printing table entries on
page.39.
Exporting cases
Use Export to create files outside the application (that is, not in the
application database) so that you can share information with other Event
Review Pro users. You can also use Export to back up information.
You can export a single case or multiple cases at a time. The Export
option is available when the case selection table is open, from the File
menu, the toolbar, or by right-clicking on a case row. In addition, if you
want to export just one case, you can do this (from the File menu or the
toolbar) when that case is open.
You can export files with or without a password. If you add a password,
anyone attempting to open the file must have the password to open it.
Philips recommends that you record the password and save it in a
secure location. If you forget the password, Customer Support
cannot “unlock” the file.
4 - Working with cases Event Review Pro User Guide

70
To export one or more case files from the case selection table
1. In the case selection table, choose the case or cases to export:
a. Click a single case.
b. CTRL-click multiple cases.
2. On the File menu, click Export.
The Export Selected Cases dialog box appears, showing the last
folder you used to export a file. If you want a different location, click
the Save in field at the top of the dialog box and navigate to that
location. The list there shows .mic files (the only supported file
format).
3. Decide how to name the exported file or files. The default name
includes a placeholder for the case ID, expressed as {caseID}. You
can accept the case ID as the file name, or you can use this
placeholder to create more descriptive names; for example, if you
type “my data {caseID} for Joe.mic” for the file name and export
two cases, one with a case ID of “abc” and one with “xyz,” then the
two .mic files will have the names “my data abc for Joe.mic” and
“my data xyz for Joe.mic.” If you type something entirely different
for the name that doesn’t include a case ID, the case ID will be
appended to the file name by the software.
4. If you want to remove confidential patient identifiers such as name,
patient ID, and age (if over 90) from the exported file (called
"redaction"), check the Remove Patient Identity box.
5. Decide whether you want to protect the file or files with a password.
If so, type it in the Password field (for security, it appears as
*****). Remember to provide the password to the recipient in a
separate communication.
6. Click Save.
A progress bar appears as the export operation proceeds, and a
confirmation message tells you that the export operation succeeded.
To export a single case file, when the case is open
1. Open a case, so that it appears on the ECG tab of the Case Editor.
2. On the File menu or toolbar, click Export.
3. Follow steps 3 through 6 in the procedure above.
4 - Working with cases Event Review Pro User Guide

71
Emailing a case
You can use the Email option or your email application to send an email
to another Event Review Pro user. You have the option of attaching the
case report, the case .MICfile, or both.
Before you use the Email option, complete the following prerequisites:
• Correctly configure Microsoft Outlook or a MAPI-compliant email
client.
• Set up the email profile in Windows, if needed. Click Help on the
email profile window for information on setting up your profile.
After you complete the profile, the profile does not appear again.
To use the Email option
1. On the case selection table, open a case. For more information, see
Displaying case details on page.61.
2. On the File menu or toolbar, click Email.
The Email window opens.
3. Complete the To field. The contact list for your email application is
not available for the Email tool.
Email saves the address. The next time you send an email to that
recipient, you can select the same address by clicking the arrow at
the end of the To field.
4. Check Email case
5. Check Attach case archive MIC file, Attach case report PDF,
or both. You must check at least one.
6. Decide whether you want to protect the file or files with a password.
If so, type it in the Password field (for security, it appears as
*****). Remember to provide the password to the recipient in a
separate communication.
7. If you want to remove confidential patient identifiers such as name,
patient ID, and age (if over 90) from the exported file (called
"redaction"), check the Remove Patient Identity box.
8. If you want, add a message.
9. Click Send.
Philips recommends that you record the password and save it in a
secure location. If you forget the password, Customer Support
cannot “unlock” the file.
4 - Working with cases Event Review Pro User Guide

72
To use your email application
1. Export the file. For more information, see Exporting cases on
page.69.
2. Note the location of the exported file. The file has a .mic extension.
3. Open your email application and create an email to the recipient of
your choice.
4. Attach the files created by Export in Step 1 to the email form.
5. Send the email.
Deleting cases
You can delete a case or multiple cases from the database. The case or
cases then disappear from the case selection table.
To delete a case or cases from the list of cases
1. Click the Patient Cases navigation button to open the case
selection table.
2. Use any of the queries to find the specific case or cases you want to
delete. For more information, see Searching for cases on page.44.
3. Click the case you want to delete, or press CTRL or SHIFT while
clicking to select multiple cases.
4. On the File menu or toolbar, click Delete .
A confirmation message might appear. For more information, see
Restoring confirmation messages on page.42.
5. If so, click Yes.
To delete a case that is open
1. On the File menu or toolbar, click Delete .
A confirmation message might appear. For more information, see
Restoring confirmation messages on page.42.
2. If so, click Yes.
The case selection table appears.
4 - Working with cases Event Review Pro User Guide

73
Reviewing cases with duplicate ECGs
The Duplicate ECGs table (in the Cases workspace)lists each case in the
Event Review Pro database for which the ECG in the case is also the ECG
in another case. The cases are listed based on the reference ID, case
ID, patient ID, case date and time, patient name, and institution.
Cases can be inadvertently duplicated when you download them
automatically. This table provides an easy way to identify these cases.
For more information, see Using the Import Service on page.138,
Use the Duplicate ECGs table to complete the following tasks:
• Delete duplicate cases. For more information, see Deleting cases on
page.72.
• Sort cases. See Sorting and grouping cases on page.62.
• Search for cases based on a variety of values. For more information,
see Filtering entries on page.37.
• Hide or display columns,. See Working with columns on page.35.
• Open cases. For more information, see Displaying case details on
page.61. You can click the Cases tab to view, modify, and delete the
information. You can use the case tabs in any order based on your
tasks within your organization.
• View and print reports. For more information, see Generating
reports on page.151.
Note:Deleting duplicate cases is not the same as merging two
related cases. Atrue duplicate case is identical in all respects; in
the case of merging cases, the cases (and their respective ECGs)
were created at slightly different times and possibly different
devices but refer to the same event. For information on merging
related cases, see Merging related cases on page.74.
4 - Working with cases Event Review Pro User Guide
74
Merging related cases
If you have two cases with data from the same incident (for example, if
one case were created by the EMTs on the scene and another by
ambulance or hospital personnel using a different device), you might
want to merge them into the same case. Event Review Pro can tell
whether two cases have ECGs recorded very close in time, and it
displays only those candidates for you to consider. In addition, you can
set further limitations on the cases that are considered merge
candidates.
This is not the same as deleting a duplicate case. A true duplicate
case has an identical ECG; in the case of merging cases, the cases
(and their respective ECGs) were created at slightly different times
and possibly on different devices but refer to the same event. For
information on deleting duplicate cases, see Reviewing cases with
duplicate ECGs on page.73.
When merge candidates have been identified, you can merge the newer
one into the older one. The newer one is then deleted. If the old case
does not contain values for certain fields and the newer one does, these
additional values are retained.
You can merge cases manually, changing the criteria for each, or you
can set Event Review Pro to merge cases automatically upon import,
based on criteria that you set. For instructions on how to merge cases
automatically, see Merging related cases automatically on page.76.
To merge related cases manually:
1. Click the Patient Cases navigation button.
2. From the left panel, under Merging and Duplicates, click Merge
Candidates.
A screen appears where you can set conditions for potential merge
candidates.
3. In the Merge Patient Cases Where area, you can decide how far
apart in time the case ECGs must be. The default is 10 minutes from
the end of one case to the start of the next; both cases must have
ECGs recorded 120 minutes or less apart to be considered. ECGs
may overlap by up to 30 minutes to be considered as merge
candidates. Click the slider and move it to the right or left to change
this time value.
4 - Working with cases Event Review Pro User Guide

75
4. In the AND section, you can specify (by checking or unchecking)
whether certain details of the cases must be equal as well, such as
Case IDs or last names. The default is that all of these items must be
equal for the two cases to be considered merge candidates.
5. You can also limit merge candidates by time—that is, how recently
the cases were created. On the Were Created in the Last line,
use the up and down arrows to change the number of days.
6. You can change the maximum number of recordings that can be
contained in the merged case. The default is 2 and the maximum is
4.
7. When you are satisfied with the limitations you have set, click
Select.
Merge candidates that fulfill all of these requirements appear in the
case list below, grouped with the older case above and any potential
newer cases below it. This process may take many minutes to
complete.
In a given group of potential merge candidates, you can merge up
to four cases. Once a case has four attached ECGs, it is not eligible
for further merging.
8. If you want to examine each of the cases before you make the
decision to merge, you must leave the Merge Candidates screen and
open the cases manually. When you are confident that the two cases
refer to the same event, click Merge.
Merging cases changes the database, and these changes are
difficult to undo.
How to separate a merged case:
It is possible to separate the ECGs in a merged case; however, any
additional case data that you entered before the merge may not be
restored to the pre-merged state after the separation.
1. Open the merged case and then, in the File menu, click Export. (For
this example, we assume you have a merged case with ECG from a
HeartStart FR3 and a HeartStart MRx.)
2. In the open case, in the Case Editor, click the ECG tab for the case
you want to separate from the current case (for example, detach the
HeartStart FR3 ECG), and then, on the ECG menu, click Detach.
3. Save the changes (for example, resulting in a case with only the
HeartStart MRx ECG).
4. Import the merged case that you exported in step 1 above.
4 - Working with cases Event Review Pro User Guide

76
5. Select the ECG tab you saved in step 3 (for example, an MRx ECG),
and then, on the ECG menu, click Detach.
6. Save the changes (for example, resulting in a case with only the
HeartStart FR3 ECG).
The previous steps preserve the original ECG waveforms from each
defibrillator in separate cases. Any patient data entered on other
tabs, including the Case ID, will be identical in both cases. Consider
changing the Case ID in one of the cases, in order to distinguish the
cases.
Merging related cases automatically
If you have two cases with data from the same incident (for example, if
one case were created by the EMTs on the scene and another by
ambulance or hospital personnel using a different device), you might
want to merge them into the same case during import. You can set
criteria for the cases that are considered merge candidates, and these
criteria will apply to all potential cases. When the criteria are fulfilled,
the two cases will be merged automatically when imported into Event
Review Pro.
When merge candidates have been identified, the newer one is merged
into the older one. The newer one is then deleted. If the old case does
not contain values for certain fields and the newer one does, these
additional values are retained.
If you want to merge cases manually, changing the criteria for each, see
Merging related cases on page.74.
To make any changes to the merging rules, you must run Event
Review Pro as an administrator.
To set ER Pro to merge cases automatically
1. On the navigation pane, click the Administration button.
1. Under Importing, click Merge Rules. A screen appears on the right
with the criteria for automatic merging.
2. By default, Event Review Pro can merge cases automatically only if
they were created within 120 minutes of each other. You can set this
time limitation lower; the default is 10 minutes. ECGs may overlap
by up to 30 minutes to be considered as merge candidates.
In the Recordings are Separated by Less Than area, click the
slider and move it to the time you want.
4 - Working with cases Event Review Pro User Guide

77
3. By default, all criteria (such as equal case IDs and patient last
names) in the AND (At Least One) area must be met as well for
automatic merging to occur. You can uncheck any of these boxes if
you do not want these criteria to be required. However, at least one
of them must remain checked.
4. In the AND section below that, you can decide whether Institution
Names and Reference IDs need to be equal as well for the case to be
considered a merge candidate. Check these boxes as appropriate.
5. You can change the maximum number of recordings that can be
contained in the merged case. The default is 2 and the maximum is
4.
6. When you are satisfied with the criteria you want for merging, check
the Enable Automatic Merging box.Only cases imported after you
enable automatic merging will be eligible for automatic merging.
Merging cases changes the database, and these changes are
difficult to undo.
How to separate a merged case:
It is possible to separate the ECGs in a merged case; however, any
additional case data that you entered before the merge may not be
restored to the pre-merged state after the separation.
1. Open the merged case and then, in the File menu, click Export. (For
this example, we assume you have a merged case with ECG from a
HeartStart FR3 and a HeartStart MRx.)
2. In the open case, in the Case Editor, click the ECG tab for the case
you want to separate from the current case (for example, detach the
HeartStart FR3 ECG), and then, on the ECG menu, click Detach.
3. Save the changes (for example, resulting in a case with only the
HeartStart MRx ECG).
4. Import the merged case that you exported in step 1 above.
5. Select the ECG tab you saved in step 3 (for example, an MRx ECG),
and then, on the ECG menu, click Detach.
6. Save the changes (for example, resulting in a case with only the
HeartStart FR3 ECG).
The previous steps preserve the original ECG waveforms from each
defibrillator in separate cases. Any patient data entered on other
tabs, including the Case ID, will be identical in both cases. Consider
changing the Case ID in one of the cases, in order to distinguish the
cases.
4 - Working with cases Event Review Pro User Guide

5
Adding case details
Use the Patient Cases navigation button to access a case and enter case
information that the defibrillator did not record. In Cases, you can do
the following tasks:
• Document events
• Document patient outcomes
• Add responder actions and observations
• Add responder notes
• Add reviewer notes
• Document patient history
• Review ECG information
• Review events
• Attach files to a case
For more information, see Working with ECGs on page.88 and Reviewing
case details on page.133. You can use the case tabs in any order based
on your tasks within your organization.
To add and review case details
1. Click the Patient Cases navigation button.
2. On the case selection table, double-click a case.
3. Click a tab.
4. Add or change information on the tab.
5. Repeat steps 3 and 4 as necessary.
6. On the File menu or toolbar, click Save.
Event Review Pro displays case detail tabs based on your
installation.
78

79
Identifying the case
Use the Overview tab to enter basic information about the patient and
the response location. The only required information is the case ID, and
the case date and time.
If you use the Case Wizard to create a case, Event Review Pro assigns a
case ID, which you can change. It also generates the case ID from the
current date and time if Case Wizard or the Import feature did not
create the case.
Different defibrillators may capture different patient information as
part of recorded ECGdata. If you use data from the HeartStart
MRx, the ECG information might include patient information (such
as name, age, and gender) that was entered by the responder.
When you import a case using Case Wizard, the Case Summary
page displays the information provided by the HeartStart MRx, and
the Case ID field displays the HeartStart MRx incident ID.
If you import a redacted case, in any report that includes that case, the
redacted case appears with a series of asterisks in the patient name
fields, and the patient ID is replaced by a series of letters and numbers,
unique to each patient ID. If the redacted age of the patient is greater
than 90, the Date of Birth field is blank and the Age field displays 90.
If the case includes audio, audio is removed from the case. If you then
import the case, the redacted information (the information that was
removed)does not appear.
After you attach the ECG, you can change any of the information except
the information in the following fields, which the defibrillator may
supply:
• Recorded “On” Time
• Defibrillator Type
• Serial Number
• Shocks
To complete the fields
1. On the Overview tab, click the field.
2. Click an option on the menu or type the information in the field.
5 - Adding case details Event Review Pro User Guide
80
If Other appears in the option list, you can type a value in the Other
text box. If your entry is the same as an option in the list, your
entry appears as shown in the option list. Click Clear to remove the
information in the field or text box.
Overview section fields table
Group or
field Description
Case ID
(Required)
When you attach an ECG, incident ID becomes the case ID.
You can change the case ID.
Time zone Initially, displays the time zone for the computer clock. The
time zone displays in the Coordinated Universal Time (UCT) or
Greenwich Mean Time (GMT) format in a similar format to the
Time Zone tab on the Windows Date and Time Properties
window. For example: (+1:00) Amsterdam, Berlin, Bern,
Rome, Stockholm, Vienna.
Click the time zone of the location where the incident
occurred.
Case date and
time
(Required)
If you attach an ECG before you save the case, this field is set
to the date and time when the device was turned on.
To change the date and time, click the value you want to
change, and then type a new value.
Site of
collapse
The list includes typical kinds of locations such as Ambulatory
or outpatient area, Hotel, Workplace, Adult intensive care unit
(ICU), and Cardiac catheterization lab (cath lab).
Location
Detail
You can use this field to be more specific about the site of
collapse. For example, consider using this field to record map
coordinates or the name of locations, such as the room
number.
Reference ID You can use this field to help identify the case. For example,
Engine 3. Maximum of 40 characters.
Institution You can use this field to identify the organization where the
case originated. For example, Valley Fire Department.
Maximum of 60 characters.
Code called
(Hospital
edition only)
The list is: Yes, No, and Unknown
Found by The list is: Nurse, Physician, Therapist, Med technician, and
5 - Adding case details Event Review Pro User Guide
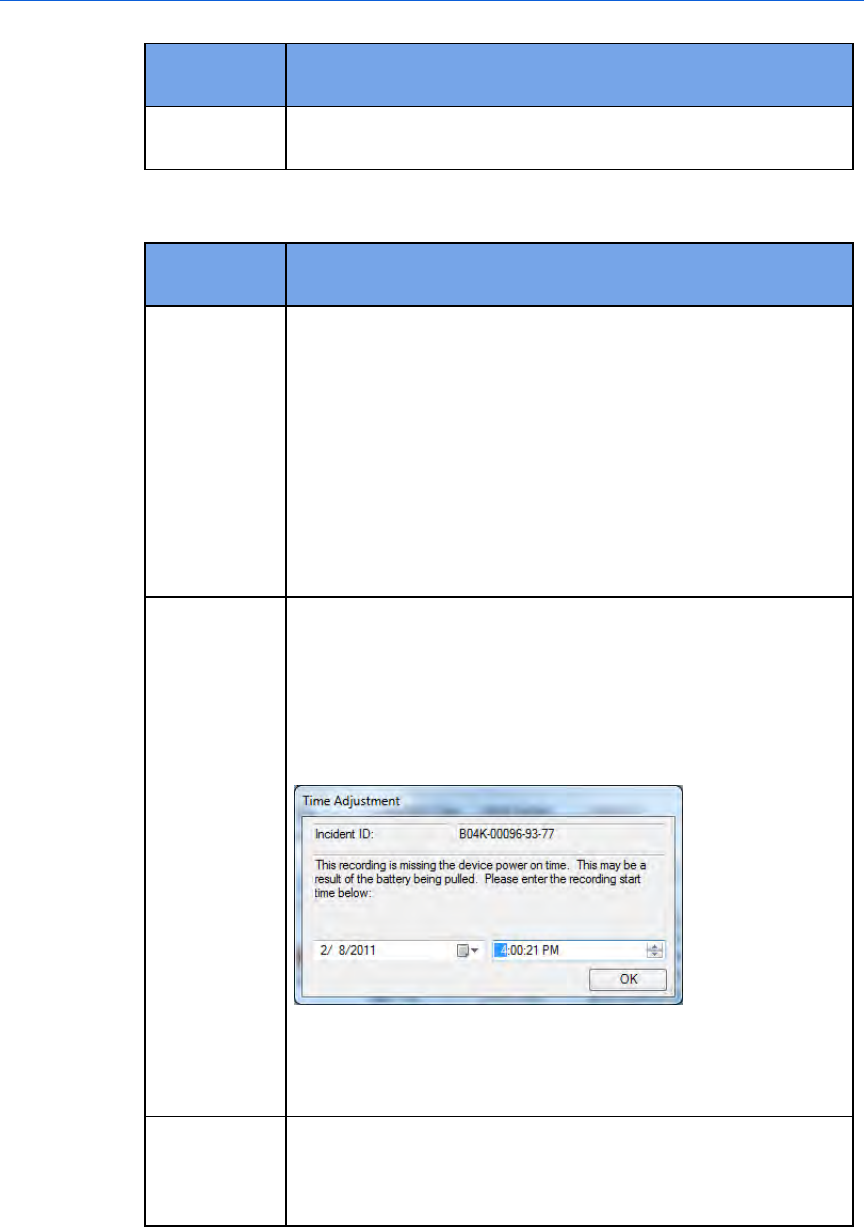
81
Group or
field Description
(Hospital
edition only)
Other
Defibrillator section fields table
Group or
field Description
Adjusted
“On”
Time
You can use this field to synchronize the defibrillator-created
date and time with the computer date and time.
Event Review Pro automatically adjusts the date and time of
each event on the ECG tab for the adjustment you enter.
Note: Event Review Pro automatically adjusts the date and
time based on the clock of the personal computer when it
receives a Bluetooth transmission from a HeartStart MRx
version 9.0 or later, or from an FR3. This adjustment records
the Recorded “On” Time. You can still use this field to override
the Recorded “On” Time.
Recorded
“On” Time
When you attach an ECG, the field displays the date and time
that the defibrillator was turned on.
HS1 and FRx only:If you removed the battery before the ECG
was attached (downloaded), Event Review Pro displays a
message requesting the case date and time. When you click
OK, you see this screen:
Set the case date and time to the Recorded “On” Time of the
ECG.
You can adjust the time in the Adjusted “On” Time field.
Defibrillator
Type
When you attach an ECG, the field displays the defibrillator
model name.
You cannot change it.
5 - Adding case details Event Review Pro User Guide

82
Group or
field Description
Serial
Number
When you attach an ECG, the field displays the defibrillator
serial number.
You cannot change it.
Shocks When you attach an ECG, the field displays the total number of
shocks delivered to the patient by this defibrillator. You cannot
change it.
Patient section fields table
Group or
field Description
Patient ID By default, the value in the Patient ID field is added based on
the patient ID from the defibrillator (if the device supports
entering such data). Otherwise, it is blank, and you can add an
ID.
The patient ID prints in report headers.
A redacted case appears with the patient ID replaced by a
series of letters and numbers, unique to each patient ID.
First name The first name prints in report headers.
If the ECG is from HeartStart MRx, this field can contain
information entered on the HeartStart MRx. You can change it.
A redacted case appears with a series of asterisks in the patient
name fields.
Middle name The middle name prints in report headers.
A redacted case appears with a series of asterisks in the patient
name fields.
Last name The last name prints in report headers.
If the ECG is from HeartStart MRx, this field can contain
information entered on the HeartStart MRx. You can change it.
A redacted case appears with a series of asterisks in the patient
name fields.
5 - Adding case details Event Review Pro User Guide
83
Group or
field Description
Date of birth Type the patient date of birth in the field.
A redacted case displays a blank field.
Age This is the age of the patient at the time of the response. Click
the format used and type the age in the field. The units of
measurements include years, months, or days. You can
change the format at any time. Event Review Pro calculates
the new value for you.
If the ECG is attached from the HeartStart MRx, this field
might contain information entered on the HeartStart MRx. You
can change it.
If you import a redacted case and the age of the redacted
patient is greater than 90, the Age field displays 90.
If the date of birth is entered, age is computed automatically,
and you may not enter it.
Height This is the height of the patient.
Click the format used by the hospital and type the height in
the field. The units of measurement include feet (ft), inches
(in), centimeters (cm), or meters (m).
You can change the format at any time.
Weight This is the weight of the patient at the time of the response.
Click the format used by your organization and type the weight
in the field. The units of measurement include pounds (lb.),
ounces (oz.), kilograms (kg), or grams (g).
You can change the format at any time.
Gender The list is:
Female
Male
Unknown
If the ECG is from the HeartStart MRx, this field might contain
information entered on the defibrillator.
5 - Adding case details Event Review Pro User Guide

84
Group or
field Description
Race The list is:
American Indian or Alaska Native
Asian or Pacific Islander
Black, Hispanic
Black, Non-Hispanic
White, Hispanic
White, Non-Hispanic
Unknown
Other
Describing the conditions at the scene
Use the Scene tab to enter the responder's initial impression of the
patient and the scene. The information on this tab appears in the Case
Report.
To complete the fields
1. On the Scene tab, click the field.
2. Click an option on the menu or type the information.
If Other appears in the option list, you can type a value in the Other
text box. If your entry is the same as an option in the list, your
entry appears as shown in the option list. Click Clear to remove the
information in the field or text box.
The following fields can accept more than one input:
• Existing medical treatments
• Past medical history
• Type of first responder
• Defibrillator types used
5 - Adding case details Event Review Pro User Guide

85
Documenting events
Use the Timeline tab to document the events that occurred during the
case, the treatments that were administered, and the notes that were
collected during the case. The Timeline tab also lists the notes that you
add to the ECG on the ECG tab.
Event Review Pro sorts events for a new case in chronological order.
You can sort events in ascending (1 to 9, a to z) or descending (9 to 1, z
to a) order.
Events and notes appear in the Event Report, on the ECG tab, and on the
Event Log tab.
If Other appears in the option list, you can type a value in the Other
text box. If your entry is the same as an option in the list, your
entry appears as shown in the option list. Click Clear to remove the
information in the field or text box.
Events that you add on the ECG tab appear on the Timeline tab as read-
only. You can edit the Event Name parameters and the Comment fields,
but not the Date and Time field.
Adding, describing, and removing events
You can add, edit, and remove an event in an open case.
To add an event
1. On the Timeline tab, click the Event Name field.
A menu showing types of events appears. You can click an event that
is specific to the phase of events, an administered treatment, and a
note added by a responder or reviewer.
2. Click an event from one of the menu columns.
Event Review Pro adds the event to the table in chronological order.
• If an event does not appear in the list of options in the Phase or
Treatment columns, click the Custom option in the appropriate
column to create one.
• Click Responder Note to document notes from a responder.
• Click Reviewer Note to document notes from a medical
director, code team leader, or operations manager.
5 - Adding case details Event Review Pro User Guide

86
• If the event displays additional fields, complete the appropriate
information for each field.
Event Review Pro displays a button to the left of the event. Click the
button to collapse or expand the additional fields that document the
event.
3. (Optional) Type text in the Comment field.
4. Repeat steps 1, 2, and 3 for each event, as necessary.
To edit an event
1. On the Timeline tab, click the appropriate event in the Event Name
field.
2. Use one of the following methods:
• On the menu, click the appropriate option.
• Type the correction.
To remove one event
1. On the Timeline tab, click the Event Name field.
2. On the menu, click Remove this Event.
The event disappears from the event table.
Sorting events
You can sort the information based on the column. Events in a new case
are first sorted in chronological order.
Click the header to sort the list of values in ascending (1 to 9, or a to z)
or descending (9 to 1, or z to a) order. A triangular symbol (arrow)
appears to indicate the sort order.
Event Review Pro adds new events based on the sort order. The
location of the event field for the new event changes depending on
the sort order. For example, if you sort events in alphabetical
order, Event Review Pro inserts the event alphabetically. The blank
event field is at the top of the table. If you sort the event by date
and time in descending order, Event Review Pro inserts the event at
the top of the table. The blank event field is at the bottom of the
table.
5 - Adding case details Event Review Pro User Guide

87
Changing the date and time of the event
You can change the date and time of an event that you entered on the
Timeline tab.
To change the event date and time
1. Click the event.
2. Click Date and Time.
3. Click the value you want to change and type the correct date or time.
Documenting the patient's outcome
Use the Outcome tab (EMS edition only) and the Follow-up tab to enter
information about the patient's outcome during and after hospitalization.
The intent for the Outcome tab is to enter information about the patient
immediately following arrest and resuscitation. This information could
be added while creating the case.
The intent for the Follow-up tab is to enter information about the patient
in the future, for instance, on discharge from the hospital, or if the
patient does not survive.
To complete the fields
1. Click the field.
2. Click an option on the menu or type the information in the field.
If Other appears in the option list, you can type a value in the Other
text box. If your entry is the same as an option in the list, your
entry displays as shown in the option list. Click Clear to remove the
information in the field or text box.
Several fields use scores for Cerebral Performance Categories (CPC)
and Overall Performance Categories (OPC). For more information, see
CPC and OPC on page.217.
EMS Edition only: Utstein reports use the Any ROSC field.
5 - Adding case details Event Review Pro User Guide

6
Working with ECGs
Use the ECG tab in the Case Editor to add, review, and print ECG
waveforms. You can also use the ECG tab to enter information and
review events.
Displayed and printed waveforms do not meet the requirements of
American National Standard ANSI/AAMI EC11:1991 (R)2007 for
display of diagnostic electrocardiograms, and may not be suitable
for diagnosis.
You can complete the following tasks on the ECG tab:
• View an attached ECG
• Add notes to an ECG
• Modify the display properties of an ECG
The ECG waveform includes recorded defibrillator events as well as
reviewer and responder notes. For information on attaching an ECG, see
AttachingECGs on page.51.
For an explanation of the Case Editor workspaces on the ECG tab, see
Understanding the Case Editor on page.88.
If the case includes 12-lead information, click the 12-lead tab within the
ECGview to see a separate waveform and accompanying information.
For details, see Working with 12-lead ECGs on page.119.
Understanding the Case Editor
When a case opens, it opens on the ECG tab, by default. The ECGtab is
the area where the waveforms are displayed.
If you navigate away from the ECG tab, you can return to it by clicking
it.
The ECGtab is one of a group of tabs on the Case Editor. Each tab in the
Case Editor provides a different work area.
The Case Editor tabs, in order, as they appear from left to right:
88
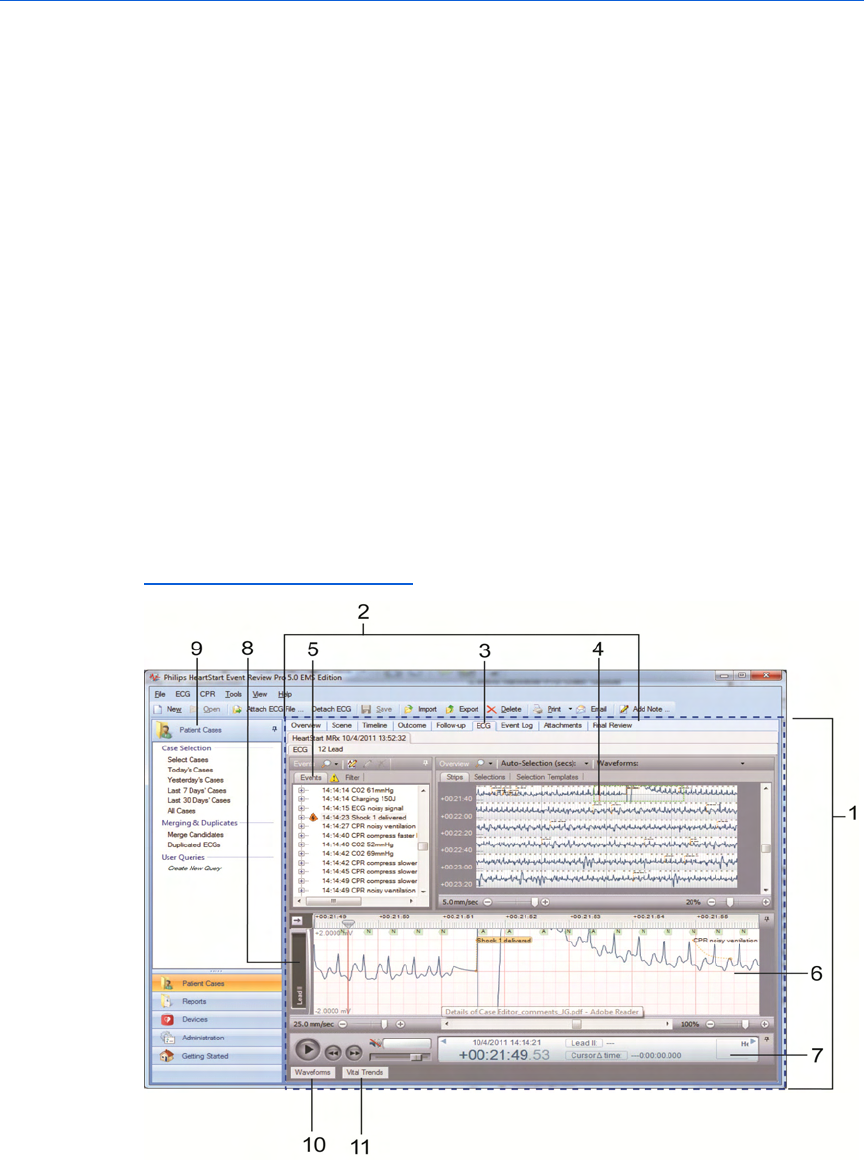
89
• Overview
• Scene
• Timeline
• Outcome (Event Review Pro EMS edition)
• Follow-up
• ECG
• Event Log
• Attachments
• Final Review
The Case Editor default view
The following illustration shows the general arrangement of the Case
Editor workspaces in the default view. The general arrangement is the
same in all languages. Refer to the table after the illustration for
definitions.
For information on viewing 12-lead ECGs in the Case Editor, see
Working with 12-lead ECGs on page.119.
Key to illustration numbers for the previous illustration:
6 - Working with ECGs Event Review Pro User Guide

90
1 The Case Editor
2 The tabs of the Case Editor, used to open different work areas
3 The ECG tab of the Case Editor, the active tab when a case is opened
4 The ECG overview pane. NOTE: For details about waveform display,
see The Case Editor Waveforms view on page.90.
5 The Events pane, used to view events in the case, ordered
chronologically. See Events pane on page.92.
6 The Rhythm strip pane. Rhythm strip shows the waveforms used for
rhythm identification, recorded by the device sequentially, in one
strip.
7 The Transport pane, used to play, rewind, or fast-forward the
waveform. If audio is present, use to play or mute audio. See
Transport pane on page.93.
8 Label for a displayed waveform, in collapsed view.
9 The Patient Cases navigation pane.
10 The Waveforms button: click to open the Waveforms pane. See
Waveforms pane on page.92.
11 The Vital Trends button: click to open the Vital Trends pane.
Navigating between the work panes in the Case Editor
When a case is first opened, the default view of the Case Editor shows
the ECG tab with the Rhythm strip pane (see item 6 in the previous
table). In the same region of the Case Editor, you can instead choose to
show the Waveforms pane, or the Vital Trends pane. Those work areas,
when not displayed, appear as buttons along the bottom of the Case
Editor. To expand one of these work areas, click the corresponding
button.
The Case Editor Waveforms view
The following illustration shows the Case Editor, with the expanded
Waveforms pane. The general arrangement is the same in all
languages. Refer the table after the illustration for definitions.
6 - Working with ECGs Event Review Pro User Guide
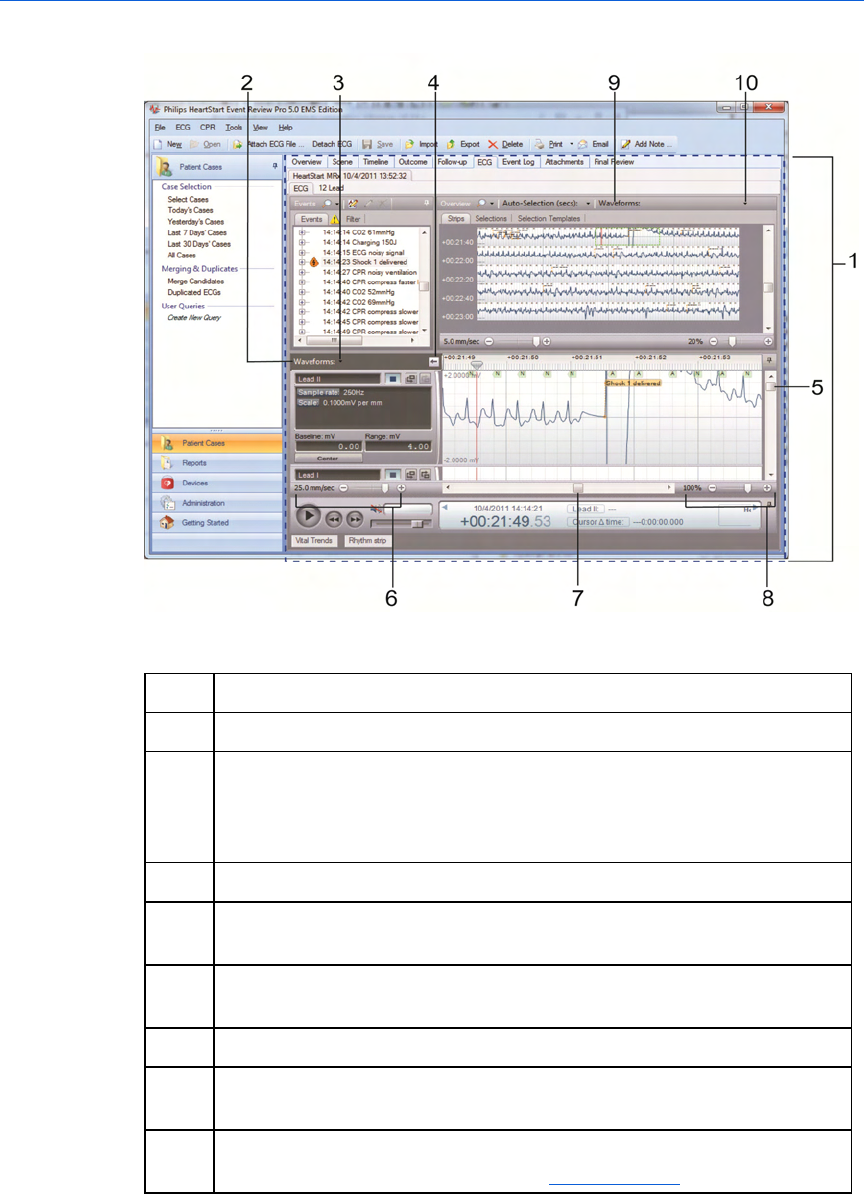
91
Key to illustration numbers for the previous illustration:
1 The Case Editor, ECG tab.
2 The expanded Waveforms pane.
3 The Waveforms pane drop-down menu: By default, all waveforms in
the case are shown, stacked vertically. Use the menu checkboxes to
hide/show waveforms by type. Changes from the default are
remembered in future sessions and cases.
4 Arrow button, used to expand or collapse the waveform controls.
5 Vertical scroll bar, used to view waveforms above or below the visible
area.
6 Time scale control, used to compress or expand the visible time range
of the waveforms.
7 Horizontal scroll bar, used to scroll the visible waveform left or right.
8 Zoom control, used to scale the waveform as a percentage of the
default 25mm/sec x 40mm.
9 The Waveforms Overview: By default, only the first waveform recorded
in the case is shown in this view. See Overview pane on page.92.
6 - Working with ECGs Event Review Pro User Guide

92
10 The Waveforms Overview drop-down menu: You can choose to overlay
multiple waveforms in this view. Check or uncheck boxes to show/hide
waveforms by type. Changes from the default are remembered in
future sessions and cases.
Events pane
All defibrillator events and notes appear in chronological order on the
Events pane. You can expand events or notes in the Events pane to
display additional details. For more information, see Using the Events
pane on page.93.
Use the Events toolbar to search for specific events or notes. You can
also add a note for an ECG. Use the event filter to control the display of
events and notes on the waveform. The event filter displays all possible
event types and categories. Check or uncheck them to control whether
they appear.
Overview pane
The Overview pane displays the ECG waveform with flagged events and
notes.
You can use the Overview toolbar to:
• Select the waveform displayed on the Overview pane.
• Select multiple waveforms to display them overlayed on one
another.
• Select portions of the ECG for detailed view on the Waveforms pane.
• Change the time scale for the ECG.
Waveforms pane
The Waveforms pane can provide a more detailed view of each
waveform. Event Review Pro can display multiple waveforms in this
pane.
Use the Waveforms pane to do the following:
• Add, edit, or delete notes
• Control the display of waveforms
• Analyze the quality of the CPR administered
6 - Working with ECGs Event Review Pro User Guide

93
Transport pane
The Transport pane at the bottom of the ECG view displays the ECG lead
information, the sweep bar time, and the cursor time relative to the
sweep bar location on the ECG.
Use the Play button to play the entire ECG.
If an audio recording is associated with the ECG, you can use the audio
controls to play the audio while viewing correlated events on the
waveform on the Waveforms pane.
You can also use the controls on the Transport pane to find and select
events on the waveform for review.
For more information, see Reviewing waveform information on
page.106.
Using the Events pane
A list of defibrillator events and notes associated with an ECG appears in
an expandable list on the Events pane. Defibrillator events can include
items such as the following:
• Shocks
• Alarms
• Monitor settings
• Equipment status
• Q-CPR events
In the Events pane, you can identify types of events, view them, search
for them, and display/hide them. You can also manage the notes that
are attached to each case.
Identifying types of events
Event Review Pro displays the notes that were entered by the user and
events recorded by the device. For more information, see Documenting
events on page.85.
The following icons identify event entries:
Symbol Meaning
Note: A user-input observation about the patient's status or the
6 - Working with ECGs Event Review Pro User Guide

94
Symbol Meaning
responder's intervention made on the Timeline tab.
Note: A user-input observation about the patient's status or the
responder's intervention made on the ECG tab.
Shock: The delivery of therapy by the defibrillator.
Device On: The time the defibrillator was turned on or returned
to use.
Device Off: The time the defibrillator was turned off.
Defibrillator alarm: An alert provided by the defibrillator. The
alarm calls attention to a patient vital sign that is beyond a preset
threshold.
Error condition: An error related to the ability of the defibrillator
or part of the defibrillator to perform its intended monitoring
function.
Viewing events
When you click an event in the Events pane, the sweep bar
automatically moves to the location of the event on the ECG strip on the
Overview and Waveforms panes. You can expand the event in the
Events pane to view event details.
To view event details, in the Events pane, click Expand next to the
event you want to view. Event details appear. You can also double-click
the event label to view details.
To expand an Events pane node
1. Click an event or event node.
2. Click the PLUS SIGN (+).
To collapse an Events pane node
1. Click an event or event node.
2. Click the MINUS SIGN (-).
To find and view an event using the scroll bar
1. Click and drag the scroll bar down the Events pane to find an event.
6 - Working with ECGs Event Review Pro User Guide

95
2. Click the event.
The sweep bar moves to the location of the event on the waveform.
3. Double-click the event.
The event expands to display event details.
Searching for events
Use the Search button on the Events toolbar to search for events or
notes.
To search for an event
1. Type or select the event name in the text box on the Events toolbar.
The search text can be any portion (substring) of the event name as
it appears in the event label. For example, to search for "patient
age," you could type "patient age," "age," or even just "patient."
2. Click Search .
If the search finds an event, it appears selected in the event pane,
and the sweep bar moves to the location of the event on the
waveform.
Hiding or displaying events
Use the Filter tab to display or hide events on the waveform on the
Overview, Waveforms, and Events panes according to criteria that you
choose.
You can display or hide events either by category or by type. If you filter
by category (for example, if you filter by Q-CPR), then all event types in
that category (CPRPads Off, CPR Intubated, etc.) are hidden as well.
By default, all events appear except CPR compression events.
The caution icon appears on the Filter tab when there is at least one
event type or category that was filtered. See the procedure below to
display some or all of these events.
To use the event filter
1. On the Events pane, click the Filter tab.
A list of event categories appears.
6 - Working with ECGs Event Review Pro User Guide

96
2. Click Expand next to an event category.
The event types in the selected category appear.
3. Uncheck the box for any event type or event category that you want
to remove from the display.
The following results occur:
• The event or events disappears from the Overview and
Waveforms panes.
• The event or events disappears from the Events pane.
• The caution icon on the Filter tab appears.
Managing notes
You can add, modify, and delete notes in the Events pane. You can also
add, edit, and delete notes directly on the ECG on the Overview or
Waveforms pane. For more information, see Managing notes on the
waveform on page.114.
To add a note in the Events pane
1. Click the part of the Events pane where you want the note to appear.
2. On the toolbar, click Add Note .
The Note window appears, with a table similar to the table on the
Timeline tab.
3. Click in the Event Name field.
4. From the list of events in the pop-up window, click and complete an
event the same as when you add events on the Timeline tab.
For more information, see Documenting events on page.85.
5. Complete the fields, as appropriate.
For example, for a Reviewer Note, type Reviewer name, Reviewer
title, and Reviewer department.
If you click Custom event, type the event name and unit, if
appropriate.
6. Click OK.
The note now appears on the event pane, the ECG waveform, and
the Timeline tab.
To modify a note in the Events pane
1. Click the note you want to modify.
6 - Working with ECGs Event Review Pro User Guide

97
2. On the toolbar, click Edit Note .
3. In the Note window, modify the note.
4. Click OK.
To delete a note in the Events pane
1. Click the note you want to delete.
2. On the toolbar, click Delete Note . The note disappears from the
Event pane, the ECG waveform, and the Timeline tab.
Viewing waveforms
You can see the entire ECG as a series of strips on the Overview pane. It
includes markers and labels for recorded events and notes. You can
point to event labels on the ECG on the Overview pane to view event
details. In the Waveformsview, you can zoom in to see an ECGsegment
of interest.
On the Overview and Waveformspanes, a vertical sweep bar indicates
the current ECG time. The sweep bar provides a visual cue to the current
position on the waveform. Click the ECG on the Overview pane to move
the sweep bar to the pointer location on the Waveforms pane. For more
information, see Working with waveforms on page.97.
You can see a close-up view of a segment of the waveform on the
Waveforms pane or the Rhythm strip pane.
To see the location of these panes in the Case Editor, see Understanding
the Case Editor on page.88.
Working with waveforms
You can manage notes on the Overview and Waveforms panes.
The following illustration shows the general arrangement of the
Overview pane. The general arrangement is the same in all languages.
The dashed box (at the black arrow, below) identifies the extents of
waveform that appears on the Waveforms pane (below the Overview
pane).
6 - Working with ECGs Event Review Pro User Guide

98
By default, the primary defibrillator waveform appears on the Overview
pane, and is checked in the Waveform drop-down menu (at the red
arrow, below) on the Overview. Check other waveforms in the menu to
display them. Menu changes will persist in future sessions and with
future cases.
To add and manage notes on the waveform, see Managing notes on the
waveform on page.114.
This ECG is intended only for basic rhythm identification. It is not
intended for diagnostic and ST segment interpretation.
To select a waveform
1. On the Overview toolbar, click the Waveform drop-down menu.
2. From the menu of all recorded waveforms in the case, click the
waveform or waveforms that you want to view on the Overview
pane.
To view event details on the waveform, hover the mouse over the event
marker on the ECG waveform. Event details appear in a pop-up window.
You can set multiple waveforms to display simultaneously. In the
Waveform drop-down menu (shown on the previous illustration),
click the check box next to each waveform that you want to display.
To remove a waveform from the overview, uncheck it in the menu.
In the Overview pane, the multiple waveforms appear overlapped,
and in different colors. Each waveform is labeled at its beginning.
6 - Working with ECGs Event Review Pro User Guide

99
Displaying and hiding waveforms
If the case has multiple waveforms, you can select which waveforms to
display or hide on the Waveforms pane.
See the location of the Waveforms pane and Waveforms drop-down
menu in the section "The Case Editor Waveforms view" in Understanding
the Case Editor on page.88.
To open waveforms for viewing on the Waveforms pane, select the
waveforms from the Waveforms drop-down menu at the top of the
Waveforms pane (the inspector must first be opened). The selected
waveform then appears. You can use the vertical scroll bar to see open
waveforms. You can also drag the border to increase the size of the
Waveforms pane.
To hide a waveform on the Waveforms pane, uncheck the waveform the
Waveforms drop-down menu at the top of the Waveforms pane.
Waveforms in the Rhythm strip pane
The waveforms used for rhythm identification are shown in the Rhythm
strip pane, arranged sequentially in a single strip, in the order recorded
by the device. All the waveforms that make up the rhythm strip are
included on the strip; therefore, there is no need for a Waveforms drop-
down menu in this pane. To display the waveforms on the Rhythm strip
pane, scroll horizontally through the timeline on the Rhythm strip pane.
The same waveforms can also be viewed individually in the Waveforms
pane.
For information on docking/undocking and pinning/unpinning areas of
the ECGdisplay, see Arranging sections of the ECGdisplay on page.99.
Arranging sections of the ECGdisplay
You can use the pinning function to maximize or minimize sections of
the ECGdisplay work area.
If you ever need to put the sections back to their default sizes and
locations, on the Tools menu, click Options, then Restore
Defaults, and then ECG Viewer and Reports. The settings will
be restored when the application is restarted.
"Pinning" and "unpinning"refer to maximizing or minimizing a section of
the work area.
6 - Working with ECGs Event Review Pro User Guide
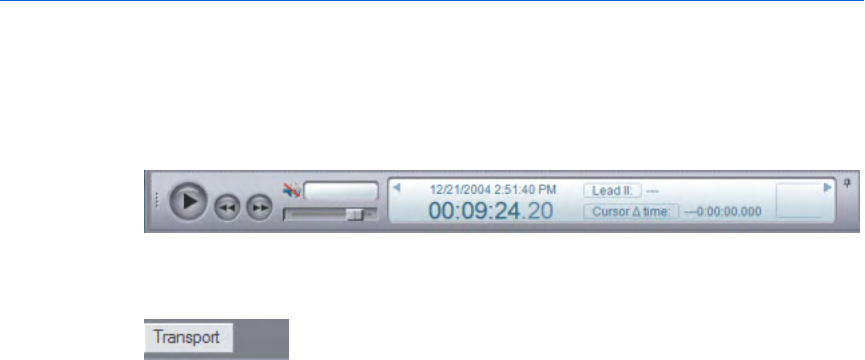
100
The following illustrations show the general arrangement of the work
area. The general arrangement is the same in all languages. A pinned
section might look like the illustration, where the pin is visible in the
upper right corner:
When you click the pin for the section, it becomes minimized; all that
you see is a tab for it at the edge of the display, as in the figure below.
Click the tab, and then click the pin, to pin it open again.
Magnifying waveforms
Use the magnifier to get a detailed view of a section on the waveform.
To use the magnifier tool
1. Hold down SHIFT and press the mouse button as you point to the area
on the waveform that you want to magnify.
A magnified view of the waveform appears, with crosshairs at the
location of the pointer.
2. Release the SHIFT button while continuing to press the mouse button,
move the pointer to another location on the waveform for closer
inspection.
The elapsed time and waveform values appear for the magnified
section, as well as the endpoints of each axis.
Changing the display time
At the top of the Waveforms pane, a ruler indicates the time scale of the
waveform displayed in elapsed time or clock time. Use the waveforms
ruler to change the time scale between Elapsed time and Clock time.
• Elapsed time is the time since the defibrillator was turned on.
• Clock time is the actual clock time based on the Adjusted Time.
The time scale appears with divisions for seconds and fractions of
seconds.
6 - Working with ECGs Event Review Pro User Guide

101
The date and time formats used in this software and in the reports
is controlled by the Windows Region and Language control panel.
Use that control panel to adjust the date and time format. For
example, use it to choose 24 hour time.
If an audio clip is associated with the waveform, the waveforms
ruler displays a blue overlay where the audio is present, in relation
to the waveforms.
To change the display time
1. Right-click the waveforms ruler.
2. On the shortcut menu that appears, click one of the following time
formats:
• Elapsed Time
• Clock Time
The display time changes according to your selection.
Changing the time scale
Use the time scale slider on the bottom left side of the Waveforms pane
to select the time scale in mm/sec. The default time scale is 25.0
mm/sec. You can also use the mouse wheel to change the time scale if
the Waveforms pane is currently in use.
The time-scale grid adjusts according to the time scale selected. At
the default time-scale setting of 25.0 mm/sec, the grid marks
indicate 1-second intervals. If you decrease the time scale, the grid
marks indicate larger intervals. For example, if you decrease the
time scale to 12.5 mm/sec, the grid marks indicate 5-second
intervals.
To change the time scale using the time scale slider, click the time-scale
slider and complete one of the following procedures:
• To increase the scale, drag the slider to the right.
• To decrease the scale, drag the slider to the left.
The adjusted scale appears in mm/sec., and the waveform display
adjusts accordingly.
6 - Working with ECGs Event Review Pro User Guide

102
To change the time scale using the mouse wheel, use one of the
following methods. Point to the waveform, hold the SHIFT key, and click
the mouse wheel to complete one of the following actions:
• To increase the scale, , hold the SHIFT key and roll the mouse wheel
forward.
• To decrease the scale, , hold the SHIFT key and roll the mouse wheel
backward.
The adjusted scale appears in mm/sec., and the waveform display
adjusts accordingly.
To reset the time scale to default, hold down CTRL and click the time-
scale slider. The time scale resets to the default scale (25.0 mm/sec.).
Scrolling through the waveform
Use the waveform scroll bar at the bottom of the Waveforms pane to
scroll through the waveform strip. You can also scroll through the strip
using key command shortcuts. For more information, see Using key
command and mouse shortcuts on page.129.
To scroll through the waveform, drag the waveform scroll bar to the
desired location on the waveform.
Customizing waveforms
Event Review Pro provides a waveform properties tool for each
waveform. This tool displays information about the waveform and
provides features to modify the waveform display. You can choose to
show or hide the tool. By default, the tool is closed; click the arrow key
on the left (see below) to open the tool.
When the waveform properties area is closed, it looks like this:
6 - Working with ECGs Event Review Pro User Guide
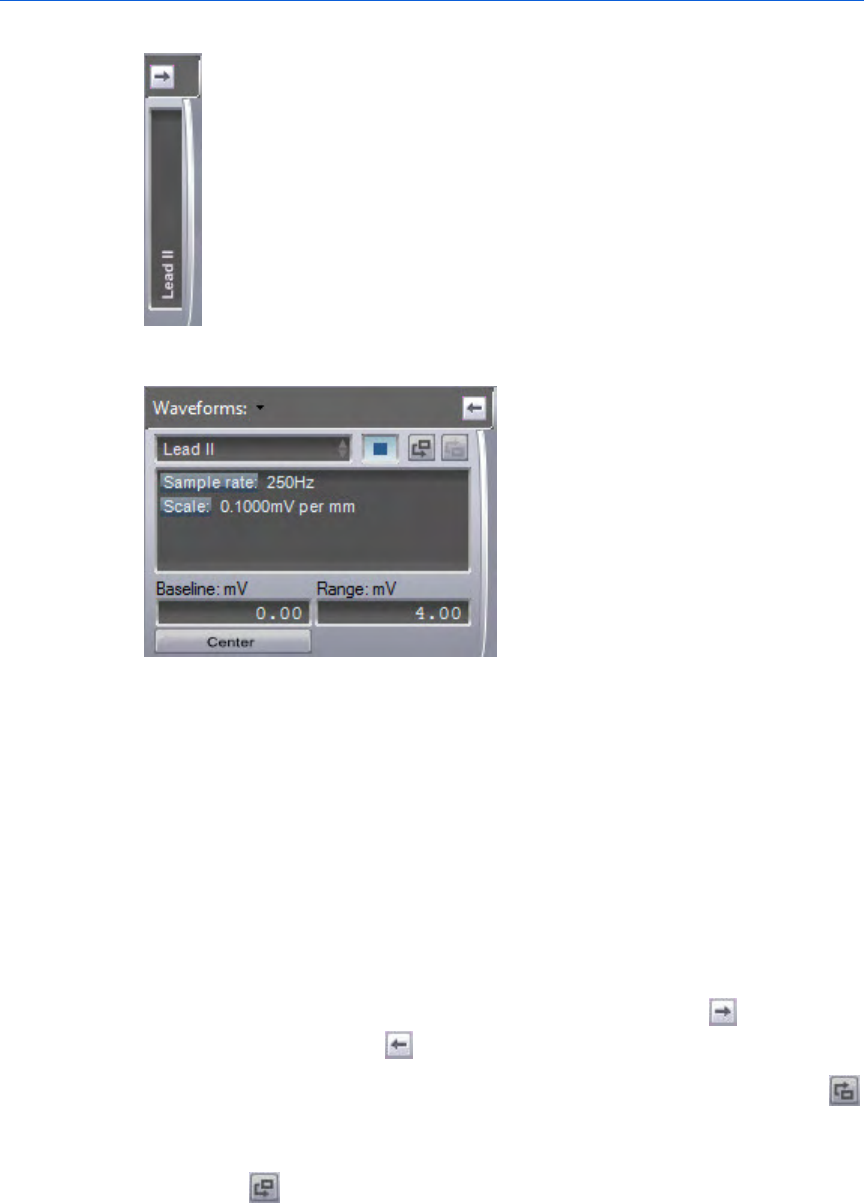
103
When the area is open, it might look like this:
The waveform properties tool displays the following information about
the current waveform:
• The waveform type or source
• The sample rate in Hz
• The current vertical offset and scale
• The scale in millivolts per millimeter (for ECG waveforms)
You can control the vertical offset and you can scale the waveforms. You
can also control the color of the waveforms.
If the ECGhas multiple waveforms, you can change the display order.
• To show the waveform properties tool, click Expand . To hide the
tool, click Collapse .
• To move a waveform up on the Waveforms pane, click Move Up
.
• To move a waveform down on the Waveforms pane, click Move
Down .
6 - Working with ECGs Event Review Pro User Guide

104
To change the color of a waveform
1. Click the color selector to the right of the waveform IDstrip.
2. In the Color window, click the desired color and click OK.
The waveform appears using the selected color.
Changing the waveform offset
The Baseline field, available in the ECG waveform when you expand the
Waveforms area with the arrow, controls the vertical offset for the
waveform. The Baseline field displays the current vertical offset in the
relevant waveform units, for example, mV for an ECG. Use the Center
button to center the waveform segment displayed in a waveform. The
waveform appears with the offset determined by the average of the
waveform values in the segment currently displayed in the waveform.
The following table provides numbers and feature names corresponding
to the images that follow.
1 Waveforms area
2 Arrow
3 Baseline field
4 Center button
5 Waveform
The following illustration shows the general arrangement of the
Waveforms area before it is expanded. The general arrangement is the
same in all languages.
6 - Working with ECGs Event Review Pro User Guide
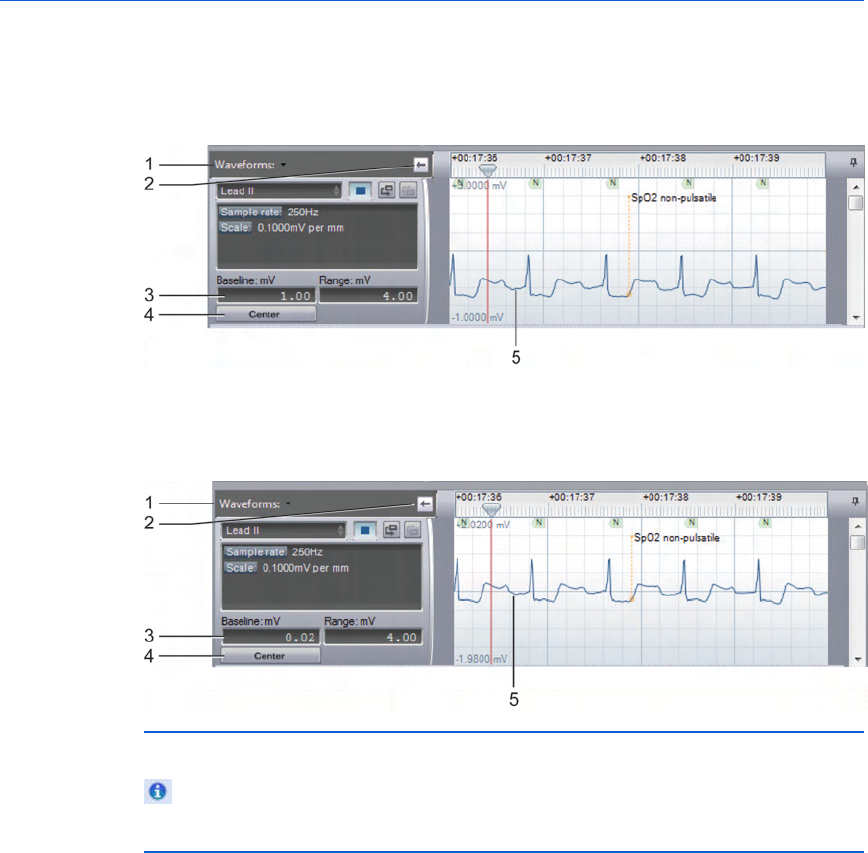
105
The following illustration shows the general arrangement of the
expanded Waveforms area, with a waveform display before centering.
The general arrangement is the same in all languages.
The following illustration shows the general arrangement of the
waveform display after centering. The general arrangement is the same
in all languages.
You can adjust multidigit scale values based on the digit selected.
For example, if the value in the Range field is 100 mV and the third
digit is increased by 1, the scale increases to 101; if the second
digit is increased by 1, the scale increases to 110.
To change the waveform vertical offset gradually, single-click the
specific digit you want to change in the Baseline field (for instance, if
you want to change 125.63 to 125.69), click the 3 so that it is
highlighted, and do one of the following:
• To increase the vertical offset, while holding the left mouse button,
move the mouse so that the cursor moves upward on the screen.
• To decrease the vertical offset, while holding the left mouse button,
move the mouse so that the cursor moves downward on the screen.
The adjusted vertical offset value appears in the Baseline field, and
modifies the vertical offset of the waveform displayed in the Waveforms
area accordingly.
6 - Working with ECGs Event Review Pro User Guide

106
To specify the waveform vertical offset, double-click the Baseline field
and type the offset value. The waveform in the Waveforms area appears
with the specified offset.
To restore the default waveform offset (the offset from the device),
hold down CTRL , and click in the Baseline field.
Changing the waveform scale
The Range field, available in the ECGwaveform when you expand the
Properties area with the arrow, controls the scale of the waveform.
You can adjust multidigit scale values based on the digit selected.
For example, if the value in the Range field is 100 mV and the third
digit is increased by 1, the scale increases to 101; if the second
digit is increased by 1, the scale increases to 110.
To change the waveform scale gradually, single-click the specific digit
that you want to change in the Range field (for instance, if you want to
change 125.63 to 125.69), click the 3 so that it is highlighted, and do one
of the following:
• To increase the waveform scale, turn the mouse scroll wheel
forward, or move the mouse so that the cursor moves upward on the
screen.
• To decrease the waveform scale, turn the mouse scroll wheel
backward, or move the mouse so that the cursor moves downward
on the screen.
The adjusted scale value appears in the Range field, and modifies the
scale of the waveform displayed in the Waveform s pane accordingly.
To specify the waveform scale, double-click the Range field and type
the scale value in mV. The waveform appears in the Waveform s pane
according to the specified scale.
To restore the default waveform scale, hold down CTRL , and click in the
Range field.
Reviewing waveform information
Use the controls on the Transport pane (shown below) to navigate
through events on an ECG waveform, play the waveform, and play an
associated audio clip, if present, while reviewing the waveform.
6 - Working with ECGs Event Review Pro User Guide

107
You can also review information about the current sweep bar and
pointer locations in the Transport pane at the bottom of the ECG view.
Navigating ECGevents on the waveform
• If you click Next , the sweep bar moves to the location of the
next event on the ECG waveform and displays the event time on the
information pane.
• If you click Previous , the sweep bar moves to the location of
the previous event on the ECG waveform and displays the event time
on the information pane.
• If you click Play , the waveform moves at the speed of real
time.
Playing the ECG audio
If an audio clip was recorded with the ECG, you can play the audio and
move along (traverse) the waveform on the Waveforms pane. The
sweep bar indicates the location on the waveform corresponding to the
current audio. You can also play and pause the audio by pressing the
spacebar if the Waveforms pane is currently in use. For more
information, see Using key command and mouse shortcuts on page.129.
• To play the ECG audio, click Play . The audio clip plays, and the
sweep bar moves through the waveform on the Waveforms pane and
indicates the real time on the information pane. If a section of the
ECGhas no audio, you will see a flashing indicator that displays "No
audio."
• To pause the ECG audio, click Pause . The audio pauses, and the
sweep bar stops at the current location on the ECG waveform.
• To change the volume of the ECG audio, drag the Volume slider to
the level you want. This adjustment is relative to the volume that
you set in the Windows audio control. If your Windows audio is
muted or the volume is very low, the Event Review Pro volume
setting cannot override it.
• To reset the ECG audio volume to the default level, hold down CTRL
and click the Volume slider.
6 - Working with ECGs Event Review Pro User Guide

108
Viewing ECG information
The date of the ECG, and the Clock time and Elapsed time for the sweep
bar location, appear in an information pane at the bottom of the ECG
view.
The waveform type and value for the current pointer location also
appears, as well as the cursor delta time. The cursor delta time is the
difference in milliseconds between the current pointer location on the
waveform and the sweep bar location.
To measure the cursor delta time, click a location on the waveform, to
place the sweep bar, then move the cursor to the left or right of the
sweep bar.
The difference between the pointer location and the sweep bar appears
in the Cursor time field on the information pane.
To measure the waveform value, move the cursor over a location on the
waveform. Event Review Pro dynamically assigns a name to the
waveform type field, according to the waveform type or source, and
displays the waveform value in the waveform value field.
The waveform value is displayed in units appropriate to the waveform
type, as in the following examples:
• mV for leads
• mmHg for blood pressure and CO2
• mm for compression
• mOhms for ventilation
Defining and viewing ECG selections
ECG selections are portions of the ECG that you need to reference for
future examination—like bookmarks. You can mark any part of an
ECGas a selection and give it a name; you can also assign it a
distinctive color to set it off. With selection templates, you can set up a
process to create selections automatically when a specific event in the
ECGoccurs. Once you have more than one selection, you can sort them
for quick reference.
You can also print a strip report of the ECGselections from the File
menu or the application toolbar. For more information, see Generating
reports on page.151 and Working with ECG reports on page.155.
Shortcut menu commands on the Waveforms pane assist in these tasks.
6 - Working with ECGs Event Review Pro User Guide

109
Creating ECG selections
If you want to isolate a section of ECGdata for separate attention (such
as focused analysis during debriefing, or printing it by itself and
inserting it in slide presentations, documents, or emails), you can create
a "selection." You can do this three different ways:
• To create an ECG selection manually, click where you want the clip
to begin on the waveform, and press and hold the mouse button as
you move the pointer to where you would like the clip to end. An
overlay highlights the selection.
• If you want to create a single selection of a predetermined length in
the part of the ECG that is on the screen at the moment, use the
Auto-selection feature, You can set the length in seconds before you
create the selection.
• If you want to set a template to create a selection automatically
every time a certain event occurs (like an alarm, a shock event, or a
ventilation), see Using selection templates on page.112.
To automatically create ECG selections
1. In the Auto-selection (secs) text box, type the ECG automatic
selection length in seconds.
2. Hold down CTRL and click the ECG where you want to create an
automatic selection.
A selection appears that is centered on the pointer location. A
colored overlay highlights the selection.
Managing ECG selections
Use the ECG menu or shortcut menu on the Overview or Waveforms
pane to manage ECG selections. To display the shortcut menu, right-
click the ECG.
In addition to printing and copying ECG selections, you can do the
following tasks:
To create an ECG selection using the shortcut menu
1. Move the sweep bar to the point in the waveform where you want
the selection to begin.
6 - Working with ECGs Event Review Pro User Guide

110
2. On the ECG or shortcut menu, click Selections, and then click Set
Selection Start.
3. Move the sweep bar by clicking the point in the waveform where you
want the selection to end.
4. On the ECG or shortcut menu, click Selections, and then click Set
Selection End.
To change the color of the ECG selection
1. Click the selection on the waveform.
2. On the ECG waveform, right-click, and then, from the menu, click
Selections and then click Selection Color.
3. In the Color window, click a color and click OK.
4. On the File menu or toolbar, click Save.
You can now export and import the case with the current ECG view.
Event Review Pro also saves the color for future selections.
To convert an ECG selection to a Q-CPR exclusion
1. Click the selection on the waveform.
2. On the ECG menu, or the shortcut menu, click Selections, and then
click Convert to Q-CPR Exclusion.
The selection is now a Q-CPR exclusion. For more information on Q-
CPRreports, see Working with Q-CPR Report Card reports on
page.158. For more information on Q-CPR exclusions from reports,
see Removing CPR exclusions on page.126.
To clear an ECG selection
1. Click the selection on the waveform that you want to remove.
2. Use one of the following methods:
• On the ECG menu, or the shortcut menu, click Selections.
• Right-click the selection to display the shortcut menu and click
Selections.
3. From the list of selection commands, click Remove Selection.
The selection disappears.
To clear all ECG selections
1. Use one of the following methods:
• On the ECG menu, or the shortcut menu, click Selections.
6 - Working with ECGs Event Review Pro User Guide

111
• Right-click the selection to display the shortcut menu and click
Selections.
2. From the list of selection commands, click Remove All Selections.
All selections disappear.
Naming, finding, and sorting ECG selections
All ECG selections appear in tabular form on the Selections tab. Use the
Selections tab to sort, assign names to, and search for selections.
You can use selection names as bookmarks. The selection name
appears at the bottom of a named selection in the Waveforms pane.
To name ECG selections
1. Click the Selections tab.
All selections appear, sorted according to time of creation.
2. Click the name field for a selection to highlight the number assigned
at creation.
3. Type a name or unique ID for the selection.
4. Repeat steps 2 and 3 for each selection.
To search for a selection
1. On the Overview toolbar, type search criteria in the Search
Selections field.
Event Review Pro searches for selections based on the name field.
The search text must match the selection name in the same order as
it appears on the Selections tab and cannot include a "wildcard" (that
is, a placeholder for an unknown part of the search string). For
example, to search for Shock 1 delivered, you can type “shock” or
“Shock 1”. Case does not matter. However, if you type only “Shock
delivered,” you will not get results. If you want to search for Shock 2
delivered, you must specify “Shock 2” to find the subsequent
selection.
2. Click Search .
The waveform selection matching the search text is now highlighted
in the Waveforms pane.
6 - Working with ECGs Event Review Pro User Guide

112
To change the sort order
1. Click the Selections tab.
All selections appear, sorted according to time of creation.
2. Click the heading of the field you want to sort by.
The selections sort themselves by the field that you chose.
3. Click the field heading again to toggle between ascending or
descending order.
While viewing ECG in the overview or Waveforms pane you can quickly
move from one waveform selection to the next, use the UP ARROW and
DOWN ARROW keys.
Using selection templates
Use selection templates to create event-based ECG selections
automatically. For instance, you can create a template that will mark all
shocks delivered, showing you 2 seconds before and after the event,
and make the selection blue.
Selection templates are saved and are available to be run every time
you import a case.
To create a selection template
1. Click the Selection Templates tab.
The headings for a selection templates table appear.
2. Click New.
A new row appears in the selection templates table.
3. From the Event Category drop-down list, click an event category.
4. Click in the Event Type field for the new selection template.
5. From the Event Type drop-down list, click an event type.
6. In the Pre-seconds field, type the number of seconds you want to
precede the event in the selection.
7. In the Post-seconds field, type the number of seconds you want to
follow the event in the selection.
8. In the Color field, click the color you want to highlight the selection.
9. Click Create.
ECG selections appear according to your criteria.
6 - Working with ECGs Event Review Pro User Guide

113
To delete a selection template
1. Click the Selection Templates tab.
The headings for a selection templates table appear.
2. Click a template and click Delete.
Copying ECGs to the Clipboard
You can copy ECG strips and ECGselections to the Clipboard. You can
then paste the strips and selections into documents created by a word
processing or graphic application. Copy the ECG strips and selections
from the ECG view.
To copy an ECG strip to the Clipboard
1. Right-click the strip in the Overview or Waveforms pane.
2. On the menu that appears, click Copy to Clipboard.
3. Paste the contents of the clipboard into a document.
To copy an ECG selection to the Clipboard
1. Left-click the selection you want to copy, to highlight it, and then
right-click to open the menu.
2. On the menu, click Selections.
3. Click Copy Selection to Clipboard.
4. Paste the contents of the clipboard into a document.
Exporting waveform data
To export a case in the Waveform Database format (.wfdb format),
which is used for data analysis, use the Export option on the File menu
or toolbar. For more information on this format, see
http://www.physionet.org.
Event Review Pro cannot import .wfdb files. For more information,
see Importing case files automatically on page.65.
To export only ECG data
1. Open a case. For more information, see Displaying case details on
6 - Working with ECGs Event Review Pro User Guide

114
page.61.
2. Click the ECG tab.
3. Click the ECG View tab.
4. On the ECG menu, click Export to WFDB.
The Save As window opens.
5. In the Save in box, navigate to the location where you want to save
the exported ECG data.
6. In the File Name box, type a name for the data files.
7. Click Save.
Event Review Pro exports the files to the folder that you name.
Managing notes on the waveform
You can add, edit, and delete notes on the ECG waveform or on the
timeline tab.
To add a note
1. Click the waveform where you want the note to appear.
The sweep bar moves to the pointer location.
2. Use one of the following methods:
• On the ECG menu, click Add Note.
• On the Events pane toolbar, click Add Note .
• Right-click the waveform to display the shortcut menu, and click
Add Note.
The Note window opens.
3. Click the Event Name field.
4. On the list of events, click and complete an event from the list the
same as when you add events on the Timeline tab.
For more information, see Documenting events on page.85.
5. Complete the fields, as appropriate.
6. Click OK.
The note now appears on the waveform, the event list, and the
Timeline tab.
6 - Working with ECGs Event Review Pro User Guide

115
NOTE: See the notes at the end of this topic, which explain fixed
versus relative times and waveform notes.
To modify a note
1. On the event list, click the note that you want to modify.
2. Use one of the following methods:
• On the Events pane toolbar, click Edit Note .
• Right-click the annotation on the waveform to display the
shortcut menu, and click Edit Note.
3. In the Note window, make your modifications to the note.
4. Click OK.
To add a quick note
1. Click the waveform where you want the note to appear.
The sweep bar moves to the pointer location.
2. Right-click the waveform to display the shortcut menu, and click
Add a quick note. (You can use the keyboard shortcut CTRL + N to
do the same thing.)
The Note field opens on the waveform.
3. Type your note in the field, and press ENTER.
The note appears on the waveform.
The most recent 100 entries are saved and become available to
auto-complete a quick note. The selections appear as you type the
note.
To delete a note
1. Click the note that you want to delete.
2. Use one of the following methods:
• On the Events pane toolbar, click Delete Note .
• Right-click the annotation on the waveform to display the
shortcut menu, and click Delete Note.
The note disappears from the waveform.
6 - Working with ECGs Event Review Pro User Guide
116
If you want to add a note with a "fixed" wall-clock time to the
ECGwaveform—that is, its time and date do not change when the
device on-time is adjusted—add the note from the Timeline tab. The
date and time field remains manually editable for notes created on
the Timeline tab.
If you want to add a note with a time relative to the device on-time,
add the note on the ECG tab. The note's time will adjust when the
device on-time is adjusted, or when the note is dragged to a new
location on the waveform. The notes added on the ECG tab also
appear in the list on the Timeline tab; however, they appear
grayed, and are not editable there.
Zooming in and out of the waveform
Use the ECG menu or shortcut menu to zoom in and out of the
waveform.
To zoom in or zoom out
1. Click the waveform.
2. Use one of the following methods:
• On the Case Editor, use the Zoom control to zoom in or out on a
waveform. For help locating the Zoom control, see "The Case
Editor Waveforms view" in Understanding the Case Editor on
page.88.
• Right-click the waveform to display the shortcut menu, and click
Zoom In or Zoom Out.
• On the numeric keypad, press PLUS (+) to zoom in or MINUS (-) to
zoom out.
To zoom in or zoom out fully
1. Click the waveform.
2. Press SHIFT and, on the numeric keypad, press PLUS (+) to zoom in
fully or MINUS (-) to zoom out fully.
6 - Working with ECGs Event Review Pro User Guide

117
Customizing the ECG display
You can configure the display of the waveforms in the Waveforms pane
according to your preferences. You can control the display of device
events, notes, and labels. You can also configure the display of the grid
and the gradient background in the waveforms.
To remove ECG information from the display
1. Click the Waveforms pane.
2. Use one of the following methods:
• Click the ECG menu.
• Right-click the ECG.
3. Click Display Items.
The display items menu opens.
4. Click the item that you want removed from display.
The item disappears from the ECG. For additional information, see
Reviewing case events on page.133.
To restore ECG information to the display
1. Click the Waveforms pane.
2. Use one of the following methods:
• Click the ECG menu.
• Right-click the ECG.
3. Click Display Items.
The display items menu opens.
4. Click the item that you want restored.
The item reappears in the ECG.
Reviewing vital trends data
On monitor/defibrillators like the HeartStart MRx, vital trends data is
associated with the ECG. You can view this data in Event Review Pro in a
table or on a chart.
The following illustration shows the general arrangement of the Table
view. The general arrangement is the same in all languages.
6 - Working with ECGs Event Review Pro User Guide

118
In Table view, you can select the time interval for the vital trends
information.
On the Vital Trends toolbar, click the Interval: drop-down menu and
click 5, 10, 15, 30, or 60 minutes from the list that appears.
The following illustration shows the general arrangement of the Chart
view. The general arrangement is the same in all languages.
In Chart view, you can select the duration of the vital trends data.
On the Vital Trends toolbar, click the Length:drop-down menu and click
10, 30, or 60 from the list that appears.
You can also choose the color and point style for each trend displayed.
To change the point style in Chart view
1. If you are not already in Chart view, click the Chart tab.
2. On the configuration pane to the left of the vital trends chart, click
the style button to the right of the trend you want changed.
The following illustration shows the general arrangement of the Point
Style area. The general arrangement is the same in all languages.
3. On the shortcut menu that appears, click Point Style.
6 - Working with ECGs Event Review Pro User Guide
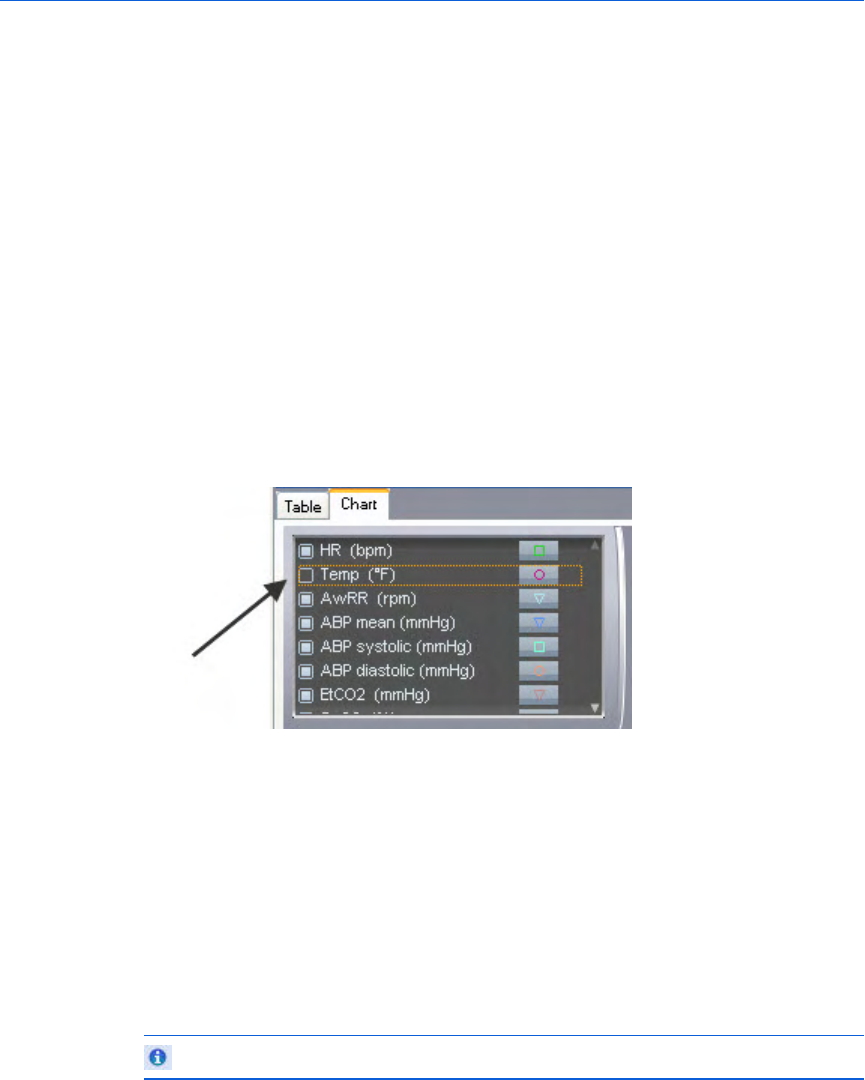
119
4. Click a point style from the list.
To change the trend color in Chart view
1. If you are not already in Chart view, click the Chart tab.
2. On the configuration pane to the left of the vital trends chart, click
the style button to the right of the trend you want changed.
3. On the shortcut menu that appears, click Color.
4. In the Color dialog box, click a color from the Basic Colorslist.
5. Click OK.
The following illustration shows the general arrangement of the Chart
view. The general arrangement is the same in all languages.
To remove trends from display in Chart view. on the vital trends
configuration pane, click the box to the left of the trends (arrow, below).
Working with 12-lead ECGs
The 12-lead data acquired during use of a defibrillator is automatically
attached to the case along with continuous ECG. You can also attach 12-
lead data that was transmitted through the Bluetooth transmission.
The 12-lead ECG and associated data appear on the 12 Lead tab, located
on the ECG tab in the Case Editor. See the 12 Lead tab location in the
illustration that follows.
Only cases with a 12-lead ECGs attached display the 12 Lead tab.
You can also double-click a 12-lead acquisition event in the event pane
to display the data on the 12 Lead tab.
6 - Working with ECGs Event Review Pro User Guide

120
The Case Editor 12 Lead tab
The following illustration shows the general arrangement of the Case
Editor 12 Lead tab. The general arrangement is the same in all
languages. Refer the table after the illustration for definitions.
Key to illustration numbers for the previous illustration:
1 The Case Editor
2 The 12 Lead tab
3 The 12-lead details, from the device recording
4 The 12-lead waveform
The 12-lead ECG appears in the middle section of the 12-Lead view. A
summary section at the top of the 12-Lead view shows the following 12-
lead data:
• Patient demographic information
• Global measurements (Rate, PR, QRSd, QT, QTc)
• Axes (P, QRS, T)
• ECG summary interpretations
6 - Working with ECGs Event Review Pro User Guide

121
• You can use the configuration section at the bottom of the 12 Lead
tab to control the display of the 12-lead ECG and display additional
12-lead ECGs attached to the case.
You can do the following tasks on the 12 Lead tab:
• Configuring the 12-lead view on page.121
• Reviewing the 12-lead ECG on page.122
You can print 12-lead reports from the File menu or application toolbar.
For more information, see Generating reports on page.151and Working
with 12-lead reports on page.156.
Configuring the 12-lead view
Use the configuration section of the 12-Lead view to control the display
of the 12-lead ECG. For information on opening the 12-Lead view, see
Working with 12-lead ECGs on page.119.
To select another 12-lead ECG, click the 12-lead drop-down list and click
a different ECG. The selected 12-lead ECG and its associate summary
information appear.
To change the time format
1. Click the Timing drop-down list to change the time format.
The following time formats appear:
• Simultaneous (European standard)
• Time Sequential (United States standard)
2. Click the time format that you want.
The 12-lead ECG appears in the selected time format.
To scale the report display
You can scale the 12-lead report from 1x, with the Scale control in the
far left position, to 3x, with the Scale control in the far right position.
You can also use the magnifying tool in the 12-Lead view, when the
Scale control is in the 1x position. For more information, see Magnifying
12-lead ECGs on page.122.
• Complete one of the following procedures:
• To increase the scale of the 12-lead ECG displayed, drag the
Scale control to the right.
6 - Working with ECGs Event Review Pro User Guide

122
• To decrease the scale of the 12-lead ECG displayed, drag the
Scale control to the left.
Reviewing the 12-lead ECG
Use the scroll bar to move through the 12-lead ECG.
If the scale has been increased so that sections of the 12-lead ECG are
outside of the current view area, drag the ECG to view the obscured
sections. A location key indicates when you have reached the limit of the
12-lead ECG in any direction.
To scroll through the 12-lead ECG, drag the scroll bar to the desired
location on the 12-lead ECG.
To use the location key
1. Click a position on the 12-lead display and hold down the mouse
button.
A four-arrow location key appears.
2. Drag the 12-lead ECG display up, down, or to the right or left to
display any obscured sections of the 12-lead ECG.
The location key indicates when you have reached the limit of the
12-lead ECG in any direction by dimming the corresponding arrow.
3. Release the mouse button.
Magnifying 12-lead ECGs
You can use the magnifier tool for a detailed view of the 12-lead ECG
waveform.
To use the magnifier tool
1. Hold down SHIFT key and click the area on the waveform that you
want to magnify.
A magnified view of the waveform appears.
2. Release the SHIFT key and while continuing to hold the mouse button,
move the pointer to another location on the waveform for closer
inspection.
3. Release the mouse button.
6 - Working with ECGs Event Review Pro User Guide

123
Working with CPR data
CPR data aquired during use of a defibrillator is automatically attached
to the case. The type of CPR data saved varies depending on the model
of defibrillator and the accessories used during the rescue.
Q-CPR data is generated by HeartStart MRxor Heartstart FR3
defibrillators used with a HeartStart CPRmeter.
zCPR data (impedance-derived CPR data) is generted by Event Review
Pro for cases recorded on HeartStart HS1 and Heartstart FRx
defibrillators, as well as for Heartstart FR3 defibrillators for cases
where a Heartstart CPR meter was not used. zCPR data does not include
compression depth or compression release information.
When a case containing CPR data is opened in Event Review Pro, the
menu at the top of the software window incudes the CPR sub-menu, to
the right of the ECG sub-menu.
You can customize and report on both Q-CPR data and zCPR data.
For detailed information, see these topics:
• Customizing CPR episodes on page.123.
• Creating CPRexclusions on page.125.
• Removing CPR exclusions on page.126.
• Adding CPR annotations on page.127.
• Adding CPR responder annotations on page.128.
Customizing CPR episodes
You can customize the Q-CPR or zCPR reports that you create. For
example, you can use commands on the shortcut menu or the CPR menu
to define an episode for CPR reporting and to mark sections of the
waveform to exclude from Q-CPR or zCPR reports.
Q-CPR or zCPR events appear on the Compression waveform.
6 - Working with ECGs Event Review Pro User Guide

124
For information on excluding sections of the waveform from
CPRreports, see Creating CPRexclusions on page.125.
To customize the CPR episode
1. If necessary, click the ECG tab and display the ECG view.
2. Click the waveform at the point where you want the episode to
begin.
3. Use one of the following methods:
• On the CPR menu, click Set Episode Start.
• Right-click the waveform to display the shortcut menu, click
CPR, and click Set Episode Start.
A vertical bar marks the start of the episode.
4. Click the waveform at the point where you want the episode to end.
5. Use one of the following methods:
• On the CPR menu, click Set Episode End.
• Right-click the waveform to display the shortcut menu, click
CPR, and click Set Episode End.
A vertical bar marks the end of the episode.
By default, the Q-CPR episode starts 1 second before the first
compression, and ends 1 second after the last compression.
By default, the zCPR episode starts at the first pads on event and
ends at the last pads off event.
For information on generating and printing Q-CPR reports, see these
topics:
• Working with Q-CPR Report Card reports on page.158.
• Working with Q-CPR Details reports on page.167.
• Working with Q-CPR Response Time reports on page.173.
For information on generating and printing zCPR Report Card reports,
see Working with zCPR Report Card reports on page.179.
6 - Working with ECGs Event Review Pro User Guide

125
Creating CPRexclusions
A CPR exclusion identifies the areas of the waveform that you do not
want to analyze. Any data inside the exclusion area is excluded from the
analysis. Use the CPR menu, the shortcut menu on the ECG tab, or
shortcut keys to create CPR exclusions.
You can also convert an ECG selection to a CPR exclusion.
To create a CPR exclusion from the CPR menu or shortcut
menu
1. If necessary, click the ECG tab and display the ECG view.
2. Click the waveform at the point where you want the exclusion to
begin.
3. Use one of the following methods:
• On the CPR menu, click Set Exclusion Start.
• Right-click the waveform to display the shortcut menu, click
CPR, and click Set Exclusion Start.
4. Click the waveform at the point where you want the exclusion to
end.
5. Use one of the following methods:
• On the CPR menu, click Set Exclusion End.
• Right-click the waveform to display the shortcut menu, click
CPR, and click Set Exclusion End.
A red overlay highlights the exclusion.
To create a CPR exclusion using shortcut keys
1. If necessary, click the ECG tab and display the ECG view.
2. Click the waveform where you want the exclusion to begin.
3. Hold down ALT and press the mouse button.
4. Drag the mouse to where you want the exclusion to end.
5. Release the key and mouse.
To create a CPR exclusion from an ECG selection, see Managing ECG
selections on page.109.
For information on generating and printing Q-CPR reports, see these
topics:
• Working with Q-CPR Report Card reports on page.158.
6 - Working with ECGs Event Review Pro User Guide

126
• Working with Q-CPR Details reports on page.167.
• Working with Q-CPR Response Time reports on page.173.
For information on generating and printing zCPR Report Card reports,
see Working with zCPR Report Card reports on page.179.
Removing CPR exclusions
You can remove a single exclusion or all exclusions.
The Remove Exclusion option
To remove a CPR exclusion
1. If necessary, click the ECG tab and display the ECG view.
2. Click the exclusion to highlight it.
3. Use one of the following methods:
• On the CPR menu, click Remove Exclusion.
• Right-click the waveform to display the shortcut menu, click
CPR, and click Remove Exclusion.
The highlighted exclusion disappears.
To remove all CPR exclusions
1. If necessary, click the ECG tab and display the ECG view.
2. Click the waveform.
3. Use one of the following methods:
• On the CPR menu, click Remove All Exclusions.
• Right-click the waveform to display the shortcut menu, click
CPR, and click Remove Exclusions.
All CPR exclusions disappear.
The Discard Changes option
The CPR menu also has a Discard Changes option.
Applying this option will reset the episode to the default values and
remove all exclusions.
In the case of zCPR, it will also reset the compression annotations to the
default state.
6 - Working with ECGs Event Review Pro User Guide

127
For information on generating and printing Q-CPR reports, see these
topics:
• Working with Q-CPR Report Card reports on page.158.
• Working with Q-CPR Details reports on page.167.
• Working with Q-CPR Response Time reports on page.173.
For information on generating and printing zCPR Report Card reports,
see Working with zCPR Report Card reports on page.179.
Adding CPR annotations
The following notes affect the calculations that appear in the Q-CPR
Report Card report.
To add CPR notes to a waveform
1. If necessary, click the ECG tab and display the ECG view.
2. Right-click the mouse to display the shortcut menu.
3. Click CPR, and then Annotations.
4. Click one of the following options to add a note, both of which appear
at the sweep-bar location:
• Ventilation (ventilation waveform only)
• PR (Perfusing Rhythm)
• VF
• VT
• PEA
• Asystole
• Compression annotation (zCPR only)
VF, VT, PEA and Asystole are for non-perfusing rhythms
To add notes using keyboard shortcuts
1. If necessary, click the ECG tab and display the ECG view.
2. On the Waveforms pane, expand the ventilation waveform.
3. Click the ventilation waveform at the point that you want to add the
note.
4. Press the following key combination:
6 - Working with ECGs Event Review Pro User Guide

128
• Press ALT+V to add a Ventilation note.(ventilation waveform
only)
• Press ALT+R to add a PR (perfusing rhythm) note.
• Press ALT+C to add a compressaion annotation (zCPR only).
To move a note to another location on the waveform, click the note on
the waveform and drag it to another location using the mouse.
For information on generating and printing Q-CPR reports, see these
topics:
• Working with Q-CPR Report Card reports on page.158.
• Working with Q-CPR Details reports on page.167.
• Working with Q-CPR Response Time reports on page.173.
For information on generating and printing zCPR Report Card reports,
see Working with zCPR Report Card reports on page.179.
Adding CPR responder annotations
In order to support filtering of data by CPR responder in Q-CPR reports,
you can add annotations to the compression waveform, noting
responders by type and name.
The reports that allow filtering by CPR responder information:
• Q-CPR Report Card report,see Working with Q-CPR Report Card
reports on page.158.
• zCPR Report Card report,see Working with zCPR Report Card
reports on page.179.
In the Q-CPR Report Card report or the zCPR Report Card report,
you can filter by named responder by selecting the named
responder the Responder field of the report customization menu.
• Q-CPR Details report, seeWorking with Q-CPR Details reports on
page.167.
In the Q-CPR Details report, when Split by responder is selected,
the Q-CPR details will appear split by named responder, in timeline
sequence.
6 - Working with ECGs Event Review Pro User Guide

129
To add annotations for CPR responders
1. If necessary, click the ECG tab and display the ECG view.
2. On the Waveforms pane, expand the Compression waveform.
3. Click the Compression waveform at the point where the a CPR
responder began delivering CPR.
4. Right-click, and then click Add Note. The Note window opens.
5. In the Note window, click once in Event Name to activate the field,
then click again to open the selection menu.
6. In the selection menu, under Response, click BLSEMSCPR or
ALSEMSCPR. The selection appears in the Event Name field.
7. In the Name field, type a name for the responder.
8. Save the case.
9. For each additional responder, advance the timeline on the
Compression waveform to the point where the next CPR responder
began delivering CPR, and repeat the above process.
Using key command and mouse
shortcuts
You can use the shortcuts in the table below to complete many of the
operations on the ECG tab:
Key command shortcuts
Audio
Play/Pause audio
Click the ECG waveform, and then press SPACEBAR
Reset audio volume to default level
Hold down CTRL and click the Volume slider
6 - Working with ECGs Event Review Pro User Guide

130
Event list
Expand selected event list nodes
SHIFT + PLUS SIGN
Collapse selected event list nodes
SHIFT + MINUS SIGN
Q-CPR
Annotate ventilation waveform with ventilation event
ALT + V
Annotate ventilation waveform with Perfusing Rhythm
ALT + R
Annotate zComp waveform with compression annotations
ALT + C
Selections
Create an automatic selection centered on the pointer location
CTRL and click waveform
Sweep bar
Move sweep bar one minute backward
PAGE UP
Move sweep bar one minute forward
PAGE DOWN
Move sweep bar one strip backward in the WAVEFORMS pane
SHIFT + PAGE UP
Move sweep bar one strip forward in the WAVEFORMS pane
SHIFT+PAGE DOWN
Move sweep bar to beginning of recording
HOME
Move sweep bar to end of recording
END
Move sweep bar one second backward
LEFT ARROW
Move sweep bar one second forward
RIGHT ARROW
6 - Working with ECGs Event Review Pro User Guide

131
Move sweep bar to previous selection
UP ARROW
Move sweep bar to next selection
DOWN ARROW
Make a notation at the sweep bar, using quick note
CTRL + N
Time scale
Time scale expand
CTRL + PLUS SIGN (+)
Time scale compress
CTRL + MINUS SIGN (-)
Zoom
Zoom in
PLUS SIGN (+)
Zoom out
MINUS SIGN (-)
Zoom in fully
SHIFT + PLUS SIGN (+)
Zoom out fully
SHIFT + MINUS SIGN (-)
Mouse shortcuts
Magnification
Display magnified view of waveform
Click the waveform; then hold down SHIFT and press the mouse
button.
Q-CPR exclusion
Create Q-CPR Exclusion
Hold down ALT and drag mouse over waveform.
6 - Working with ECGs Event Review Pro User Guide

132
Time scale
Expand time scale
Press the SHIFT key and move the mouse wheel backward.
Compress time scale
Press the SHIFT key and move the mouse wheel forward.
Vertical offset
Increase vertical offset
Click the BASELINE field and move the mouse cursor up.
Decrease vertical offset
Click the BASELINE field and move the mouse cursor down.
Waveform scale
Increase vertical waveform scale
Click the RANGE field and move the mouse cursor up.
Decrease vertical waveform scale
Click the RANGE field and move the mouse cursor down.
6 - Working with ECGs Event Review Pro User Guide

7
Reviewing case
details
The following topics present information about reviewing cases.
Reviewing case events
Use the Event Log tab to view all the defibrillator events and user notes
that are associated with the ECG. The events that appear on the
Timeline and ECG tabs are in the Event Log.
In the Event Log, you can do the following:
• View details for an event.
• Sort and group the defibrillator events and notes that a responder or
reviewer added to the case.
• Filter events and notes to display only those that fit your selection
criteria.
• Print the events for the open case. See Printing table entries on
page.39.
To display the event log
1. On the case selection table, click a case to open it. For more
information, see Displaying case details on page.61.
2. Click the Event Log tab.
The table that appears is a log of events for the case in the Event
Review Pro database.
Viewing event details
To view event details, double-click an event. The details appear on the
ECG tab.
133

134
The sweep bar identifies the event. For more information, see Working
with waveforms on page.97.
Hiding and displaying events
You can hide or display the list of events for the open case from the
shortcut menu.
To use the shortcut menu
1. Open a case. For more information, see Displaying case details on
page.61.
2. To display a list of options, use the View menu or, on the Event Log
tab, right-click a row in the table.
3. Click Refresh, Expand All, or Collapse All.
To refresh, hide, and display events
• To refresh the list of events, on the View menu or shortcut menu,
click Refresh.
• To hide event details, click Collapse -to the left of the event.
• To hide all event details, on the View menu or shortcut menu, click
Collapse All.
• To display event details, click Expand +to the left of the event.
• To display all event details, on theView menu or shortcut menu,
click Expand All.
Sorting, grouping, and filtering events
You can change the way information displays in the list of events. You
can sort and group the list of events, and you can filter them so that only
the events that meet the criteria you select appear.
For more information, see Working with columns on page.35, Grouping
and sorting entries on page.35, Filtering entries on page.37, and
Removing filters on page.39.
Printing case events
You can print the events for any case saved in the database.
7 - Reviewing case details Event Review Pro User Guide

135
To print a list of events for a case saved in the database
1. Click the Reports navigation button.
2. On the Reports navigation pane, click the Case Events report from
the list of reports in the Cases group. For more information, see
Working with case reports on page.155 and Working with ECG
reports on page.155.
Attaching files to a case
Use the Attachments tab to attach files to the open case. For example,
you can manage a text document, a .pdf file, or a graphics file.
Use the Attachments menu to attach files, open the attachments, and
remove attached files from the case.
No attachment file can be larger than 10 Mb.
To view an attachment, you must have the associated application
on your computer. For example, to open a picture, you must have
an application that can open graphic files.
Displaying the Attachments tab
Use the following procedure to display the Attachments tab.
To display the Attachments tab
1. Open a case. For more information, see Displaying case details on
page.61.
The Case Editor opens, to the ECGtab.
2. On the Case Editor, click the Attachments tab.
A table of the files that are attached to the case appears. The table is
blank when there are no attachments in the Event Review Pro
database that are associated with the case.
Adding and removing attachments
Use the following procedures to manage the attachments that are
associated with the open case.
7 - Reviewing case details Event Review Pro User Guide

136
To attach a file
1. On the Attachments menu, click Attach.
2. In the Attach File window, navigate to the file location.
3. Click Open.
Depending on the size of the file, it can take a few seconds for the
file to show in the Attachments list.
To remove an attached file
1. On the Attachments tab, click the file description in the
Attachments table.
2. On the Attachments menu, click Detach.
Viewing file contents
To display the contents of an attached file
Use one of the following methods:
• On the Attachments menu or toolbar, click Open.
• Double-click the row in the Attachments table.
The attachment opens in the associated application.
Printing the attachments in an open case
You can print the contents of the files attached to an open case when you
have the associated application installed on your computer. Open the
attachment in this associated application and print using its features.
You might not be able to print all attachments, such as a video clip.
You can also print the list of attachments as they appear on the
Attachments tab.
To print the list of attached files
1. On the File menu or toolbar, click Print.
2. On the Print menu, click Table.
3. In the Print window, click the printer, page range, and number of
copies.
7 - Reviewing case details Event Review Pro User Guide

137
4. Click Print or OK.
Completing a final case review
After the case is reviewed, you can add reviewer notes under the Final
Review tab. However, at any time during the case, you can update this
information, or add more.
This screen can contain up to two pages' worth of information. You can
also copy and paste information from other applications and retain the
character formatting (such as bold or italic).
To perform a final review
1. Click the Final Review tab.
2. Place the cursor in the Reviewer Notes pane.
3. Do any of the following:
• Type your information.
• Copy from another application such as a Microsoft Word
document and paste the information into the Review pane.
4. In the Reviewer Name field, type your name.
5. In the Review Date field, type the date.
6. Check the Case Reviewed box.
7. Do either of the following:
• On the toolbar, click Save.
• Exit the Review Notes pane. When the application asks if you
want to save the information, click OK.
For cases with the Case Reviewed box selected, the Reviewed
column in the Patient Cases table displays a Yes. Sorting the Patient
Cases table by the Reviewed column is a method to quickly see
which cases have been reviewed. For information about sorting
cases, see Sorting and grouping cases on page.62.
7 - Reviewing case details Event Review Pro User Guide

8
Using the Import
Service
Use the Import Service on the Administration navigation pane to import
cases into the Event Review Pro database automatically. The case data
can come from several sources:
• Case files exported by other users of Event Review/Event Review
Pro or Data Messenger
• Cases exported by third-party ePCR software
• From the MRx via batch LANdata transfer (BLDT)
• Via HTTP, using Data Messenger
See Configuring the Import Service on page.141 for information on your
configuration options.
When the Import Service detects that there is a case file with the
correct extension, it automatically creates a case using the patient
demographic information and the ECG from the case file.
Event Review Pro saves the case to the database and lists it on the case
selection table. The Import Service moves the file to the archive folder
in the inbox folder.
To make any changes to the Import Service, you must run Event
Review Pro as an administrator.
To manage inbox folders, see Managing Import Service inboxes on
page.139.
To manage the working folder for HTTPtransfer, see Using the
HTTPImport Service on page.143.
To filter the device data, so that the only data imported is of certain
case types, see Managing Import Service inboxes on page.139.
To specify how long to keep the content in archive folders before it is
purged, see Managing Import Service inboxes on page.139.
138
139
If the imported case contains a duplicate ECG, the case appears on the
Duplicated ECGs table. For more information, see Reviewing cases with
duplicate ECGs on page.73.
For information on how to monitor system usage, see Working with the
System Log on page.145.
If you want to configure and run an Import Service on another
machine that does not have Event Review Pro installed on it, see
the Server Pack documentation in the Implementation guide.
Case files that are password-protected cannot be imported with the
Import Service.
Managing Import Service inboxes
Use the Import Service workspace to add (up to 100), view, and remove
inboxes.
If the Import Service is running when you add or delete inboxes, it
detects the change and updates the folders that are monitored. You do
not need to restart the Event Review Pro 5.0 Import Service.
If Import Service is not running when you add or delete inboxes, the
changes become effective the next time that Import Service runs.
To make any changes to the Import Service, you must run Event
Review Pro as an administrator.
You can also filter the device data that is imported so that it includes
only certain case types (the default is to import all data).
By default, the content in archive folders is retained for 7 days.
However, you can decide how long to keep this data and purge the
folders after a designated number of days. For more information, see
Managing Import Service archives on page.142.
To view the Import Service inboxes
1. On the navigation pane, click the Administration navigation button.
2. On the Administration navigation pane, click Import Service.
To add an inbox
1. Display the Import Service workspace.
8 - Using the Import Service Event Review Pro User Guide

140
2. In the area below the Inbox folder box, click Add.
The Browse for Folder window opens.
3. Use one of the following methods:
• Navigate to an existing folder.
Note: Not all networked folders can be found by browsing. In
this case, type in the full path name of the folder.
• Navigate to the location for the new folder, click Make New
Folder, and type a name for the folder.
Make sure that the Import Service has read/write permission for
this folder.
4. Click OK.
The path to the folder in the Inbox folder area now appears.
5. Click Save.
The action is complete.
HeartStart MRx monitors that use batch LAN data transfer use the
directory that is configured by the FTPservice. This directory must
be designated as an inbox folder for it to import automatically to
Event Review Pro.
To remove inbox folders
1. Display the Import Service workspace.
2. In the list of inbox folders, click the one you want to remove and
click Remove.
The folder no longer appears in the list.
To filter the device data that is imported
1. Display the Import Service workspace.
2. In the Import Service Filters area, click the Import Device Data
that Includes Any of the Following button, and then check as
many of the boxes below as you want.
Device data that does not include any of the checked items will be
excluded from import.
8 - Using the Import Service Event Review Pro User Guide

141
Configuring the Import Service
The default installation does not set up the Import Service to start
automatically. If you need to import cases automatically, you must set
up the Import Service from Windows.
To set up the Import Service from Windows
1. Complete the typical Event Review Pro installation.
2. Navigate to the Windows Control Panel.
3. Click Administrative Tools.
4. Double-click Services.
5. Scroll to the Event Review Pro 5.0 Import Service and double-
click it to open it.
The Event Review Pro 5.0 Import Service Properties window
appears.
6. If you want the Import Service to run automatically when the
computer starts, under the General tab, change the notation in the
Startup Type column to Automatic and click Apply.
7. If you are using HTTP:Click the Log On tab and, under Log On As,
click This Account. Type a named administrator and password in
the appropriate boxes, and click Apply.
8. Click the General tab, click Start, and click OK.
To start the Import Service manually
1. Navigate to the Windows Control Panel.
2. Click Administrative Tools.
3. Double-click Services.
4. Right-click Event Review Pro 5.0 Import Service.
5. Click Start.
The System Log lists any Import Service activity. For more
information, see Working with the System Log on page.145.
If the Import Service can't connect, it will write the error(s) to the
Event Viewer (under Windows Logs / Applications).
8 - Using the Import Service Event Review Pro User Guide

142
The Import Service pauses for three minutes at the time of startup.
After processing all inboxes, the Import Service then pauses for one
minute before checking for new files. Processed files are moved to
archive files, which are stored in one of four possible directories:
• Cases successfully imported: Archive/YYYYMMDD/Imported
• Cases partially transmitted: Archive/YYYYMMDD/Incomplete
Transmission
• Cases not imported because of filters: Archive/YYYYMMDD/Not
Imported
• Cases not supported, or corrupted:Archive/YYYYMMDD/Error
All four types are deleted when the purge days have been exceeded (if
the purge limit is set).
Managing Import Service archives
After a file is imported successfully to the database, Import Service
moves the file to archive files, which are stored in one of four possible
directories:
• Case types successfully imported: Archive/YYYYMMDD/Imported
• Case types partially transmitted: Archive/YYYYMMDD/Incomplete
Transmission
• Case types not imported because of filters: Archive/YYYYMMDD/Not
Imported
• Case types not supported, or corrupted:Archive/YYYYMMDD/Error
To make any changes to the Import Service, you must run Event
Review Pro as an administrator.
You can purge these folders automatically after a designated number of
days—between 1 and 31 days. All four types are deleted when the purge
days have been exceeded (if the purge limit is set).
To purge archive folders
1. Display the Import Service workspace.
2. Below the Inbox Folders area, check the Purge Archive Folders
After box and type the number of days in the box following. You can
choose any number between 1 and 31.
8 - Using the Import Service Event Review Pro User Guide
143
All archive folders will be purged that number of days after the
contents are created.
Using the HTTPImport Service
The Import Service, working in conjunction with HeartStart Data
Messenger, supports the import of case data using HTTP. After you set
up HTTP, you can transfer case data from Data Messenger.
For more information, see the HeartStart Data Management
implementation guide.
To make any changes to the Import Service, you must run Event
Review Pro as an administrator.
When you use HTTP, you must set a special type of inbox folder, called a
working folder; you can have only one working folder. Use the Import
Service workspace to set, change, or clear the working folder.
For the Import Service to work properly with HTTP, you must run it
under a named administrator (not the default local service). See
Configuring the Import Service on page.141.
If you are importing from Data Messenger, you must open a TCP
port in the Windows firewall on the computer that is running Import
Service, and this port must correspond to the port used by Data
Messenger. By default, this is port 7080.
To enable the HTTPimport service and set the working folder
1. Display the Import Service workspace. For more information, see
Managing Import Service inboxes on page.139.
2. In the HTTP Import Working Folder area, click Set.
The Browse for Folder window opens. In the Browse for Folder
window, navigate to a folder. Make sure that the Import Service has
read/write permission for this folder.
3. Click OK.
The path to the folder in the HTTP Import Service area appears.
4. Click Save.
The action is complete.
8 - Using the Import Service Event Review Pro User Guide

144
To change the working folder
1. Display the Import Service workspace. For more information, see
Managing Import Service inboxes on page.139.
2. In the HTTP Import Working Folder area, click Set.
3. Use one of the following methods to change the working folder:
• Navigate to a different existing working folder.
• Navigate to the location for the new folder, click Make New
Folder, and type a name for the folder.
Make sure that the Import Service has read/write permission for this
folder.
4. Click OK.
The path to the folder in the HTTP Import Service area appears.
5. ClickSave.
The action is complete.
If you change the working folder while files are still waiting in the
previous working folder to be imported, those files will not be
imported.
To disable the HTTP Import Service and clear the working
folder
1. Display the Import Service workspace. For more information, see
Managing Import Service inboxes on page.139.
2. In the HTTP Import Working Folder area, click Clear.
The path to the folder in the HTTP Import Service area disappears.
3. Click Save.
The action is complete.
If you disable the import service while files are still in the working
folder waiting to be imported, those files will not be imported.
8 - Using the Import Service Event Review Pro User Guide

9
Working with the
System Log
You can view the System Log to see the status of all Event Review Pro
activity. You can sort, group, and filter the list of entries. A filter shows
only those entries that meet the criteria you select.
This section provides an overview of how to use the system log.
You can change the default layout of the System Log. This change
persists the next time you use the navigation pane or workspace. For
more information, see Resizing panes and workspaces on page.41.
To display the system log
1. On the navigation pane, click the Administration navigation button.
2. On the Administration navigation pane, click System Log.
The system log appears.
The System Log lists Event Review Pro activity. For each action, the
following information is included:
• The type of activity, for example, Information or Error
• A description of the action
• The date and time when the action occurred
• The user logon name
If a customer support representative asks you to send the System Log,
on the Help menu, click Email with System Log, and send the email
message that appears. The System Log is attached to it by default.
The software retains the last 10,000 system log entries.
For information about working with the log entries, see:
• Working with columns on page.35
• Grouping and sorting entries on page.35
• Filtering entries on page.37
145
10
Working with
reports
Use reports to evaluate the timeliness of, effectiveness of, and trends in
your emergency system response. You can generate a report for an
open case or directly from the database.
Event Review Pro generates reports from case information.
If you import a redacted case, in any report that includes that case,
the redacted case appears with a series of asterisks in the patient
name fields, and the patient ID is replaced by a series of letters and
numbers, unique to each patient ID. If the redacted age of the
patient is greater than 90, the Date of Birth field is blank and the
Age field displays 90.
Report type availability is based on your installation. The following
categories of report types are available (though not all cases include
data for all reports):
Report category Based on
Case Information that appear on various tabs in the
Case Editor
See Working with case reports on page.155.
ECG ECG and other waveforms that appear on the
ECG tab in the Case Editor
See Working with ECG reports on page.155.
12-lead 12-lead waveforms that appear on the
12Lead tab in the Case Editor
See Working with 12-lead reports on
page.156.
CPR Quality of CPR information
See Working with Q-CPR Details reports on
147

148
Report category Based on
page.167.
See Working with Q-CPR Report Card reports
on page.158.
See Working with Q-CPR Response Time
reports on page.173.
See Working with zCPR Report Card reports on
page.179.
Selecting CPR Guidelines for reporting on
page.150.
Vital Trends Vital trends information that appears on the
ECGtab in the Case Editor
See Working with Vital Trends reports on
page.183.
(EMS Edition only) Response
times
Emergency response times
See Working with Response Times reports on
page.183.
(EMSEdition only)Utstein Utstein guidelines for reporting on aggregated
out-of-hospital cardiac arrests
See Working with Utstein reports on
page.186.
Self-Test Device self-test history
See Working with Device Self-test History
reports on page.187.
Selecting cases for reports
The Reports workspace provides a list of available report types, and the
Select Cases area, where you can filter the cases you want to report on
by date and by content.
The Select Cases area appears when you select a report type. The
logical options for the chosen report type are preselected. For example,
if the 12-Lead report type is chosen, then, in Select Case, the ECG and
12-Lead options are preselected.
10 - Working with reports Event Review Pro User Guide
149
Filter your case selection by defining the date range for the reports you
want to include. Date selections are at the top of Select Cases.
Date refers to the case date, usually the device-on date, not the
date a case was imported.
Two methods are available for specifying dates:
• From the last (1-365) days
This date is calculated from today's date, and saved across sessions,
for the specific report type.
• From (dd/mm/yyy) To (dd/mm/yyy)
Enter the earlier date in the From field.
This date range is saved for the current session, for the specific
report type.
More filtering options are grouped under With and And Have. Use
them to further define the list of cases to include in your report.
Use the selection menus, text fields, or check boxes adjacent to each
item to select the filters you want to add.
Some of the Device Data properties are preselected based on the report
type selected from the Reports navigation pane. For example, if 12-Lead
is selected in the Reports navigation pane, the checkbox "12-Lead" is
preselected, and cannot be edited.
To start the search and display matching cases
1. When the selection criteria are set, click Select to retrieve the
cases.
Cases matching the selection criteria appear in the table at the
bottom of the work area.
Note: You can print the table by right-clicking in the table and
clicking Print Table.
You can also export the table with all columns by right-clicking the
table and then clicking Export to Excel. This creates a Microsoft
Excel file in XLSX format.
For information about generating reports once the cases are selected,
see Generating a report using the Reports workspace on page.152.
10 - Working with reports Event Review Pro User Guide

150
Selecting CPR Guidelines for reporting
By default, the CPR guidelines used for reporting are the CPR guidelines
that were used by the defibrillator during an incident.
The Q-CPR and zCPR reports include a selection menu for Guidelines.
The menu default is Device Guidelines. You may select different
guidelines. The guidelines you select will be used to calculate the results
displayed in the report.
Guideline options include:
• Device Guidelines
• AHA 2005
• AHA 2010
• AHA 2015
• ERC 2005
• ERC 2010
• ERC 2015
There are options available for custom guidelines. If custom guidelines
are configured, they appear at the bottom of the Guidelines menu.
Custom CPR Guidelines
You can use the Administration workspace to create custom CPR
guidelines and save them for selection from the Guidelines menu of the
Q-CPR or zCPR reports.
In order to create custom CPR guidelines, you must be a member of
the Administrators group, or, when starting Event Review Pro,
right-click Run as administrator.
Configure custom CPR Guidelines in the Administration workspace. See
Using the Administration workspace on page.25.
To configure a custom CPR Guideline
1. From the Navigation pane, click Administration to open the
Administration workspace.
10 - Working with reports Event Review Pro User Guide

151
2. In the Administration workspace menu, under User CPR Guidelines,
click Create New Guidelines.
A CPR Guidelines setup space opens on the right.
3. In the Title field, enter a name for your custom CPR Guideline.
4. In the Description field, enter your description.
5. Under Depth, adjust the slider to set an upper and lower limit for
CPR depth.
6. Under Rate, adjust the slider to set an upper and lower limit for CPR
rate.
7. Click Save to save your custom CPR Guideline.
Your guideline is saved by name at the bottom of the User CPR
Guidelines list on the Administration workspace menu.
To delete a custom CPR Guideline
1. From the Navigation pane, click Administration to open the
Administration workspace.
2. In the Administration workspace menu, under User CPR Guidelines,
right-click the name of the custom CPR Guideline you want to delete.
3. Click Delete from the pop-up menu.
The custom CPR Guideline is deleted.
Generating reports
There are two approaches to generating a report; selecting a case and
then choosing a report type, or selecting a report type and then choosing
a case. The procedure is different for each approach.
• To select a case and then choose a report type, see Generating a
report for an open case on page.151.
• To select a report type and then choose a case, see Generating a
report using the Reports workspace on page.152.
Generating a report for an open case
You can generate a report for an open case.
10 - Working with reports Event Review Pro User Guide

152
To generate a report for an open case
1. Open a case. For more information, see Displaying case details on
page.61.
2. On the File menu or toolbar, click Print, and then click Report, and
select a report type.
The report opens in the Report Preview window.
Use the tools in Report Preview to customize the report settings, and to
view, print, or export the report. See Working in Report Preview on
page.153.
Generating a report using the Reports
workspace
Use the Reports workspace to choose a report type, search the database
for available cases, and then generate a report.
To open the Reports workspace, click the Reports navigation button.
Aselection list of the available report types appears on the Reports
navigation pane.
To generate a report from the database
1. From the Reports navigation pane, select a report type.
Follow the instructions for selecting a case or cases in Selecting
cases for reports on page.148.
2. After setting up your case selection search in the Select Cases area,
click Select.
The application locates the required case or cases.
• For single-case report types, a list of matching cases in the database
opens at the bottom of the Reports workspace.
When this happens, double-click the case row to open the report in
the Report Preview window.
• For aggregate report types, when you click Select, the report opens
in the Report Preview window.
Use the tools in Report Preview to customize the report settings, and to
view, print, or export the report. See Working in Report Preview on
page.153.
10 - Working with reports Event Review Pro User Guide

153
Working in Report Preview
The Report Preview window opens when a report is generated.
User-configurable settings, specific to each report type, are located on
the customization tab of the Report Preview window. For reports with
user-configurable options, in the customization tab area, choose the
parameters to customize the report. The report regenerates with the
settings.
Customized settings persist for a report type. For example, when you
open an ECG pre- and post-shock report, you can choose to display a
grid, or hide it. Your custom settings are re-applied the next time you
open an ECG pre- and post-shock report. At that time, you can keep the
settings, or change them.
All the tools for viewing, printing or exporting a report are available on
the toolbar of the Report Preview window.
To view a report that has multiple pages, click the
first/previous/next/last page icons in the Navigation section.
To find specific text in the report, click Find, type a string to search for,
specify any search options, and click Find Next.
Click one of the print options to print the report being previewed:
• Click Print to select a printer, number of copies, and other printing
options. When you have made these specifications, click Print.
• Click Quick Print to send the report directly to the default printer
without making changes.
Click Export to to export a report to a file. A window appears allowing
you to specify details.
The following table explains the function of each toolbar icon:
Button Description
Click Print or press CTRL+P to select a printer, number of copies,
or other printing options before printing.
Click Quick Print to send the document directly to the default
printer without making changes.
Click First Page or press CTRL+HOME to navigate to the first page
of the document.
10 - Working with reports Event Review Pro User Guide
154
Button Description
ClickPrevious Page or pressPAGEUP to navigate to the previous
page of the document.
Click Next Page or press PAGEDOWN to navigate to the next page
of the document.
Click Last Page or press CTRL+END to navigate to the last page of
the document.
Click Many Pages to select the page layout and to arrange the
document pages in preview.
Click Zoom Out to see more of the page at a reduced size.
Click Zoom to change the zoom level of the document preview.
Click Zoom In to get a close-up view of the document.
Click Export To to export the document in PDF format, and save it
to a file on a disk. A window to specify parameter settings for the
export file appears.
Printing reports
Use Print or Quick Print on the Report Preview toolbar to print a report.
See Working in Report Preview on page.153.
Exporting reports
If you frequently review a report or want to track your system’s
performance, generate the report, and then use the Export tool to save
the report as a file.
On the Report Preview toolbar, use Export to PDF to export a report to
a file.
See Working in Report Preview on page.153.
10 - Working with reports Event Review Pro User Guide

155
Working with case reports
Case reports include detailed case information based on the information
collected on the tabs in the Case Editor.
You can generate the reports in the Cases category when a case is open.
For information on how to generate a report, see Generating reports on
page.151.
The Case report
The Case report, also called the Case details report, shows information
from tabs in the Case Editor. You can choose to append all the available
report types for that case into one report, rather than printing each one
separately. To do so, in Report Preview, on the Case Customization tab,
check all the report types that you want to include. Then, on the tab for
each of these report types, select the parameter settings you want to
include in the Case report.
The Case events report
The Case events report lists events from the Event log in the Case
Editor. In Report Preview, on the Case Customization tab, you can
choose to hide or show event parameters.
For more information, see the following topics:
• Working in Report Preview on page.153
• Printing reports on page.154
• Exporting reports on page.154
Working with ECG reports
ECG reports are based on the ECG strip on the ECG tab in Cases.
For information on how to generate a report, see Working with reports
on page.147.
You can choose which waveforms are visible. The Rhythm strip is
shown with a red grid. Other waveforms are shown with a blue grid.
ECGFull Disclosure
The ECG Full Disclosure report includes the entire ECG.
10 - Working with reports Event Review Pro User Guide

156
The report uses the following parameter settings:
• Change the scale of the waveform
• Display grid
• Events
• Notes
• Beat labels
• Selected waveforms
• Overlay waveforms
• Scale
• Elapsed time/Clock time
• Orientation (Portrait or Landscape)
Dashes in the report indicate periods during the report when there is no
waveform data.
Pre- and Post-shock
The Pre- and Post-shock report documents the ECG segments
surrounding each shock event.
The report uses the following parameter settings:
• Change the scale of the waveform
• Grid
• Events
• Notes
• Beat labels
• Selected waveforms
• Presenting seconds
• Closing seconds
• Preshock seconds
• Postshock seconds
Working with 12-lead reports
12-lead reports are based on 12-lead ECG recordings in a case, if
present.
10 - Working with reports Event Review Pro User Guide
157
View 12-leads on the 12 Lead tab in the Case Editor. For information on
viewing 12-leads, see Working with 12-lead ECGs on page.119.
You can print 12-lead reports from the File menu or application toolbar.
For more information, see Generating reports on page.151. When the
print preview of the report is open, the parameter settings appear at the
top of the preview.
In the 12-lead report you can choose from the following parameter
settings:
Demographics
Select one of the following:
• Original The default selection, the report shows the patient
information recorded by the device.
• Override report with case information Patient information
entered on the Overview tab in the Case Editor is shown in the
report, in place of patient information recorded by the device. This
setting could be helpful in correcting a patient name, for example.
The change appears only in the report. The information recorded by
the device is not altered.
• Redact patient information Patient information recorded by the
device is not shown in the report. Reference ID, if entered, is not
redacted. The change appears only in the report. The information
recorded by the device is not altered.
Timing
Select one of the following ECG display methods:
• Simultaneous The defualt selection
• Time Sequential
Display Grid
Check this box to add a grid behind the ECG waveforms. The grid is
visible in the on-screen preview and when printed.
10 - Working with reports Event Review Pro User Guide

158
Working with Q-CPR Report Card
reports
The Q-CPR Report Card report is based on the quality of the CPR that a
patient received during the episode.
Customizing the Q-CPR Report Card report
Once selected, the following settings, except Responder, persist when
the application is reopened.
• GuidelinesIn the Guidelinesmenu you can select the CPR
Guidelines used to generate the report. The default is Device
Guidelines, the CPR guidelines that were used by the defibrillator
during the incident.
Other available guideline options are AHA 2005, 2010, or 2015, ERC
2005, 2010, or 2015, or Custom (if configured). Up to 15 custom
guidelines can be saved. Custom CPR Guidelines are set in the
Administration workspace. For details, see Selecting CPR Guidelines
for reporting on page.150.
• DepthYou can select the compression depth units, inches or
millimeters. You can toggle between inches and millimeters. The
default setting is inches.
• ResponderYou can filter results by responder (limit the results to
CPR performed by a specific responder) by selecting a CPR
responder from this menu. The default is All, and the report displays
statistics for the entire episode, including all responders. You can
filter by responder if responders are annotated.
In order to filter by responder, you need to annotate the
compression waveform with responder type, and enter the
responder name in the name field. For details, see Adding CPR
responder annotations on page.128.
• Ignore episode markersCheck this option if you want to ignore
the episode markers and report on the entire recording from device
on to device off.
When this option is unchecked, the episode start and end times are
set to one second prior to the first compression event, and one
second after the last compression event. The user can manually
adjust the episode start and end times from the ECG viewer.
10 - Working with reports Event Review Pro User Guide

159
By default, this option is unchecked (do not ignore).
• Split by intubationYou can choose whether to split the intervals
by intubation events. By default this is checked (split).
• Display/Hide report sectionsYou can choose whether to show
parts of the report.
• Toggle to show or hide Q-CPR tabular details.
By default this is checked (show).
• Toggle to show or hide ventilation data.
By default this is checked (show).
• Toggle to show or hide compression strips.
By default this is checked (show).
Information in the report
The report contains information about the quality (rate and depth) of
CPR in the episode.
The report information is presented in these pages:
• Information in the Q-CPR Report Card chart on page.159.
• Information in the Q-CPR Report Card details on page.163.
• Information in the Q-CPR Report Card compression strips on
page.166
Information in the Q-CPR Report Card chart
The following illustration shows the general arrangement of the Q-CPR
Report Card chart page. The general arrangement is the same in all
languages. Refer to the table after the illustration for definitions.
10 - Working with reports Event Review Pro User Guide
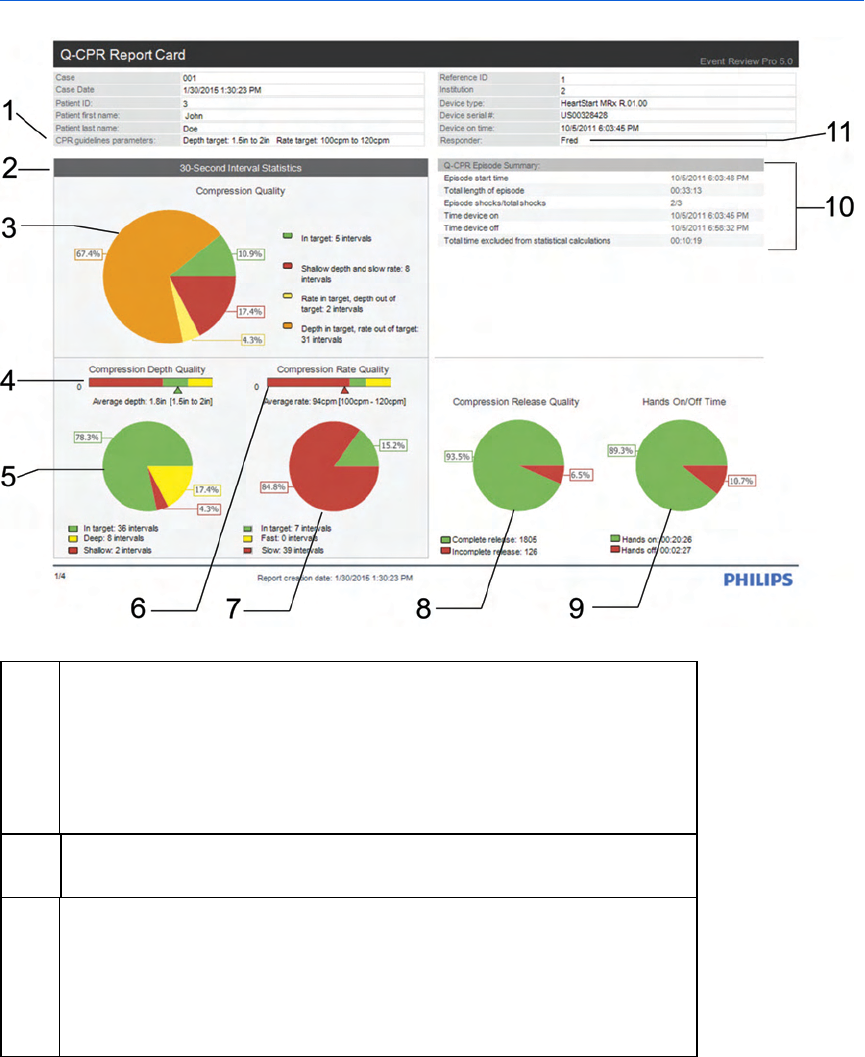
160
Key to illustration numbers for the previous illustration:
1 CPR guideline parameters This field shows the CPR guideline
depth and rate targets used to calculate the CPR quality shown in the
report. When no upper target is specified, only the lower target is
shown.
Note that when Guidelines other than Device are selected,
this field appears highlighted in yellow.
2 30-Second IntervalStatistics All the statistics in this box are
calculated based on 30-second intervals.
3 Compression Quality The charts in this box show the overall
compression quality for all intervals in the episode. For the overall
compression quality chart, the compression depth quality chart, and
the compression rate quality chart, the labels in each slice show the
percentage of intervals in each category. The color legend below the
charts shows the actual number of intervals in each category.
10 - Working with reports Event Review Pro User Guide

161
4 Compression Depth Qualitygauge This gauge shows the average
compression depth over the entire episode. The segment colors
correspond to the colors of the chart below. The marker beneath the
gauge is green if the average depth is in the target zone, red if below
the target zone, and yellow if above the target zone. The label shows
the actual average depth value, as well as the lower and upper (if
applicable) targets.
5 Compression Depth Quality chart This chart shows the
compression depth quality for all intervals in the episode. Intervals
with an average compression depth greater than or equal to the lower
target and less than or equal to the upper target (if applicable) are
depicted in the green slice. Intervals with an average compression
depth less than the lower target are depicted in the red slice. Intervals
with an average compression depth greater than the upper target (if
applicable) are depicted in the yellow slice.
6 Compression Rate Quality gauge This gauge shows the average
compression rate over the entire episode. The segment colors
correspond to the colors of the chart below. The marker beneath the
gauge is green if the average rate is within the target zone, red if below
the target zone, and yellow if above the target zone. The label shows
the actual average rate value, as well as the lower and upper (if
applicable) targets.
7 Compression Rate Quality chart This chart shows the
compression rate quality for all intervals in the episode. Intervals with
a compression rate greater than or equal to the lower target and less
than or equal to the upper target (if applicable) are depicted in the
green slice. Intervals with a compression rate less than the lower
target are depicted in the red slice. Intervals with a compression rate
greater than the upper target (if applicable) are depicted in the yellow
slice.
8 Compression Release Quality chart This chart shows the
percentage of compressions with complete release versus incomplete
release (leaning) for the episode. The legend shows the actual number
of compressions with complete and incomplete releases.
9 Hands-On/Hands-Off Time chart This chart shows the amount
of hands-on versus hands-off time for the episode. Green represents
hands-on time , and red represents hands-off time. The slice labels
show hands-on and hands-off time as percentages of the entire
episode time. The legend shows the actual amount of hands-on and
hands-off time in hours, minutes and seconds.
10 Q-CPR Episode Summary This section contains a summary of the
episode. This information is duplicated on the details page.
10 - Working with reports Event Review Pro User Guide

162
11 Responder This field shows the CPR responder by name. The
selection is made in the Responder menu on the Q-CPR Customization
area in the report. When All is selected in the Responder menu (the
default selection), an empty string appears in this field.
10 - Working with reports Event Review Pro User Guide

163
Information in the Q-CPR Report Card details
The following illustration shows the general arrangement of the Q-CPR
Report Card details page. The general arrangement is the same in all
languages. Refer to the table after the illustration for definitions.
Key to illustration numbers for the previous illustration:
1 Q-CPR Episode Summary
• Episode start time: The episode start time defaults to 1 second
prior to the first compression event. The user can adjust the episode
start time from the ECG tab in the Event Review Pro Case Editor.
• Total length of episode: Total time from episode start to episode
end. The episode end time defaults to 1 second after the last
compression event. The user can adjust the end time from the ECG
tab in the Event Review Pro Case Editor. In addition, the user can
ignore the episode markers and make the episode correspond to the
entire recording from device on to device off.
• Episode shocks/total shocks: Shows both the total number of
shocks that lie between episode start and episode end and the total
number of shocks in the recording.
• Time device on: Device-on time as recorded by the device.
• Time device off: Device-off time as recorded by the device.
• Total time excluded from statistical calculations: Total time
of all exclusion periods. The user can add and adjust exclusion periods
using the ECG tab in the the Event Review Pro Case Editor.
10 - Working with reports Event Review Pro User Guide

164
2 Compression Data
• Total number of compressions: Total number of compressions
in the entire episode.
• Total compressions with in-target depth: Total number of
compressions in the entire episode, where the depth was greater
than or equal to the lower target and less than or equal to the upper
target.
• Total compressions with insufficient depth: Total number of
compressions in the entire episode, where the depth was less than
the lower target.
• Total compressions with incomplete release: Total number of
compressions in the entire episode, where there was an incomplete
release (leaning).
• Compressions with complete release [percentage]:
Percentage of compressions with complete release in the entire
episode.
• Average compression rate [/min] [xx-yy]: Average
compression rate of the entire episode, where xx is the lower rate
target and yy is the upper rate target.
• Average depth [xx u to yy u] or [at least xx u]: Average
compression depth over the entire episode, where xx is the lower
target, u is the units (inches or mm), and yy is the upper target.
• In-target depth [percentage]: Percentage of compressions
over the entire episode, where the depth was greater than or equal to
the lower target and less than or equal to the upper target.
• Average compression counts [/min]: Average number of
compression events per minute over the entire episode.
10 - Working with reports Event Review Pro User Guide
165
3 Flow/Hands On Time
• Flow time [percentage]: Flow time as percentage of the entire
episode. Flow time represents blood-flow time which includes the
hands-on time, and the perfusion time during hands-off time as
annotated by the user.
NOTE: Flow time is calculated from the time a PR annotation is made
to the time any non-perfusion annotation is made.
• Hands-on time [percentage]: Hands-on time as percentage of
the entire episode. Hands on time is the time with active chess
compression.
• Average no-flow time before shock[s]: Average no-flow time
before each shock in the episode.
• Average no-flow time after shock[s]: Average no-flow time
after each shock in the episode.
• No-flow time: No-flow time in hours, minutes, and seconds over
the entire episode. No-flow time is time between compressions where
no perfusion is recorded. The user can identify perfusion by
annotating the ECG with PR (perfusing rhythm) annotations.
• Hands-off time: Hands-off time in hours, minutes, and seconds
over the entire episode.
4 Ventilation Data
• Total number of ventilations: Total number of ventilations in
the entire episode.
• Total time before intubation: Total time in hours, minutes,
seconds before intubation.
• Total time after intubation: Total time in hours, minutes,
seconds after intubation.
• Average ventilation rate before intubation [/min]: Average
ventilations per minute before intubation.
• Average ventilation rate after intubation [/min]: Average
ventilations per minute after intubation.
10 - Working with reports Event Review Pro User Guide

166
5 Defibrillation data
• Time from power-on to first shock: Time in hours, minutes,
and seconds from device power-on to the first shock.
• Total analysis and shock-delivery time: Total time in hours,
minutes, and seconds, where the device is either in analysis mode or
delivering a shock (AED devicesonly).
• Average analysis and shock delivery time: Average analysis
and shock delivery time in hours, minutes, and seconds for all episode
shocks (AED devicesonly).
Information in the Q-CPR Report Card
compression strips
The following illustration shows the general arrangement of the Q-CPR
Report Card Compression strips page. The general arrangement is the
same in all languages. Refer to the table after the illustration for
definitions.
Key to illustration numbers for the previous illustration:
10 - Working with reports Event Review Pro User Guide

167
1 Rate Quality BandThis band contains rate markers that show the
average rate for each 30 second segment. The target rate is shown
with a shaded strip: green = in-target, red = below target, yellow =
above target. The band range is between 30 and 150cpm so that the
target range can be distinguished. Rates below 30cpm are not visible
(but will be shaded red).
2 CPR Quality Band The CPR quality band shows the overall quality for
each 30 second segment. The segments are color coded: red = depth
and rate out of target, green = depth and rate in target, yellow = rate
in target, depth out of target, orange = depth in target, rate out of
target.
3 Depth Quality Band This band contains the compression waveform.
The target depth is shown with a shaded strip: green = in-target, red =
below target, yellow = above target.
4 Rate Bar The height of each line connected to the marker represents
the rate. Ideally the end of the line should be within the shaded area.
5 30 Second Interval The bands are comprised of 30 second intervals.
Each interval is marked with a white vertical line.
6 Incomplete Release Marker Compressions with incomplete release
are marked by a red down-arrow with a bar across the top.
7 Responder Switch Annotation Each responder CPR annotation is
marked with an icon with the name of the responder.
8 Key to symbols and color codes
Working with Q-CPR Details reports
You can see details about Q-CPR data for a single episode in the Q-CPR
Details report. To see what data is included in this report, see Q-CPR
Details report data on page.169.
If you want to export the Q-CPR Details report data to a spreadsheet,
select the Q-CPR Details Tabular Export report. You can compile
details for multiple cases and display them with the results.
In Report Preview, use the settings at the top of the preview to
customize the way the data is presented in the report. See Working in
Report Preview on page.153.
Customizing the Q-CPR Details report
Once selected, the following settings persist when the application is
reopened.
10 - Working with reports Event Review Pro User Guide
168
• GuidelinesIn the Guidelinesmenu you can select the CPR
Guidelines used to generate the report. By default, the report uses
the CPR guidelines that were used by the defibrillator during the
incident, Device Guidelines.
The available options are AHA 2005, 2010, or 2015, ERC 2005, 2010,
or 2015, or Custom (if configured). Up to 15 custom guidelines can
be saved. Custom CPR Guidelines are set in the Administration
workspace. For details, see Selecting CPR Guidelines for reporting
on page.150.
• DepthYou can select the compression depth units, inches or
millimeters. The default setting is inches. You can toggle from inches
to millimeters.
• Split by responderBy default, the option is unchecked: the report
will display statistics on the entire episode for all CPR responders in
a single block. You can choose to split the results by reponder.
Before you can split by responder, you need to annotate the
compression waveform (right-click the waveform and then click
Add Note), and add a name in the responder name field. For
details, see Adding CPR responder annotations on page.128.
• Ignore episode markersBy default, this is unchecked. Check this
option if you want to ignore the episode markers and report on the
entire recording from device on to device off (default is unchecked).
The episode start and end times default to one second prior to the
first compression event and one second after the last compression
event respectively. The user can manually adjust the episode start
and end times from the ECG viewer.
• Split by intubationYou can choose whether to split the intervals
by intubation events. By default this is checked.
• IntervalsYou can select the interval used to calculate statistics.
Click either No intervals, 30 second intervals, or 60-second
intervals.
Information in the report
The report contains detailed information about CPR in tabular form.
See Q-CPR Details report data on page.169.
10 - Working with reports Event Review Pro User Guide
169
Q-CPR Details report data
This section provides a description of the Q-CPR data that appear in the
Q-CPR Details report.
You can create a Q-CPR Details Tabular Export report that includes data
from multiple cases, with the results separated by case. This data can
be exported to a Microsoft® Excel spreadsheet.
Q-CPR statistics section
Statistic
label Description
Type The time period for the data: Episode, Period, or Interval.
Start The start time of the statistics coverage, in milliseconds from
the start of the first compression event.
Length The length of the statistics covered time (up to one second
after the last compression event), in milliseconds.
NFT The no-flow time (NFT), in milliseconds.
NFT% The no-flow time ratio. NFT divided by length, expressed in
percent.
FT The flow time (FT), in milliseconds. It is defined as the length
of the statistic minus the NFT, in milliseconds.
FT% The flow time ratio. Flow time divided by the length,
expressed in percent.
Compression section
Statistic label Description
Comp The total number of compressions.
Correct The total number of compressions that are in-target and
are not leaning.
Deep The number of compressions that are too deep.
Shallow The number of compressions that are too shallow.
Depth The average depth of compression, in millimeters.
10 - Working with reports Event Review Pro User Guide

170
Statistic label Description
Leaning The number of compressions with incomplete release.
Rate The average compression rate, per minute. The rate is
computed over the active compression time. The time
without compression activities is excluded.
Duty Cycle The compression duty cycle.
In Target % The ratio of adequate count to total count.
Ventilation section
Statistic
label Description
Vent The total number of ventilations.
Vent Rate The average ventilation rate, per minute. If the statistics are
divided into pre- and post intubation periods, the ventilation
rate is for the specific pre-intubation and post-intubation
period. If not, the ventilation rate is for the entire episode.
dt The average inflation time, in milliseconds.
dz The average inflation level, in mOhms.
Too Fast The total number of ventilations that are too fast (too short in
duration).
No Sign of Circulation (NSC) section
Statistic
label Description
NSC The total No Sign of Circulation (NSC) time, in milliseconds.
Comp The average compression rate during the NSC time.
NFT/NSC The ratio of No Flow Time (NFT) to No Sign of Circulation
(NSC).
Summary section
Statistic
label Description
Shock count The total number of shocks in the episode.
Shocks
included
Number of shocks included in this shock summary statistics.
10 - Working with reports Event Review Pro User Guide

171
Statistic
label Description
Power on to
1st shock
Time from power‐on to first shock, in milliseconds.
Analysis +
Delivery time
total
Total analysis and shock delivery time, in milliseconds.
Analysis +
Delivery time
avg
Average time from start of analysis to shock delivery, in
milliseconds.
Avg pre HOT Average hands off time before shock delivery, in milliseconds.
Avg post HOT Average hands off time after shock delivery, in milliseconds.
Total count Total number of analyses in the episode.
Total included Number of analyses included in this analysis summar
statistics.
Auto total Number of auto‐initiated analysis included in this analysis
summary statistics.
Manual total Number of manually initiated analysis included in this analysis
summary statistics.
Result
incomplete
Number of analyses indicated no shock decision was made.
Result shock
ind
Number of analyses indicated shock was advised.
Result no
shock ind
Number of analyses indicated no shock was advised.
Total time Total analysis time, in ms (does not include those with
incomplete results)
Avg time Average time for the analyses to make the shock or no shock
decision, in ms (does not include those with incomplete
results).
Pre HOT total Total pre shock hands on time in milliseconds.
Pre HOT avg Total hands off time before start of analysis, in milliseconds.
Post HOT total Total hands off time after start of analysis, in milliseconds.
Post HOT avg Average hands off time after the start of analysis, in
milliseconds.
10 - Working with reports Event Review Pro User Guide
172
Shock statistics section
Statistic
label Description
Index Shock index within the whole incident case.
Start Shock start time in milliseconds.
Analysis time Time from the start of analysis event to the shock delivery
event, in milliseconds.
Pre HOT Hands off time (HOT) before the shock delivery, in
milliseconds.
Post HOT Hands off time (HOT) after the shock delivery, in milliseconds.
Analysis statistics section
Statistic
label Description
Start Time the analysis started, in milliseconds, from the start time.
Analysis time Length of time, in milliseconds, of the analysis.
Type Analysis initiated manually, or automatically (in AED mode.)
Result The analysis result.
Pre HOT Hands off time (HOT) before the analysis started, in
milliseconds.
This is defined as the shortest time of:
• Time from last compression to start of analysis.
• Time from start of the period to start of the analysis.
• Time from last shock to the start of analysis.
• Time from the ending of last analysis to the start of this
analysis.
• Time from the start of last analysis to the start of this
analysis.
10 - Working with reports Event Review Pro User Guide

173
Statistic
label Description
Post HOT Hands off time (HOT) before the analysis started, in
milliseconds. The time is measured from when the analysis
started, not from when the analysis was completed.
This is defined as the shortest time of:
• Time from start of analysis to next compression.
• Time from start of analysis to end of the period.
• Time from start of analysis to next shock.
• Time from start of analysis to start of next analysis, in this
case, the analysis time is used instead.
Working with Q-CPR Response Time
reports
The Q-CPR Response Times report shows overall CPR performance for
all cases in a given time range (up to a maximum of 24 months) as well
as trends over the same period.
Depending on the number of cases included in the time range, this
report may take hours to generate.
Customizing the Q-CPR Response Times report
Once selected, the following settings persist when the application is
reopened.
• Start Date You can specify the start date for the time range. The
report includes cases from this date. The default setting is today's
date minus two years.
• End Date You can specify the end date for the time range. The
report includes cases up to and including this date. The default
setting is today's date.
• Compression Count Threshold You can select the compression
count threshold. The report will include only cases that have more
than this number of compressions. The default setting is 1
compression.
10 - Working with reports Event Review Pro User Guide

174
• Depth You can select the compression depth units, inches or
millimeters. The default setting is inches. You can toggle from inches
to millimeters.
• Institution Only cases that match this Institution value will be
included in the report. This information comes from the Institution
field, on the Overview tab.
• Reference ID Only cases that match this Reference ID value will
be included in the report. This information comes from the
Reference ID field, on the Overview tab.
Graphical information in the Q-CPR Response Times report
The report contains an overview page and a details page, each
presenting information in a graphical format.
• Information in the Q-CPR Response Times overview on page.174
• Information in the Q-CPR Response Times details on page.178.
Information in the Q-CPR Response Times
overview
The following illustration shows the general arrangement of the
overview. The general arrangement is the same in all languages. Refer
to the table after the illustration for definitions.
10 - Working with reports Event Review Pro User Guide

175
Key to illustration numbers for the previous illustration:
1 Start date: The start of the date range. The report includes cases with
a case date greater than or equal to this date.
2 End date: The end of the date range. The report includes cases with a
case date less than or equal to this date.
3 Total cases: The total number of cases included in the report results.
4 Cases with 12-leads: The total number of cases with 12-leads in
the report results.
5 Average shocks per case: Average shock events per case for the
cases included in the report results.
6 Cases with shocks:The total number of cases included in the report
results which include shocks.
7 Cases with Q-CPR: The total number of cases included in the report
results which include Q-CPR data.
10 - Working with reports Event Review Pro User Guide
176
8 Compression threshold/cases excluded:This field shows 2
values.
The first shows a compression event count threshold. Cases with a
total number of compressions below this threshold are excluded from
Q-CPR calculations, but are not excluded from the other statistics
included in the report.
The second value shows the total number of cases that were excluded
from Q-CPR calculations.
9 Overall CPR quality chart: This chart shows the overall
compression quality for all intervals in the date range.
Intervals with both compression depth and rate in target are depicted
in green.
Intervals with both compression depth and rate out of target are
depicted in red.
Intervals with compression depth in target and rate out of target are
depicted in orange.
Intervals with compression rate in target and depth out of target are
depicted in yellow.
10 Compression depth quality chart: This chart shows the
compression depth quality for all intervals in the date range.
Intervals with an average compression depth greater than or equal to
the lower target and less than or equal to the upper target (if
applicable) are depicted in the green slice.
Intervals with an average compression depth less than the lower
target are depicted in the red slice.
Intervals with an average compression depth greater than the upper
target (if applicable) are depicted in the yellow slice.
The average compression depth is shown above the pie chart.
Note that, unlike in the Q-CPR report card, the depth targets are not
shown. This is because the data may contain input from multiple
devices using different CPR guidelines.
11 Hands on/off time chart: This chart shows the amount of hands
on/off time for the date range. Green represents hands-on time and
red represents hands-off time.
10 - Working with reports Event Review Pro User Guide

177
12 Compression rate quality chart: This chart shows the compression
rate quality for all intervals in the date range.
Intervals with a compression rate greater than or equal to the lower
target and less than or equal to the upper target (if applicable) are
depicted in the green slice.
Intervals with a compression rate less than the lower target are
depicted in the red slice.
Intervals with a compression rate greater than the upper target (if
applicable) are depicted in the yellow slice.
The average compression rate is shown above the pie chart.
Note that, unlike in the Q-CPR report card, the rate targets are not
shown. This is because the data may contain input from multiple
devices using different CPR guidelines.
13 Average time to first 12-lead: Average time to first 12-lead for
each month, for cases with 12-lead data. No point is drawn if there are
no cases with 12-leads in a given month.
14 Average time to first shock: Average time to first shock for each
month, for cases which include shock events. No point is drawn if there
are no cases with shock events in a given month.
10 - Working with reports Event Review Pro User Guide
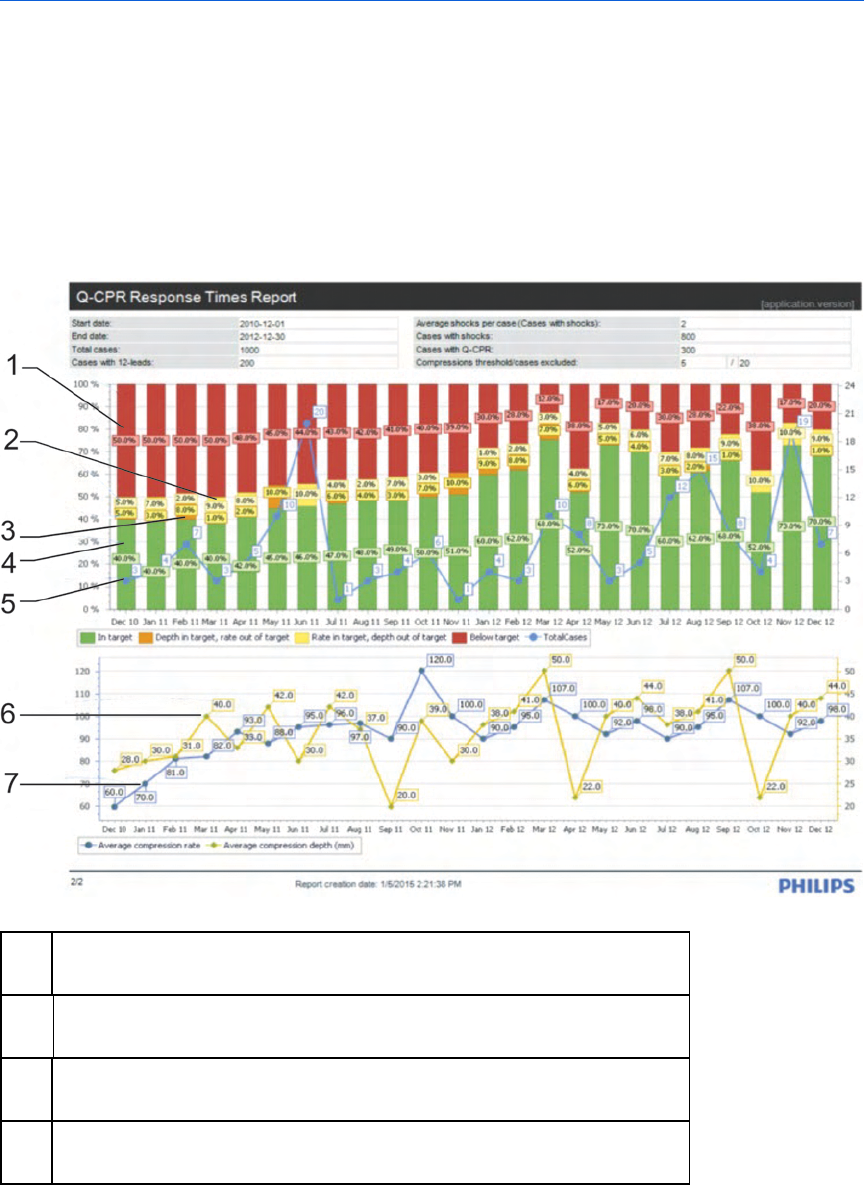
178
Information in the Q-CPR Response Times
details
The following illustration shows the general arrangement of the CPR
Details. The general arrangement is the same in all languages. Refer to
the table after the illustration for definitions.
Key to illustration numbers for the previous illustration:
1 Below target: (Red) This chart section shows the percentage of cases
in the period with average CPR depth and rate below target.
2 Rate in target: (Yellow) This chart section shows the percentage of
cases in the period with average CPR rate but not depth in target.
3 Depth in target: (Orange) This chart section shows the percentage of
cases in the period with average CPR depth but not rate in target.
4 In target: (Green) This chart section shows the percentage of cases
in the period with average CPR depth and rate in target.
10 - Working with reports Event Review Pro User Guide

179
5 Total cases: This line graph shows the total number of cases with CPR
for each month. The right vertical axis represents total cases.
6 Average compression depth: This line graph shows the average
compression depth over all cases with CPR data for each reported
month.
7 Average compression rate: This line graph shows the average
compression rate over all cases with CPR data for each reported
month.
Working with zCPR Report Card
reports
The zCPR Report Card report is based on the impedance data recorded
by the defibrillator during the episode. It is used to view CPR rate data
and hands-on/hands-off time for cases where the HeartStart Q-CPR
meter was not used.
zCPR should not be used for determining chest compression depth
or chest compression release.
Customizing the zCPR Report Card report
Once selected, the following settings persist when the application is
reopened.
• Guidelines In the Guidelinesmenu you can select the CPR
Guidelines used to generate the report. The default setting is
AHA2010, and only the CPR rate guidelines apply, because
impedance data does not capture CPR depth information.
Other available guideline options are AHA 2005 or 2015, ERC 2005,
2010, or 2015, or Custom (if configured). Custom CPR Guidelines are
set in the Administration workspace. For details, see Selecting CPR
Guidelines for reporting on page.150.
• ResponderYou can filter results by responder (limit the results to
CPR performed by a specific responder) by selecting a CPR
responder from this menu. The default is All, and the report displays
statistics for the entire episode, including all responders. You can
filter by responder if responders are annotated.
10 - Working with reports Event Review Pro User Guide

180
In order to filter by responder, you need to annotate the
compression waveform with responder type, and enter the
responder name in the name field. For details, see Adding CPR
responder annotations on page.128.
• Interval In seconds. Choose 10, 15 or 30 (default).
• Ignore episode markersCheck this option if you want to ignore
the episode markers and report on the entire recording from device
on to device off. When this option is unchecked, the episode start
and end times are set to one second prior to the first compression
event, and one second after the last compression event. The user
can manually adjust the episode start and end times from the ECG
viewer. By default, this option is unchecked (do not ignore).
• Display compression stripsYou can choose whether to show or
hide compression strips in the report. By default this is checked
(show).
Information in the report
The report contains information about the rate of CPR in the episode.
• Information in the zCPR Report Card chart on page.180.
• Information in the zCPR Report Card compression strips on
page.182.
Information in the zCPR Report Card chart
The following illustration shows the general arrangement of the zCPR
Report Card chart page. The general arrangement is the same in all
languages. Refer to the table after the illustration for definitions.
10 - Working with reports Event Review Pro User Guide

181
Key to illustration numbers for the previous illustration:
1 CPR guideline parametersThis field shows the CPR guideline rate
targets used to calculate the CPR quality shown in the report. When no
upper target is specified, only the lower target is shown.
2 Compression Rate Quality gauge This gauge shows the average
compression rate over the entire episode. The segment colors
correspond to the colors of the chart below. The marker beneath the
gauge is green if the average rate is within the target zone, red if below
the target zone, and yellow if above the target zone. The label shows
the actual average rate value, as well as the lower and upper (if
applicable) targets.
3 Compression Rate Quality chartThis chart shows the compression
rate quality for all intervals in the episode. Intervals with a
compression rate greater than or equal to the lower target and less
than or equal to the upper target (if applicable) are depicted in the
green slice. Intervals with a compression rate less than the lower
target are depicted in the red slice. Intervals with a compression rate
greater than the upper target (if applicable) are depicted in the yellow
slice.
4 Hands-On/Hands-Off Time chartThis chart shows the amount of
hands-on versus hands-off time for the episode. Green represents
hands-on time , and red represents hands-off time. The slice labels
show hands-on and hands-off time as percentages of the entire
episode time. The legend shows the actual amount of hands-on and
hands-off time in hours, minutes and seconds.
10 - Working with reports Event Review Pro User Guide

182
5 CPR Episode SummaryThis section contains a summary of the CPR
episode.
6 Compression DataThis section contains a summary of the
compression events.
7 Responder This field shows the CPR responder by name. The
selection is made in the Responder menu on the Q-CPR Customization
area in the report. When All is selected in the Responder menu (the
default selection), an empty string appears in this field. his
8 Hands-On/Hands-Off Time table This section contains a summary
of the hands on time periods.
9 Defibrillation Data This section contains a summary of the episode
shocks.
Information in the zCPR Report Card
compression strips
The following illustration shows the general arrangement of the zCPR
Report Card compression strips page. The general arrangement is the
same in all languages. Refer to the table after the illustration for
definitions.
Key to illustration numbers for the previous illustration:
10 - Working with reports Event Review Pro User Guide

183
1 Rate Quality Band This band contains rate markers that show the
average rate for each interval of 10, 15, or 30 seconds in length. The
target rate is shown with a shaded strip: green = in-target, red =
below target, yellow = above target. The band range is between 30
and 150 cpm so that the target range can be distinguished. Rates
below 30 cpm are not visible (but will be shaded red).
2 zComp This band contains the compression waveform.
3 Rate Bar The height of each line connected to the marker represents
the rate. Ideally the end of the line should be within the shaded area.
4 Interval The bands are comprised of intervals of 10, 15, or 30
seconds in length. Each interval is marked with a white vertical line.
5 Key to symbols and color codes
Working with Vital Trends reports
The Vital Trends report provides case information based on the vital
trends information that appears on the ECG tab. The information
appears in a table format.
The report displays the following information:
• Case ID, device, and patient information
• Vital trends in a table format
The report uses the interval parameter setting. An interval can be 1, 5,
10, 15, 30, or 60 minutes in duration.
Working with Response Times reports
The EMS edition provides Response Times reports.
You can generate Response Times reports to assess your system’s
overall response performance and compare performance with service-
level commitments.
The type of report determines the parameter settings you can specify.
For information on how to generate a report, see Generating reports on
page.151.
Event Review Pro saves the parameter settings and displays them the
next time you select the report. You can select or clear any of these
fields.
10 - Working with reports Event Review Pro User Guide

184
The following fields are used and incorporated in Response Times
reports:
• Call Receipt
• Bystander CPR
• Dispatcher Began AED or CPR Instruction
• BLS Unit Notified
• BLS Vehicle Mobile
• BLS Arrival at Scene
• BLS EMS CPR
• ALS Unit Notified
• ALS Vehicle Mobile
• ALS Arrival at Scene
• ALS arrival at Patient
• ALS EMS CPR
Certain database fields must contain data in order to generate the
Response Time reports. Ensure that you have entered the following
Timeline events for each case you want to report on:
• Call Receipt
• ALS/BLS Unit notified
• ALS/BLS Vehicle mobile
• ALS/BLS Arrival at scene
• ALS/BLS Arrival at patient
• ALS/BLS EMS CPR
Time to first shock is calculated from the device data.
Average Response Times – Total System
This report summarizes the average response times for key milestones
in your system’s response protocols. You can specify the date range and
site of collapse to limit the cases you want to summarize in the reports.
The report uses the following parameter settings:
• Start date
• End date
• Site of collapse
10 - Working with reports Event Review Pro User Guide

185
Percentile Response Times –Total System
This report summarizes response times achieved in a specific
percentage of responses. You can specify the date range and site of
collapse to limit the cases you want to summarize in the report. You
must specify the percentile.
The report uses the following parameter settings:
• Start date
• End date
• Site of collapse
• Percentile (required, median is 50%)
Average Response Times
This report summarizes, for a specific unit, the average response times
for key milestones in your response protocols. You can specify the date
range and site of collapse to limit the cases you want to summarize in
the reports.
The report uses the following parameter settings:
• Start date
• End date
• Site of collapse
• Unit name
Percentile Response Times
This report summarizes, for a specific unit, the response times achieved
in at least a specific percentage of responses. You can specify the date
range and site of collapse to limit the cases you want to summarize in
the report. You must specify the percentile.
The report uses the following parameter settings:
• Start date
• End date
• Site of collapse
• Unit
• Percentile (required, median is 50%.)
10 - Working with reports Event Review Pro User Guide

186
Working with Utstein reports
The EMS edition provides Utstein reports.
These reports follow the Utstein guidelines for reporting information on
out-of-hospital cardiac arrests. They use the recommended terms and
follow the Utstein template approach.
The type of report determines the parameter settings that you can
specify. For information on how to generate a report, see Generating
reports on page.151.
Event Review Pro saves the parameter settings and displays them the
next time you select the report.
Certain database fields must contain data in order to generate the
Utstein reports. Ensure that you have entered the following Scene
details for each case you want to report on:
• Resuscitation attempted
• Initial rhythm
• Witnessed by
• Cardiac etiology
• Confirmed cardiac arrest
• Bystander CPR
Ensure that you have entered the following Outcome details for each
case you want to report on:
• Any ROSC
• Admitted to ICU/ward
• Discharged
• Alive at one year
• Patient death
Unwitnessed with Bystander CPR
The Unwitnessed with Bystander CPR report summarizes the
unwitnessed cardiac arrests that are in your system.
The report uses the following parameter settings:
• Start date
10 - Working with reports Event Review Pro User Guide
187
• End date
• Location
• Population served by EMS system
Witnessed with Bystander CPR
The Witnessed with Bystander CPR report summarizes witnessed
cardiac arrest in which a bystander administered CPR.
The report uses the following parameter settings:
• Start date
• End date
• Location
• Population served by EMS system
Witnessed without Bystander CPR
The Witnessed without Bystander CPR report summarizes witnessed
cardiac arrests in which no bystander administered CPR.
The report uses the following parameter settings:
• Start date
• End date
• Location
• Population served by EMS system
Working with Device Self-test History
reports
Event Review Pro generates a Device Self-test History report for self-
test data imported from a specific AED device. For information about
importing device self-test data, see Importing device self-test data
using the Device Self-test Wizard on page.209.
Device self-test data can be imported from HeartStart AED devices.
However, the importation of device self-test data from HeartStart
monitor/defibrillators, such as the HeartStart MRx, is not
supported.
10 - Working with reports Event Review Pro User Guide

188
To generate a Device Self-test History report from the Reports
navigation area
1. From the Reports navigation area, click Device Self-Test History.
The All Devices table opens, showing a list of devices with imported
self-test data.
2. Select the table row for the device you want to report on.
3. From the File menu or the toolbar, click Print and then click
Report.
Report Preview opens, displaying the Device Self-test History
report. See Working in Report Preview on page.153.
To generate a Device Self-test History report from the Devices
navigation area
1. From the Devices navigation area, click All Devices to open the All
Devices table, showing a list of devices with imported self-test data.
2. Select the table row for the device you want to report on.
3. From the File menu or the toolbar, click Print and then click
Report.
Report Preview opens, displaying the Device Self-test History
report. See Working in Report Preview on page.153.
The Device Self-test History report includes the following columns of
self-test information:
• Sequence Number
• Date
• Time
• Relative Time
• Description
• Status
10 - Working with reports Event Review Pro User Guide

11
Customer support
Philips strives to provide you with excellent customer service and
technical support. Software updates for the integrated applications are
available from the application Help menu. From the Help menu, click
Check for Updates.
Customer support is available through email, Internet, and telephone.
Email product support is available in English only at:
eventreview.support@philips.com. In addition, if a customer support
representative asks you to send the System Log, on the Help menu,
click Email with System Log and send the email message that appears.
The System log is attached to it by default.
Internet product support is available at the following address:
http://www.philips.com/DataManagementSupport
For telephone assistance outside the United States, please call your
sales representative or local response center. You can also navigate to
technical support telephone numbers for data management products at
this address:
http://www.healthcare.philips.com/main/services/response_center
For telephone support in English only, you can call the following
numbers:
• 800-722-9377 (option 3), inside the United States
• +1 770-510-1130
See the following table for contact information:
Region Address Telephone number
United States Philips Healthcare 3000
Minuteman Road
Andover, Massachusetts
01810-1099
800-722-9377 (option
3)
+1 770-510-1130
189
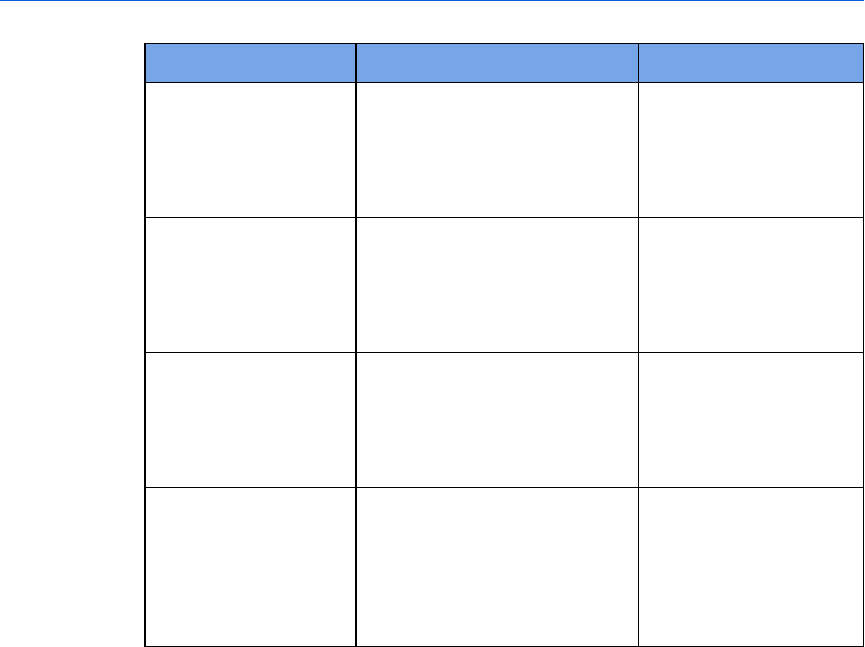
190
Region Address Telephone number
Canada Philips Healthcare, a Division
of Philips Electronics Ltd.
281 Hillmount Road
Markham, Ontario, Canada
L6C 2S3
+1(800) 291-6743
Authorized EU
Representative
Europe, Middle East,
and Africa
Philips Medizin Systeme
Boeblingen GmbH Cardiac and
Monitoring Systems
Hewlett-Packard Strasse 2
71034 Boeblingen, Germany
(+49) 7031 463-2254
Latin America Philips Medical Systems Ltda.
Av. Dr. Marcos Penteado
Ulhôa Rodrigues, 401
Parte 16 – 06460-040 –
Barueri/SP, Brazil
0800 7017789
Asia Pacific Philips Electronics Hong Kong
Ltd. 6/F, Core Building 1
1 Science Park East Avenue
Hong Kong Science Park
Shatin. New Territories, Hong
Kong
+852 2821 5888
Comments or suggestions?
Please send your feedback and suggestions to:
eventreview.support@philips.com
Supported help
Customer support technicians provide help for the following:
• Explaining the proper use of application features and answering your
questions about how the application works
• Explaining the proper installation and maintenance of the application
• Assisting you in selecting and configuring card readers
Unsupported help
Customer support technicians do not provide help for the following:
11 - Customer support Event Review Pro User Guide

191
• Interpreting ECG or medical data. Please call your medical director
or clinical specialist.
• Repairing hardware. The support technicians can help you determine
if you have a hardware problem, but they cannot help you fix
problems that are not related to the Philips HeartStart application
software.
• Troubleshooting defibrillators. Instead, call Philips Customer
Support and ask for defibrillator support.
• Troubleshooting non-Philips products.
Helping us help you
You can help our technicians give you good support by following these
steps:
1. Call from a phone near your computer.
2. Start Event Review Pro.
3. Have the following information:
• Windows version.
• The application version number. This is available from the Help
menu. Click the About option.
• A written copy of the error message text.
• The activity and task you did when the error occurred.
11 - Customer support Event Review Pro User Guide
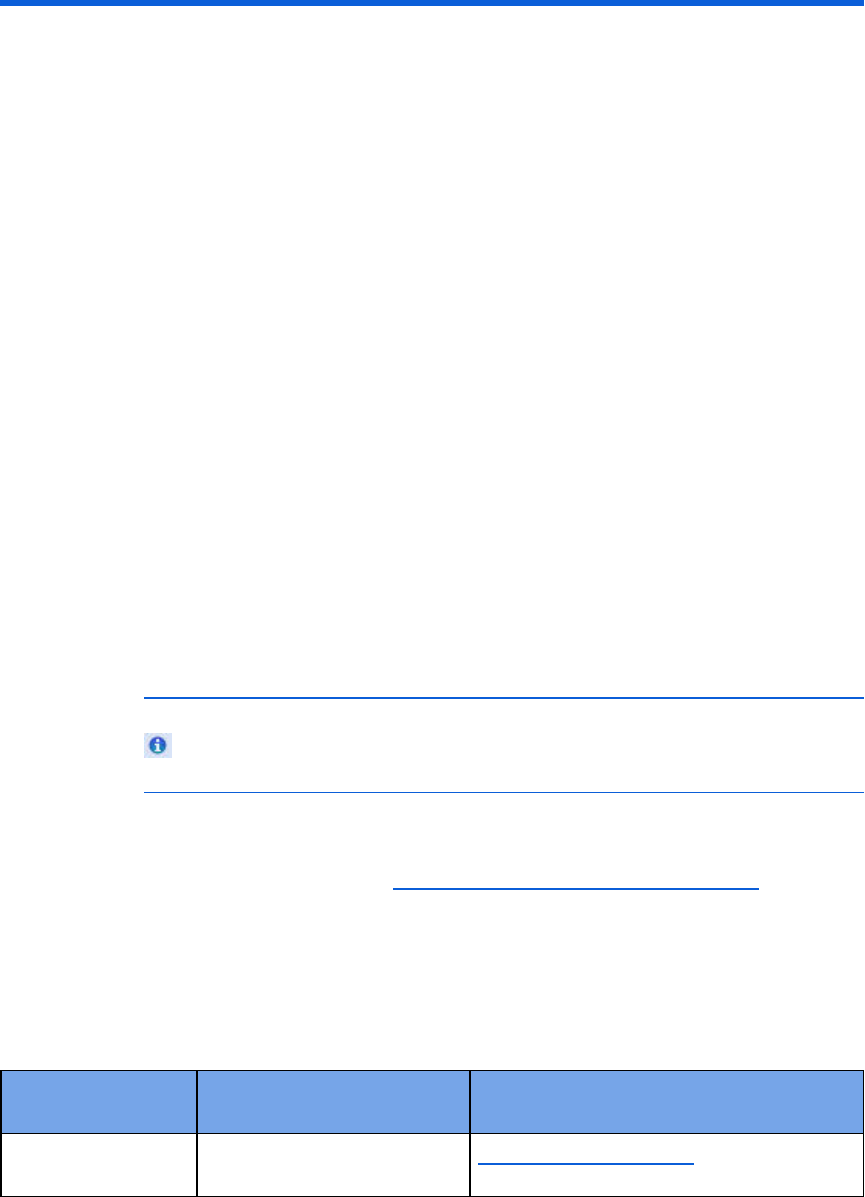
12
Working with
defibrillators
A wide variety of HeartStart defibrillators can transfer data to Event
Review Pro, and data transfer methods vary by model.
Prepare for transferring data from your defibrillator to Event Review Pro
by reviewing the following information, and ensuring that you have
everything you need to perform the task before you need to do so.
Supported defibrillators
Familiarize yourself with the methods for transferring data from your
defibrillator by reading the instructions that came with the device.
You can download product owners' manuals for supported defibrillators
from the Philips support Web site.
Event Review Pro documentation uses the HS1 or HS1 family of
defibrillators to refer to HeartStart Home, HeartStart OnSite, and
HeartStart HS1 Defibrillators.
To download products and services materials
1. Navigate to this site: http://www.philips.com/productdocs
2. Click Resuscitation/Defibrillators products.
3. Click the defibrillator to display a page that lists the product user and
service materials.
The defibrillators and the data transfer methods available for them are
shown in the following table:
Data source
Method used to read data
Instructions
HeartStart FR3 Secure digital (SD) card or
Bluetooth connection
Reading FR3 series cards on page.195
192
193
Data source
Method used to read data
Instructions
Attaching an ECG using an FR3 Bluetooth
transmission on page.56
HeartStart FR2 Compact Flash data card Reading FR2 series cards on page.200
HeartStart FRx Infrared (IrDA) connection Downloadingan ECG from an infrared
connection on page.58
HeartStart HS1 Infrared (IrDA) connection Downloadingan ECG from an infrared
connection on page.58
HeartStart MRx Card reader or
Bluetooth connection
Reading HeartStart MRx cards on
page.196
Downloadingan ECG using a HeartStart
MRx Bluetooth transmission on page.57
HeartStart XL ATA flash data card Reading HeartStart XL cards on page.201
HeartStart XL+ USB data drive Transferring HeartStart XL+ data on
page.201
Efficia DFM100
defibrillator/monitor
(where available)
USB data drive Transferring Efficia DFM100 data on
page.202
Selecting accessories for data
transfer
If your HeartStart defibrillator model uses a data card or a data drive to
transfer defibrillator data, refer to the following quick reference to
ensure that you have the necessary accessories.
Quick reference to data cards and drives
The following table shows the defibrillators with their associated data
cards. If your computer did not come installed with the appropriate
software or adapter for the transfer method, you can use an adapter or
reader.
Not all defibrillator models use data cards or drives. Only
defibrillators that use data cards or drives are included in the
following table.
12 - Working with defibrillators Event Review Pro User Guide
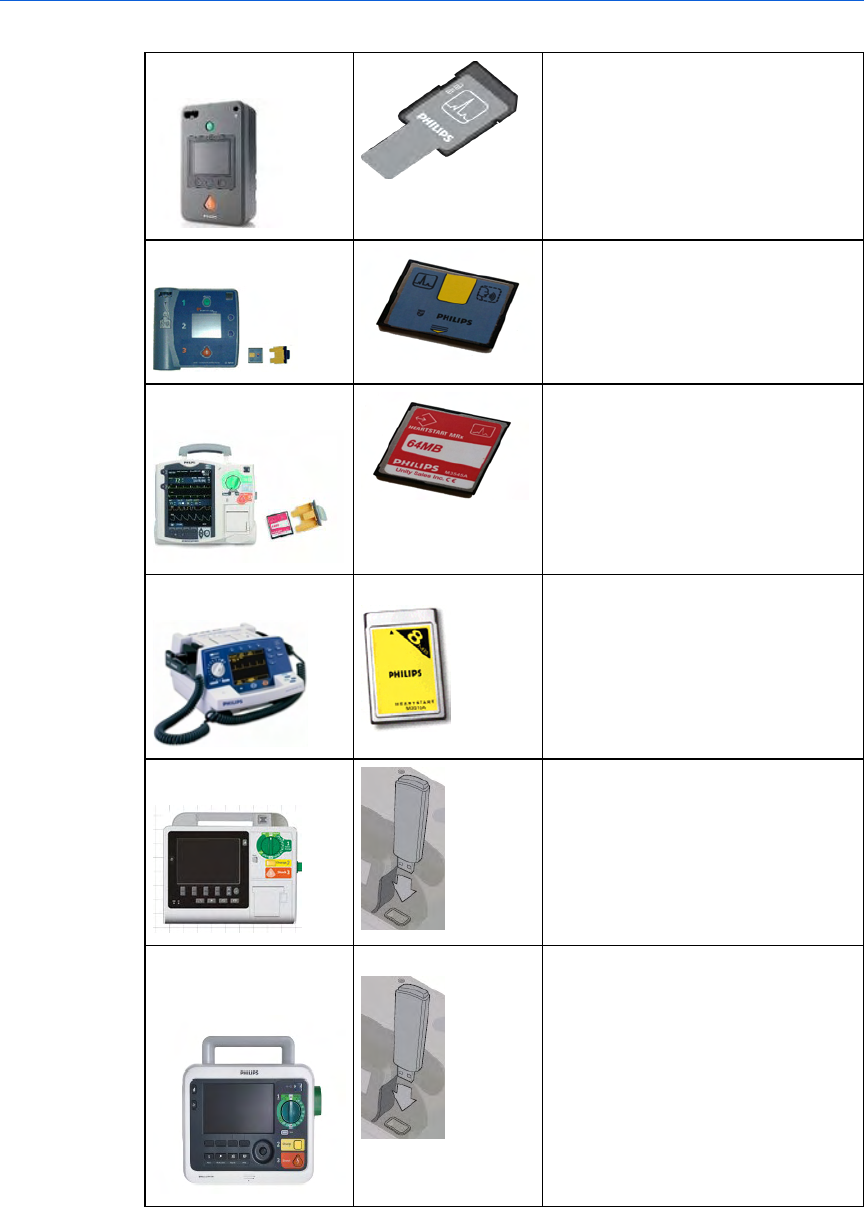
194
HeartStart FR3 FR3 defibrillators use Philips
secure digital SDdata cards, with
the SD data-card reader.
HeartStart FR2 FR2 defibrillators use the Compact
Flash cards with the Compact
Flash card reader.
HeartStart MRx HeartStart MRx
monitor/defibrillators use Compact
Flash cards with the Compact
Flash card reader.
HeartStart XL XL defibrillators use ATAflash
cards.
HeartStart XL+ XL+ defibrillators use Philips
USBdata drives.
Efficia DFM100
(Where available)
Efficia DFM100
monitor/defibrillators use Philips
USBdata drives.
12 - Working with defibrillators Event Review Pro User Guide

195
Reading FR3 series cards
The FR3 series defibrillators store information on a SD card. SD card
readers are available through your Philips sales representative or local
distributor.
To read a data card
1. One end of the data card has a series of contacts. Plug this end
firmly into the card reader.
2. Check in Windows File Explorer to see that the card was recognized.
When Windows recognizes the card, it assigns a drive letter to the
card.
At this point, Event Review Pro can read the data on the card.
3. If the card does not appear in Windows File Explorer, remove the
card from the reader, and then insert it into the card reader again.
Retrieving FR3 data using Bluetooth
transmission
You can download data from a HeartStart FR3 to the computer through
Bluetooth transmission. All data contained on the HeartStart FR3 is
transferred during the transmission, and it also remains on the device.
Preparing for Bluetooth transfer
Ensure that you have the option for Bluetooth wireless transfer for your
HeartStart FR3. Confirm that you have a Bluetooth card installed in the
defibrillator. For more information, see the most current release of the
HeartStart FR3 Instructions for Administrators.
Pairing the Bluetooth option for the FR3 occurs automatically. When data
matching the device type, serial number, date, and time appears in the
Attach ECG window, that is your confirmation that Bluetooth wireless
transfer has occurred.
You must have a supported adapter for Bluetooth transmission.
Most Microsoft®-compatible Bluetooth adapters are suitable. You
can order an adapter from your Philips representative.
12 - Working with defibrillators Event Review Pro User Guide

196
To transfer data from the HeartStart FR3
1. From an open case, click Attach ECG file.
The Attach ECG window opens, and the Case Wizard begins scanning
for any device in Bluetooth communication range.
Data transfer begins as soon as the FR3 is ready for data transfer.
2. For assistance in preparing your FR3 for data transfer, click the
Bluetooth icon, and then click the image of the HeartStart FR3, to
start the Bluetooth Wizard.
Follow the instructions in the wizard as it guides you through the
steps necessary to prepare the FR3 for data transfer.
Note: The Bluetooth Wizard, launched by clicking the Bluetooth icon
in the Attach ECG window, does not initiate Bluetooth
communication; it is simply a tutorial.
3. Watch the Attach ECG window.
When data transfer occurs, all the data on the FR3 appears in the
Attach ECG window, with columns for device type, serial number,
and date and time, for use in identifying the data.
If no data is on the FR3, a message appears indicating that there is
no patient data.
4. Click the data to attach to your case, and then click Open.
The data is added to the case.
5. Remember to save the case.
Reading HeartStart MRx cards
The HeartStart MRx defibrillator stores information in internal memory.
To open the information in Event Review Pro, transfer the information
from the internal memory to an external CompactFlash card and then
download it to Event Review Pro.
Follow the instructions in the HeartStart MRx Monitor/Defibrillator
Instructions for use guide. After you transfer the information to a
CompactFlash card, you can read the card in a card reader.
CompactFlash cards and card readers are available through your
Philips sales representative or local distributor.
12 - Working with defibrillators Event Review Pro User Guide

197
To read a data card
1. Remove the data card from the HeartStart MRx data card tray.
2. One end of the data card has a series of perforations. Insert this end
firmly into the card reader.
3. Check in Windows File Explorer to see that the card was recognized.
When Windows recognizes the card, it assigns a drive letter to the
card.
Event Review Pro can read the data on the card.
4. If the card does not appear in Windows File Explorer, remove the
card from the card reader, and then insert it into the card reader
again.
Setting up Bluetooth transmissions
for the HeartStart MRx
You can transmit HeartStart MRx data to Event Review Pro through
Bluetooth® wireless transmission.
You must have a supported adapter for Bluetooth transmission.
Most Microsoft®-compatible Bluetooth adapters are suitable. You
can order an adapter from your Philips representative.
To transfer the information from the defibrillator to Event Review Pro,
you must set up the Bluetooth transmission on the defibrillator and the
receiving computer that has Event Review Pro installed.
If your computer is not equipped with Bluetooth capability, it will require
a Bluetooth dongle with a USB connector. The dongle connects the
computer and the defibrillator.
Event summaries and HeartStart MRx 12-lead reports might contain
information that could be considered Patient Healthcare Information
(PHI) or patient-identifiable data. Handle the information in accordance
with HIPAA or your local patient privacy requirements.
12 - Working with defibrillators Event Review Pro User Guide

198
Bluetooth option prerequisites for the
HeartStart MRx
The prerequisites for Bluetooth use are slightly different for the MRx and
FR3 defibrillators.
The Bluetooth card on the HeartStart MRx is inside of the defibrillator.
Confirm that you have a Bluetooth card installed and that the Event
Summary Bluetooth transmission feature is enabled.
For implementation information, see "Support for MRx Bluetooth FTP
using the Microsoft Bluetooth stack" in HeartStart Data Management
Solutions Implementation Guide.
For detailed information on setting up the HeartStart MRx for Bluetooth
transmission, see the most current release of the following
documentation:
• HeartStart MRx Instructions for Use Addendum
• HeartStart MRx Instructions for Use
• HeartStart MRx Data Transmission Implementation Guide
Pairing and testing the HeartStart MRx
Bluetooth option with the computer
Use this information to pair and test the Bluetooth option for HeartStart
MRx.
Make sure that the application computer is turned on and that the
Bluetooth software and adapter are installed and visible or
discoverable.
To pair the HeartStart MRx Bluetooth option with the computer
1. On the HeartStart MRx main menu, click Bluetooth Devices.
2. Click Add Device.
The defibrillator searches for your computer.
If your computer is not listed after the search, your computer
Bluetooth is not enabled or set up correctly. Refer to the Bluetooth
documentation to troubleshoot the setup.
3. Click the name of your computer and then press Enter.
4. Type a passkey for the Bluetooth option, using the number menu.
12 - Working with defibrillators Event Review Pro User Guide
199
The passkey is a user-defined character sequence, such as 000, or
1234. For information on this sequence, see the documents listed in
the Bluetooth option prerequisites for the HeartStart MRx on
page.198.
5. At the top of the number menu, click Done.
6. On your computer, immediately watch for a pop-up message.
7. Click the pop-up message.
8. Type the same Bluetooth passkey that you typed on the defibrillator
in step 4.The defibrillator and the computer now have a Bluetooth
connection, and you are ready to test it.
9. Click Next and then Finish.
10. On the main menu of the HeartStart MRx, click File Transfer and
then Enter.
HeartStart MRx displays the "transmission test passed" message.
11. Click Acknowledge.
12. Scroll to Exit and click Enter.
13. On the main menu of the HeartStart MRx, click Other, Data
Management, and then Acknowledge.
14. After changing into Data Management mode, the HeartStart MRx
displays a list of summary case data with the most recent incident at
the top. Select the incident that you want to transmit.
15. Click Send and then All Event Data.
16. On your computer, in the Attach ECG dialog box, watch for a display
that includes the HeartStart MRx folder, and open it to see the data
that has been transferred.
Sending the HeartStart MRx Bluetooth
transmission
You can download a case from HeartStart MRx to the computer through
a Bluetooth transmission.
Before downloading, make sure that the HeartStart MRx has been paired
with your computer. See Pairing and testing the HeartStart MRx
Bluetooth option with the computer on page.198 for information on
pairing and testing the connection.
12 - Working with defibrillators Event Review Pro User Guide

200
To make a Bluetooth transmission
1. On the HeartStart MRx, place the defibrillator in the Data
Management mode. If necessary, use the HeartStart MRxdocuments
listed in Bluetooth option prerequisites for the HeartStart MRx on
page.198.
2. Use Next Item or Prev Item to navigate to the incident of your
choice.
3. Press Menu, and then navigate to Send.
4. Press Send.
5. Navigate to the information you want to transmit to Event Review
Pro and press Enter.
6. The first time you send a Bluetooth transmission, a popup message
appears, including an option to Always Allow Authorization. If you
want to do this, check the box; if you do not, the checkbox will
appear every time you send a transmission.
7. After the HeartStart MRx transmits the incident to the computer, you
can attach the ECG from the HeartStart MRx in Event Review Pro.
For more information, see Downloadingan ECG using a HeartStart MRx
Bluetooth transmission on page.57.
Reading FR2 series cards
The FR2 series defibrillators store information on a Compact Flash card.
Card readers are available through your Philips sales representative or
local distributor.
To read a data card
1. One end of the data card has a series of perforations. Plug this end
firmly into the card reader.
2. Check in Windows File Explorer to see that the card was recognized.
When Windows recognizes the card, it assigns a drive letter to the
card.
Event Review Pro can read the data on the card.
3. If the card does not appear in Windows File Explorer, remove the
card from the reader, and then insert it into the card reader again.
12 - Working with defibrillators Event Review Pro User Guide

201
Reading HeartStart XL cards
The HeartStart XL defibrillator stores information on an ATA flash card.
Read the documentation for the defibrillator to learn how to remove the
card from the defibrillator.
You can read these cards in an ATA flash card reader or a PC Card
(PCMCIA) reader. If the computer is not equipped with an adapter, you
can add one as an external or internal card reader. The external card
reader plugs into the desktop USB port.
To read a data card
1. One end of the card has a series of perforations. Insert this end
firmly into the card reader.
2. Check in Windows File Explorer to see that the card was recognized.
When Windows recognizes the card, it assigns a drive letter to the
card.
The Philips Data Management Solutions application and Event
Review Pro can read the data on the card.
3. If the card does not appear in Windows File Explorer, remove the
card from the card reader, and then insert it into the card reader
again.
Transferring HeartStart XL+ data
The HeartStart XL+ defibrillator uses a USBdata drive to transfer event
summary data. The USBdata drive connects to a USBport on the
computer running Event Review Pro.
You can export data from the HeartStart XL+ defibrillator to the
USBdata drive using a menu selection in the Data Management mode.
Once the data is exported, you can transfer it to the computer running
Event Review Pro. See Attachingan ECG from a USB port on page.56.
To export event-summary data from the HeartStart XL+
defibrillator to the USB data drive
1. Locate the USB data port on top of the HeartStart XL+ defibrillator
and insert the USB data drive. For more information, see the
HeartStart XL+instructions for use.
12 - Working with defibrillators Event Review Pro User Guide

202
2. Confirm that you are in Data Management mode.
3. Press the Menu Select button.
The Internal Memory menu appears.
4. Use the navigation buttons to select the Export All option.
5. Press the Menu Select button to start the task.
The data is exported to the USB data drive.
Transferring Efficia DFM100 data
The Efficia DFM100 defibrillator/monitor (where available) uses a
USBdata drive to transfer event summary data. The USBdata drive
connects to a USBport on the computer running Event Review Pro.
You can export data from the Efficia DFM100 defibrillator/monitor to the
USBdata drive using a menu selection in the Data Management mode.
Once the data is exported, you can transfer it to the computer running
Event Review Pro. See Attachingan ECG from a USB port on page.56.
To export event-summary data from the Efficia DFM100
defibrilator/monitor to the USB data drive
1. Locate the USB data port on the Efficia DFM100 defibrillator/monitor
and insert the USB data drive. For more information, see the Efficia
DFM100 Defibrillator/Monitor Instructions for Use.
2. Confirm that you are in Data Management Mode. When first entering
Data Management Mode, the Internal Memory Screen is displayed.
3. From the Internal Memory Screen, press the Smart Select knob to
enter the Internal Memory Menu.
4. Use the knob to navigate to the Export All menu option.
5. Press the Smart Select knob again to start the task.
The data is exported to the USB data drive.
Determining the HS1 and FRx case
date and time
Event Review Pro documentation uses the HS1 or HS1 family of
defibrillators to refer to HeartStart Home, HeartStart OnSite, and
HeartStart HS1 Defibrillators.
12 - Working with defibrillators Event Review Pro User Guide

203
The HS1 family of defibrillators and FRx defibrillators do not have a
real-time clock that keeps the defibrillator date and time. Instead, the
defibrillator has a continuously running timer that starts when you insert
the battery. Event Review Pro uses this timer and the computer date and
time to calculate case and event time. The timer resets when you
remove the battery. Therefore, do not remove the battery until after
you have downloaded the patient data to the application.
Each time that Event Review Pro communicates with an HS1 and FRx
defibrillator through IrDA, it uses the date/time of the computer clock
and the timer of the HS1/FRx defibrillator to synchronize the date/time
of all events and data since the battery was last inserted. This is called
the "time sync point." If Event Review Pro does not find a time sync
point, you need to set the case start time.
The time sync point might not match the date and time of the
computer by 15 to 30 seconds.
Using infrared connections for the
HS1 and FRx
Event Review Pro documentation uses the HS1 or HS1 family of
defibrillators to refer to HeartStart Home, HeartStart OnSite, and
HeartStart HS1 Defibrillators.
The HS1 family of defibrillators and the FRx defibrillator use an infrared
(IrDA) communications port to transfer information. For information on
how to determine the case date and time, see Determining the HS1 and
FRx case date and time on page.202.
The infrared port is located on the lower right side of the defibrillator. To
transfer information, the receiving computer must be set up for infrared
communication.
Some computers have infrared ports. If the computer does not have an
infrared port or the port does not work with the defibrillator, you can
add an infrared adapter.
Use an infrared adapter that connects to a USB port, if you require an
adapter.
12 - Working with defibrillators Event Review Pro User Guide

204
Setting up an infrared adapter
The Event Review Pro software was tested with ACTiSYS adapters. More
information is available at the following Web site:
http://www.actisys.com
To set up the infrared adapter
1. Read the instructions that came with the infrared adapter.
2. Use the CD that comes with the infrared adapter to run the driver
setup program on the Windows operating system.
3. The New Hardware Wizard guides you through setting up the
adapter.
Setting up the infrared connection
Downloading an ECG and device history information from an HS1 or FRx
defibrillator requires an infrared connection between Event Review Pro
and the defibrillator.
To transfer information, you must put the HS1 or FRx defibrillators into
the Administration mode.
The following discussion explains how to set up each defibrillator to
transfer information. The process is similar for the HS1 family of
defibrillators and the FRx defibrillator. However, there are enough
differences to warrant separate sections.
To set up the IrDA connection between an FRx defibrillator and
Event Review Pro
If the FRx does not receive a transmission within 3 minutes of starting
Administration mode, it cancels the mode. At that point, you must start
over.
1. In Event Review Pro, complete one of the following procedures:
a. Use the Case Wizard to create a case, up to the point where you
see the Attach ECGdialog box.
b. Create a case for the infrared transmission, and then (on the
toolbar) click Attach ECGfile.
c. Open an existing case for the transmission, and then (on the
toolbar) click Attach ECGfile.
12 - Working with defibrillators Event Review Pro User Guide

205
2. Remove the pads connector.
3. If you haven't done so already, insert the battery into the
defibrillator.
You will hear voice prompts to plug in pads connectors. Disregard
these prompts in this instance. These messages end once you place
the defibrillator in Administration mode.
4. On the defibrillator, press and hold the blue Information button,
and wait for three tones.
5. Release the blue Information button.
The voice message announces Administration. The defibrillator
enters data transfer mode.
6. Position the defibrillator to communicate with the computer.
The defibrillator and computer should be between 4 and 24 inches
apart and aligned with each other, with an unobstructed path
between them. For help aligning the defibrillator and the computer,
click the IrDA icon on the Attach ECGwindow and follow screen
instructions.
You can now send and receive information between the defibrillator
and the computer. The device announces transferring data.
7. When sending and receiving information is complete, plug the pads
connector in again. You can now turn off the defibrillator.
For more information, refer to the defibrillator documentation.
To set up the IrDA connection between an HS1 defibrillator and
Event Review Pro
Event Review Pro documentation uses the HS1 or HS1 family of
defibrillators to refer to HeartStart Home, HeartStart OnSite, and
HeartStart HS1 Defibrillators.
If the HS1 does not receive a transmission within 3 minutes of starting
Administration mode, it cancels the mode. At that point, you must start
over.
1. In Event Review Pro, complete one of the following procedures:
a. Use the Case Wizard to create a case, up to the point where you
see the Attach ECGdialog box.
b. Create a case for the infrared transmission, and then (on the
toolbar) click Attach ECGFile.
c. Open an existing case for the infrared transmission, and then (on
the toolbar) click Attach ECGFile.
12 - Working with defibrillators Event Review Pro User Guide

206
2. Remove the pads cartridge. Locate the latch at the top, and then
slide the latch to the right to release the pads cartridge.
3. If you haven't done so already, insert the battery into the
defibrillator.
4. You will hear voice prompts to plug in the pads cartridge. Disregard
these prompts in this instance. These messages will be silenced once
you place your defibrillator in Administration mode. After the battery
is inserted, the defibrillator automatically turns on.
5. On the defibrillator, press and hold the blue Information button,
and then wait for three tones.
6. Release the blue button.
A voice message announces Administration.
7. Briefly press the blue Information button again.
The voice message says Mode 1.
8. Position the defibrillator to communicate with the computer.
The defibrillator and computer should be at least 12 inches apart and
aligned with each other. For help aligning the defibrillator and the
computer, click the IrDA icon on the Attach ECGwindow and follow
screen instructions.
You can now send and receive information between the defibrillator
and the computer. The device announces sending data.
9. When sending and receiving information is complete, reinstall the
pads cartridge. You can now turn the defibrillator off.
For more information, refer to the defibrillator documentation.
Understanding voice and system
messages
Defibrillators and Windows use messages to announce the current state.
Defibrillator voice messages
Philips designed the defibrillators to ensure that they are always ready
for use in an emergency.
Event Review Pro documentation uses the HS1 or HS1 family of
defibrillators to refer to HeartStart Home, HeartStart OnSite, and
HeartStart HS1 Defibrillators.
12 - Working with defibrillators Event Review Pro User Guide
207
Remember to insert the HS1 pads cartridge or plug in the FRx pads
connector when you finish transferring information. This will ensure
that the defibrillator is ready for use on the next patient.
At several points in the Event Review Pro instruction, you are instructed
to ignore the defibrillator voice message that says "Insert the pads
cartridge (for HS1)" or "No connector installed. Plug in pads connector
(for FRx)." The message is there to ensure that you are aware of the
defibrillator's current state. If you remove the HS1 pads cartridge or the
FRx pads connector, the defibrillator is not available for use on the
patient. When the defibrillator enters the Administration mode, the
message stops.
You can put the FR3 into Admin mode without removing pads.
System messages
If Windows detects an active wireless device, it starts the Windows
Wireless Link. Event Review Pro does not use this application and cannot
disable it. As a result, the Windows Wireless Link might display
messages. These messages do not apply to your current task. You can
ignore them.
Emailing device history data
If you ask for assistance from Philips Customer Support, you may be
asked to retrieve the device history data (self-test data) from a specific
defibrillator. Use the following steps (or do as directed by Customer
Support) to retrieve and email this data.
Email a device self-test file to customer support only when
instructed to do so by a Philips support technician.
To email device self-test data to Customer Support
1. From the Help menu, click Email with Device Self-Test File.
The Device Self-Test Wizard appears .
2. Set up the device that you are sending data from. For details on how
to set up your device to transmit data, see Set up an AED device to
transfer self-test data on page.208.
12 - Working with defibrillators Event Review Pro User Guide

208
3. From the list of devices, click the one that you want to send data for
and click Next.
An email message box appears, with the device history data
appearing as an attachment.
4. Type any comments you want to add to the message.
5. Click Next.
The message is sent and a status message appears.
Set up an AED device to transfer self-test data
The process for setting up an AED device to transmit data varies by
model. See the following for information by model.
HS1 and FRx devices
If you are sending data from an FRx or HS1 device, put the device into
Administration mode. For details on setting up the connection, see
Setting up the infrared connection on page.204.
FR2 devices
If you are sending data from an FR2 device using a data card, the data
card must be installed, and the Battery Insertion Test (BIT) must be run
before the self-test data is available to transfer. See the instructions
that came with the FR2 device for further information about running BIT.
When the device history data file is on the card, insert the card into a
card reader attached to your computer.
FR3 devices
When sending self-test data from an FR3 device by any method, before
the self-test data is available to transfer, the data card must be installed
in the device, and the User-Initiated Test (UIT) must be run. (See the
instructions that came with the FR3 device for further information about
running UIT.)
Transfer FR3 self-test data using the data card
Remove the data card from the FR3 and insert the card into a card
reader attached to your computer.
Transfer FR3 self-test data using the Bluetooth connection
Turn on the FR3 device, wait for the voice prompts, put the device
into Administration mode, press the option button, and select
Wireless Data Transfer. (See the instructions that came with the
FR3 device for further information about putting the device into
Wireless Data Transfer mode.)
12 - Working with defibrillators Event Review Pro User Guide

209
Importing device self-test data using
the Device Self-test Wizard
The Devices workspace provides tools for transferring self-test data
from Philips HeartStart AEDs to the Event Review Pro database, and
tables where you can view and manage the self-test data.
Device self-test data can be imported from HeartStart AED devices.
However, the importation of device self-test data from HeartStart
monitor/defibrillators, such as the HeartStart MRx, is not
supported.
The Device Self-test Wizard
Use the Device Self-test Wizard to transfer self-test data from the
HeartStart FR3, FR2 series, FRx, or HS1 AEDs.
Event Review Pro documentation uses the HS1 or HS1 family of
defibrillators to refer to HeartStart Home, HeartStart OnSite, and
HeartStart HS1 Defibrillators.
Opening the Device Self-test Wizard
1. In the navigation pane, click Devices.
The Devices pane opens.
2. On the Devices pane, click Device Self-Test Wizard.
The Device Self-test Wizard opens.
3. Follow the steps in the wizard, clicking Next to move to the next
section.
Preparing the AED to transfer data
You can use the Device Self-test Wizard to transfer data wirelessly from
the FR3 device using Bluetooth wireless data transfer.
When sending self-test data from an FR3 device by any method, before
the self-test data is available to transfer, the data card must be installed
in the device, and the User-Initiated Test (UIT) must be run. (See the
instructions that came with the FR3 device for further information about
running UIT.)
12 - Working with defibrillators Event Review Pro User Guide

210
Turn on the FR3 device, wait for the voice prompts, put the device into
Administration mode, press the option button, and select Wireless
Data Transfer. (See the instructions that came with the FR3 device for
further information about putting the device into Wireless Data Transfer
mode.)
Click the icon only if you want setup instructions; clicking an icon
does not start data transfer.
To use the Wizard to transfer self-test data
1. In the wizard, navigate to the Device Selection page, and then
prepare your defibrillator for data transmission.
When the user selects a transfer method (for example, Bluetooth, or
IrDA), and clicks Next, the wizard then begins data acquisition.
2. Allow the transmission to complete before moving the device.
When the self-test data is transferred, a message appears in the
wizard: "All tasks have been successfully completed", and the All
Devices table opens.
3. Return the defibrillator to ready-to-use state.
The All Devices table
When the Device Self-test Wizard competes data transfer, devices
whose self-test data has been acquired through the Device wizard
appear in the All Devices table. In each row, the table shows a device
icon, device serial number, device manufacture date, and the date that
the last self test data was acquired, to assist you in locating the correct
self-test data.
To open the All Devices table directly
• On the Devices pane, click All Devices to open the All Devices
table directly.
To view device self-test data
1. In the All Devices table, double-click a row to view the self-test
data for that device.
The Self-test Results table opens, with self-test data for the selected
device. At the top of the table, the device model and serial number
appear.
2. To print the data, click Print on the toolbar or in the File menu.
12 - Working with defibrillators Event Review Pro User Guide

211
The Self-test Results table
The Self-test Results table includes the following columns of self-test
information:
• Sequence Number
• Date
• Time
• Relative Time
• Description
• Status
For information about sending self-test data to Philips Customer
Support, see Emailing device history data on page.207.
12 - Working with defibrillators Event Review Pro User Guide
13
Managing the
database
Event Review Pro stores the application database in Microsoft SQL
Server 2014 SP1 Express Edition. This is a free version that Philips
installs by default. It limits the database size to 10GB. The number of
cases that can be stored in a database of this size varies depending on
the length of captured ECGs and whether or not the file includes audio.
The versions sold by Microsoft are limited only by the size of the hard
disk.
The Event Review Pro database is low-maintenance, compared to many
other databases. Nonetheless, attention to routine maintenance tasks
will help to ensure database integrity.
It is the database administrator’s responsibility to set up the
appropriate database management features and to check that they
execute successfully.
Back up the database on a regular basis (preferably every day) to tape
or some other medium. Disaster-recovery experts recommend that you
store the backup tapes somewhere safe, so that disasters such as fire or
theft cannot harm them. Without a recent backup, you have no chance
of recovery after a catastrophe, such as a disk failure or fire. Use your
organization's backup and recovery policy to set up this procedure.
Microsoft has procedures and tools for managing SQL Server.
For information on migrating data from an older version of the
application, see Migrating cases from previous versions on page.214.
212

213
Using the Event Review Pro database
on a remote database server
Event Review Pro supports use of a remote database that is installed on
another machine. Multiple instances of Event Review Pro can share the
database.
You must install the same version of Event Review Pro on each client
machine connected to a shared database.
Philips recommends that Information Technology (IT) personnel
install and configure the remote database server.
Use this feature with care. Before implementing the application on a
shared database server, keep the following in mind:
• The application saves only the changes made by the most recent
user to save the case. For example, when more than one user
opens the same case, makes changes, and saves the case, the
application saves the changes made by the last user to save the
case.
• Event Review Pro installed on a client machine cannot run when
the shared database is unavailable, such as when the database
server is down, or the network is disconnected (if the shared
database resides on another computer).
Using Microsoft SQL
You can purchase the SQL Server software to set up a shared database
environment for Event Review Pro.
For information about installing and setting up a shared database, refer
to the HeartStart Data Management Solutions Implementation Guide,
available from Philips customer support.
The following information is intended for users who have already set up
their databases.
Changing the database server
You can change the Event Review Pro database server in the following
ways:
13 - Managing the database Event Review Pro User Guide

214
To change the database server from the Tools menu
1. From the Programs menu, right-click Event Review Pro and click
Run as Administrator.
2. On the Tools menu, click Database Server.
The Configure SQLServer for Event Review Pro 5.0 window opens,
showing the current database name.
3. In the SQLServer name box, use the down arrow to display a list
of all available SQL Servers on the network.
4. Click the SQL server name that you want.
5. To verify the connection, click Test.
A message from the SQL server software appears, noting success or
failure. If Event Review Pro displays a failure message, refer to your
SQL Server documentation or your local database administrator for
instructions.
6. If you see a success message, click the database you wish to use,
then click OK.
This database must use the Event Review Pro 5.0 schema. You
cannot use an older database that uses a different schema.
A message appears stating that you must restart Event Review
Proto use the new database server.
To verify the database server on the About box of Event
Review Pro
1. Start Event Review Pro.
2. On the Help menu, click About Event Review Pro.
3. On the Server line, confirm that Event Review Pro is connected to
the correct server database.
Migrating cases from previous
versions
You can migrate cases from HeartStart Event Review 3.5, 4.2 , or 4.3,
and from Event Review Pro 3.5, 4.0, 4.1, 4.2, or 4.3 to the Event Review
Pro 5.0 database.
13 - Managing the database Event Review Pro User Guide

215
HeartStart Event Review 4.3 and HeartStart Event Review Pro 4.3 had
limited geographical distribution.
If the source database is on another machine, see your
ITprofessional for assistance.
Steps for migrating the data
1. Identify the database from which the cases are to be migrated—the
source database.
2. Event Review Pro creates a list of all the cases from the source
database and compares them (using the case ID) to the cases in the
current Event Review Pro 5.0 database (the destination database).
Cases that are not already in the destination database are marked
for migration.
3. Migrate the marked cases.
Use the following procedures to complete the migration.
To select and identify the source database
1. In the main workspace, click the Administration button.
2. In the Administration navigation pane, click one of the following
options:
• Migrate Records from Event Review 3.5
• Migrate Records from Event Review 4.2
• Migrate Records from Event Review 4.3
• Migrate Records from Event Review Pro 3.5
• Migrate Records from Event Review Pro 4.0
• Migrate Records from Event Review Pro 4.1
• Migrate Records from Event Review Pro 4.2
• Migrate Records from Event Review Pro 4.3
A database selection dialog box appears, displaying only those
computers with the expected, well-known instance and database
names, as shown in this table:
Event Review Pro
version
SQLServer instance
name Database name
3.5 HeartStart ERPro35
4.0 Philips ER40
13 - Managing the database Event Review Pro User Guide

216
Event Review Pro
version
SQLServer instance
name Database name
4.1 Philips ER41
4.2 PHILIPS42 ER42
4.3 PHILIPS42 ER43
3. Click the appropriate source database.
4. (Recommended) To test the accessibility of the source database,
click Test.
Amessage appears reporting the success or failure of the test.
5. Click OK to continue the migration process.
To view and confirm the list of cases to be migrated
1. Once you have selected the source database, Event Review Pro
connects to this database and displays a list of all the cases. The
ones to be migrated have the + sign.
2. If a case with the same case ID already exists in the destination
database, the case will not be migrated. If you still want to migrate
such a case, cancel the migration, rename or delete the case in the
destination database, and then restart the migration process.
To migrate the cases
1. Click OK.
The data migration begins. This takes some time, particularly if you
have many cases.
2. If you need to cancel migration, click Cancel. You can restart the
migration process at another time. Because any already migrated
cases will have the same case ID as those in the source database,
those cases will not be migrated again.
The migration status of each case is logged in the System Log,
including any errors.
The successfully migrated cases are now in the database, and
appear in the case selection table.
13 - Managing the database Event Review Pro User Guide
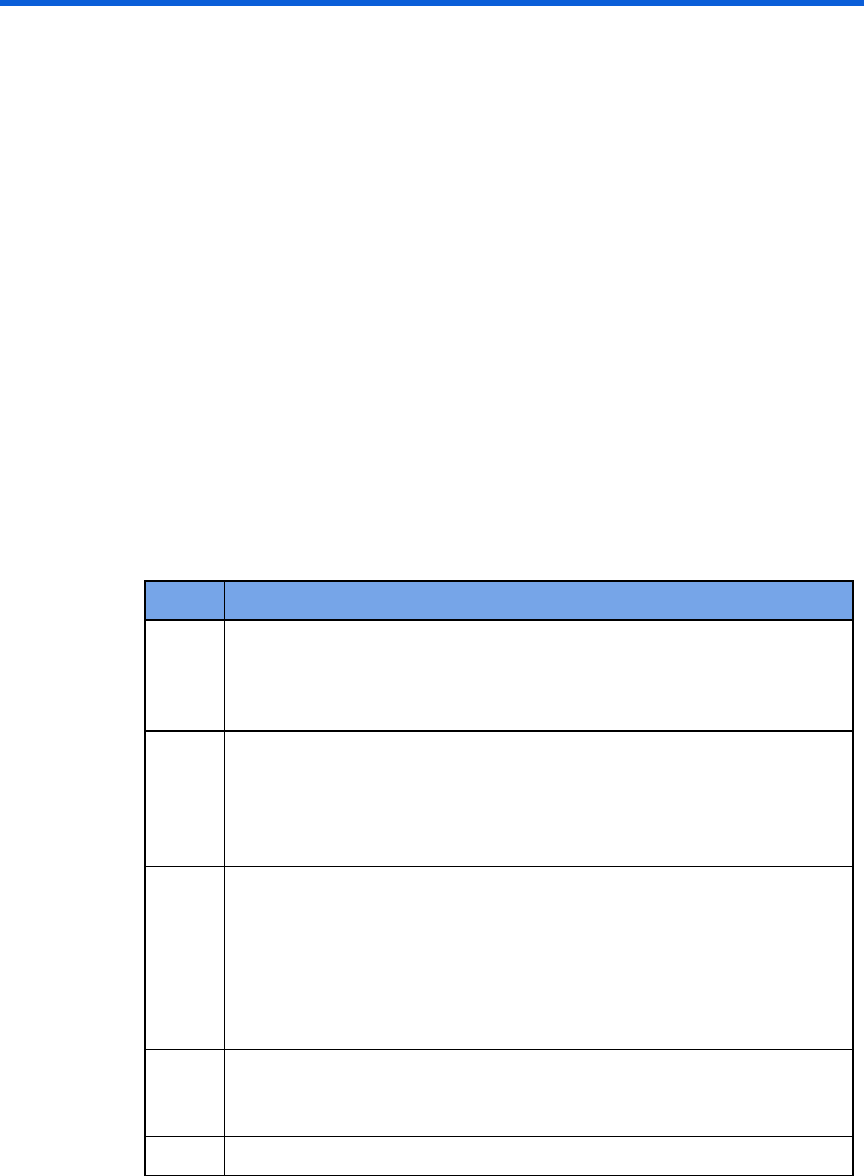
14
CPC and OPC
The Cerebral Performance Categories (CPC) and Overall Performance
Categories (OPC) are two scores that are used when reporting cardiac
arrest. The Follow-up tab uses these categories.
EMSEdition only:The two scores are the recommended guidelines for
uniform reporting of information from out-of-hospital cardiac arrest in
the Utstein-style templates. (Cummins RO, Chamberlain DA, Abramson
NS, et al. AHA Medical/Scientific Statement - Recommended guidelines
for uniform reporting of information from out-of-hospital cardiac arrest:
the Utstein style. Circulation 1991;84:960-975.)
This section explains the scores for each category.
Cerebral performance categories table
Score Description
1 Good cerebral performance: Conscious. Alert, able to work and
lead a normal life. May have minor psychological or neurological
deficits (mild dysphasia, nonincapacitating hemiparesis, or minor
cranial nerve abnormalities).
2 Moderate cerebral disability: Conscious. Sufficient cerebral
function for part-time work in sheltered environment or independent
activities of daily life (dressing, traveling by public transportation, and
preparing food). May have hemiplegia, seizures, ataxia, dysarthria,
dysphasia, or permanent memory or mental changes.
3 Severe cerebral disability: Conscious. Dependent on others for
daily support because of impaired brain function (in an institution or
at home with exceptional family effort). At least limited cognition;
includes a wide range of cerebral abnormalities from ambulatory with
severe memory disturbance or dementia precluding independent
existence to paralytic and able to communicate only with eyes, as in
locked-in syndrome.
4 Coma, vegetative state: Not conscious. Unaware of surroundings,
no cognition. No verbal or psychological interactions with
environment.
5 Death: Certified brain dead by traditional criteria.
217

218
Overall performance categories table
Score Description
1 Good overall performance: Healthy, alert, capable of normal life.
Good cerebral performance (CPC1) plus no or only mild functional
disability from noncerebral organ system abnormalities.
2 Moderate overall disability: Conscious. Moderate cerebral disability
alone (CPC2) or moderate disability from noncerebral system
dysfunction alone or both. Performs independent activities of daily life
(dressing, traveling, and food preparation). May be able to work part-
time in sheltered environment but disabled for competitive work.
3 Severe overall disability: Conscious. Severe cerebral disability alone
(CPC3) or severe disability from noncerebral organ system
dysfunction alone or both. Dependent on others for daily support.
4 Coma, vegetative state: Not conscious. Unaware of surroundings, no
cognition. No verbal or psychological interactions with environment.
5 Death: Certified brain dead by traditional criteria.
14 - CPC and OPC Event Review Pro User Guide
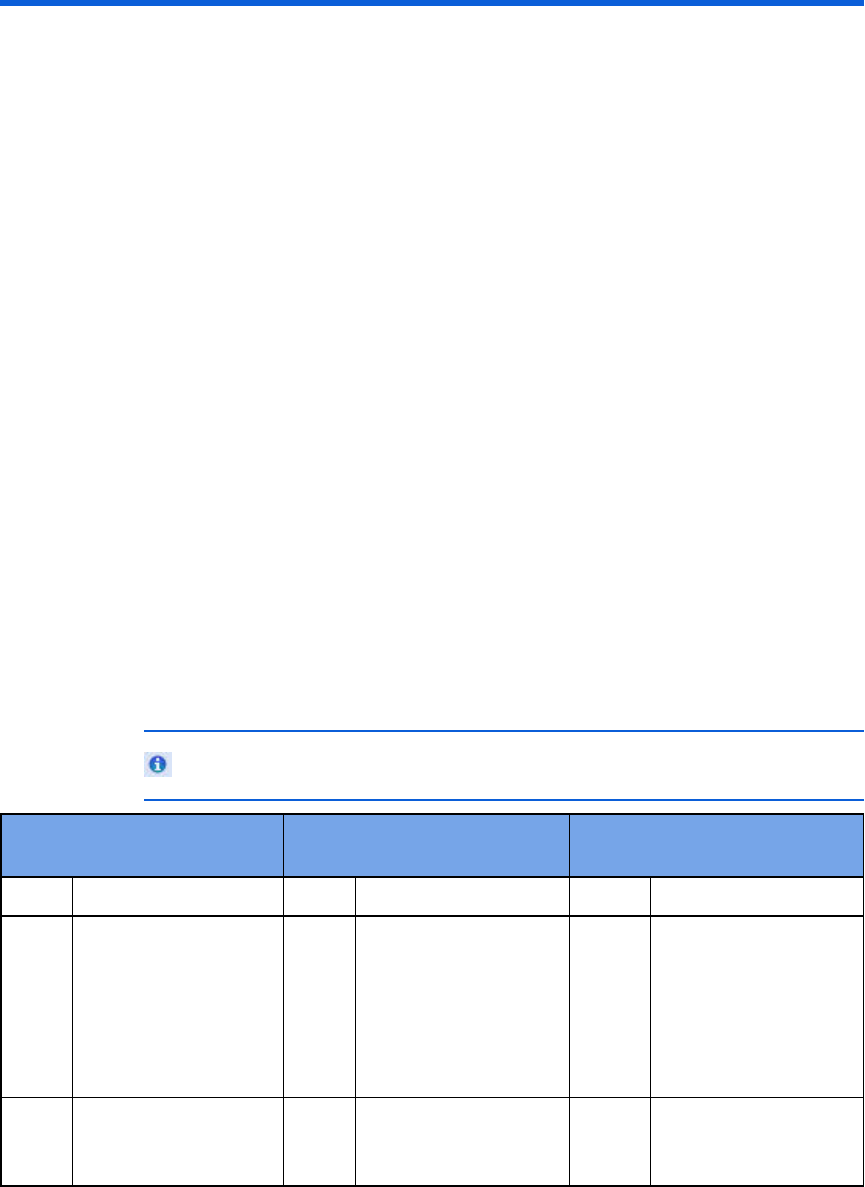
15
Glasgow Coma
score
The Glasgow Coma score is a score given to head trauma victims based
on the Glasgow Coma Scale (GCS). The total score is based on
examination of eye opening, verbal output, and motor (movement)
responses to different modalities and intensity of stimulation. You give
each of these three attributes a score, and then Event Review Pro totals
the three scores.
The choices in the GCS Total field are:
0 (3) (total up to 3)
1 (4-5) (total from 4 to 5)
2 (6-8) (total from 6 to 8)
3 (9-12) (total from 9 to 12)
4 (13-15) (total from 13 to 15)
Unknown
A score of “unknown” indicates that one or more response scores do
not have an entry or have an entry of “unknown.”
Eye opening Verbal output
Motor (movement)
responses
Score Finding Score Finding Score Finding
6 Obeys commands
or
Spontaneous or
purposeful
Patient follows
commands
5 Oriented
or
Coos or babbles
5 Purposeful
or
Withdrawal from
219
220
Eye opening Verbal output
Motor (movement)
responses
Normal, fluent,
appropriate speech
touch
Patient locates pain
on own body
4 Spontaneous
Eyes open without
stimulation
4 Confused
or
Irritable and
continually crying
Confused but fluent
speech
4 Withdraws from pain
Patient pulls away
from pain
3 To voice
Eye opening to loud
noise
3 Inappropriate
or
Cries to pain
Mumbling, occasional
word recognizable
3 Flexion to pain
or
Abnormal flexion to
pain
Patient flexor
postures
2 To pain
Eye opening to pain
only
2 Incomprehensible
or
Moans to pain
Vocalizations but not
verbalizations (no
words)
2 Extension to pain
Patient extensor
postures
1 None
No eye opening
1 None
No vocalization or
verbalization
1 None
No motor response
15 - Glasgow Coma score Event Review Pro User Guide

16
Glossary
ACI-TIPI
For acute cardiac ischemia time-insensitive predictive instrument. A
Philips software tool in that can provide a second opinion and decrease
the time between the onset of a patient’s acute cardiac ischemia (ACI)
symptoms and the treatment of interventional cardiology. The ACI-TIPI
feature computes a patient’s likelihood of having an ACI based on the
patient’s age, gender, chest pain status, and acquired 12-lead ECG.
When the defibrillator runs the ACI-TIPI analysis, the interpretative
block on the 12-lead report includes ACI-TIPI data.
Impedance
Commonly denoted as Z. Electrical resistance.
License Manager
The License Manager allows you to type in your registration key to
activate HeartStart software.
PEA
Pulseless Electrical Activity.
PR
Perfusing Rhythm.
PST
For Periodic Self Test. A self-administered test mode that a HeartStart
defibrillator runs to ensure that all defibrillator components are
functional and work properly. Test results are Pass, Warning, or Call
customer support.
Q-CPR
Q-CPR is a Laerdal registered trade mark. The name was derived from
Quality of Cardiopulmonary Resuscitation
URL
URL stands for Uniform Resource Locator, an address for a resource on
the Internet. URLs are used by Web browsers to locate Internet
resources.
221

222
XML
Stands for eXtensible Markup Language. This file format has a set of
rules for encoding documents in machine-readable form.
zCPR
Impedance-derived cardiopulmonary resuscitation quality. zCPR derives
compression rate, hands on time and hands off time from the
impedance recorded across the AED therapeutic pads. Unlike Q-CPR,
zCPR does not provide compression depth or compression release
calculations.
16 - Glossary Event Review Pro User Guide
.
.cod file 43
.hic file 43, 65
.mic file 43
.wfdb file format 113
1
12-lead information 60, 119, 121
attaching 52
magnifying 122
reviewing 122
12-lead reports 156
A
activating the product 14
Internet 15
within 60 days 17
Administration workspace tasks 25
annotations
CPR responder 128
ventilation 127
ATA flash card reader 201
attachments 34, 53
Bluetooth 53
ECGs 48, 51, 54-58, 60, 201
printing 136
Attachments table 34
audio, playing on ECGs 107
Average Response Times—Total System
report 184
Average Response Times report 185
B
Bluetooth 119, 195, 197, 199
attaching ECGs 53, 56-57
automatic synchronization 67
erasing data source 67
exchange folders 58
pairing and testing 198
prerequisites 198
C
card readers 193
Case Details report 155
Index
223

Case Editor tabs 28, 88
Case Editor, default view 28, 89
Case Editor, viewing 12-leads 120
case ID 70
case selection table 26, 33
Case Summary 48
Case Wizard 47, 51
cases 43, 48, 62
adding details 78
adding patient information 87
attaching files 135
creating 43, 47, 50
defined 19, 43
deleting 72
duplicate ECGs 73
emailing 71
exporting 69
hiding and displaying 63
identifying 79
importing automatically 65
importing manually 64
merging automatically 76
merging manually 74
migration from earlier versions 214
opening 50
printing 69
reviewing events 133
saving 66
sorting and grouping 62
CDR file, sending to Customer Support 207
Cerebral Performance Categories (CPC) 217
color
ECG selections 110
vital trends display 119
CompactFlash card reader 196
compatibility 18
confirmation messages, restoring 42
CPR
adding notes to ventilation channel 127
creating exclusions 125
customizing episodes 123
removing exclusions 126
report data 169
response times 173
CPR guidelines 150
cumulative device records, sending to
Customer Support 207
cursor delta time 108
customer support 189
emailing System Log to 145
D
data card for attaching ECGs 55, 193
data migration from older versions 18
data source, erasing 67
data, migration from earlier verions 214
database 212
remote server 213
verifying location 213
date and time
adjusting defibrillator 66
events, changing 87
defibrillators
adjusting date and time 66
supported 192
transferring self-test data 209
voice and system messages 206
detaching ECGs 61
device tests 207
Do Not Show This Message Again 42
document conventions 21
downloading ECGs 51
Duplicate ECGs table 139
E
ECG Pre- and Post-shock report 156
ECG selections 108
color 110
creating 109
managing 109
naming and sorting 111
Index Event Review Pro User Guide
224

Q-CPR 110
templates 112
ECGs 88
12-Lead view 121-122
adding 51, 54-58, 60
attaching 48, 51, 54-58, 60, 201
changing time scale 101
Channels pane 32, 92
copying to clipboard 113
customizing display 117
detaching 61
duplicates 73, 139
Events pane 31, 92
navigating on waveform 107
Overview pane 31, 92
playing audio 107
printing reports 108
removing 61
reports 155
shortcuts 129
tab 88
Transport pane 32, 93
view 28, 88
viewing information 108
vital trends 117
Efficia DFM100 56
Efficia DFM100 defibrillator/monitor 54
email
cases 71
reports 50
EMS edition 9
erasing data source 67
events 34
adding 85
changing date and time 87
documenting 85
editing 85-86
expanding and collapsing nodes 94
expanding list 93
filtering 134
filtering entries 37
finding and viewing 94
grouping 36, 134
hiding and displaying 95, 134
icons 93
managing notes 96
printing 134
removing 85
searching for 95
sorting 36, 86
Events pane 31, 92
Excel
exporting Q-CPR Details Report data 169
exporting table data 40
exclusions for Q-CPR
creating 125
removing 126
exporting files 49
password protection 49
F
F1, using for Help 20
fields, completing 40
files, adding to case 135
files, attaching ECGs from 60, 135
filtering events 134
filtering imported device data 140
filters
removing 39
Final Review tab
Case Reviewed box 137
G
Getting Started options 23
Glasgow Coma Score (GCS) 219
grouping events 134
guidelines 150
Index Event Review Pro User Guide
225

H
HeartStart FR2 defibrillators 56
attaching ECGs 54
data cards 200
HeartStart FR3 defibrillators 53-54, 56
Bluetooth 195
data cards 195
synchronizing clock via data erasure 69
HeartStart FRx defibrillators 55, 58
Admin mode 204
case date and time 203
HeartStart Home defibrillators 55
HeartStart HS1 defibrillators 55, 58
Admin mode 205
case date and time 203
HeartStart MRx monitor/defibrillators 48,
53-54, 57, 79, 119
Bluetooth 198-199
data cards 196
HeartStart OnSite defibrillators 55
HeartStart XL defibrillators
attaching ECGs 54
data cards 201
HeartStart XL+ defibrillators 56, 69, 201
attaching ECGs 54
data cards 201
help 20, 22, 189
hiding and displaying cases 63
Hospital edition 9
HTTP, using for Import Service 143
I
icons for events 93
Import Service 65, 138, 143
configuring 141
filtering data 140
managing inboxes 139
purging archive folders 142
importing files 138
automatically 65
manually 64
infrared connections 53, 58, 203-204
installation 8
IrDA connections 53, 58, 203
K
key command shortcuts 129
L
license types 9
M
merging related cases manually 74
migration of older data 8, 214
mouse 129
N
navigation pane 24, 26
minimizing 24
new features, version 5.0 19
notes, managing 96
Index Event Review Pro User Guide
226

O
offset, waveform 104
online help 21
Overall Performance Categories (OPC) 217
Overview pane 31, 92
P
password protection 49, 65, 69, 139
patient identity, removing 49
patient information, adding 87
PC card reader 201
PCMCIA card reader 201
pediatric option for FR2 56, 60
Percentile Response Times—Total System
report 185
Percentile Response Times report 185
pinning and unpinning 99
point style, vital trends view 118
printing
case information 69
reports 49
product support 189
purging archive folders for imported device
data 142
Q
Q-CPR data 123
Q-CPR Report Card report 158
queries, creating 46
queries, deleting 47
quick note 115
R
redaction 70-71, 79, 147
removing ECGs 61
removing the application 17
reports 147
12-lead 156
case details 155
ECGs 155
emailing 50
exporting 154
generating for open case 151
generating from database 152
printing 50
Q-CPR 158
Response Times 183
Select Cases 148
toolbar icons 153
Vital Trends 183
Reports
12 lead 156
resetting ECG display layout 99
resizing panes and workspaces 41
Response Times report 183
Reviewed column in Patient Cases table 137
reviewer notes, adding to a case 137
S
saving cases 42, 66
scale, waveform 106
search for cases
queries 44, 46
Quick Selections 44
search criteria 44
selections templates, ECGs 112
Index Event Review Pro User Guide
227

self-test
All Devices table 210
Device Self-test Wizard 209
emailing data to customer support 207
Self-test Results table 211
tranferring device data 209
shortcuts 129
starting the product 23
synchronization, defibrillator and computer
date and time 69
System Log 34, 145
emailing to Customer Support 145
filtering entries 37
grouping and sorting entries 36
printing entries 39
removing filters 39
system requirements 9
T
tables
duplicate ECGs 73
printing 69
Tablet Mode 41
Tablet PC 41
telephone support 189
time
changing on waveform 100
changing scale 101
Timeline tab 85
tooltips 20
transferring self-test data from a device 209
Transport pane 32, 93
U
uninstalling
current version software 17
Unwitnessed with Bystander CPR
report 186
updates, checking for 13
upgrade 8
USB data drive, attaching ECGs from 56,
201
using Select Cases for reports 148
Utstein guidelines 186, 217
Utstein reports 186
V
ventilation channel, adding notes to 127
vertical offset, changing 104
vital trends 117
color 119
point style 118
Vital Trends report 183
W
waveforms 97
audio 101
changing color 104
changing display time 100
changing scale 106
displaying and hiding 99
displaying in channel 103
ECG information 108
exporting data 113
magnifying 100
magnifying waveforms 100
measuring value 108
navigating ECG events 107
notes 114
offsets 104
selecting 98
viewing 97
viewing ECG information 108
zooming in and out 116
Index Event Review Pro User Guide
228


