
Use of Foreign Comparators in Bioequivalence
Studies for Health Canada
Scott Appleton, PhD
Manager, Division of Biopharmaceutics Evaluation
Pharmaceutical Drugs Directorate
Health Canada
SBIA Meeting
February 28, 2024

Outline
• Canadian Food and Drug Regulations
• Development of HC’s Approach to Foreign Comparators
• Earlier
• Recent
• Risk associated with using foreign comparators
• Case Study
• International Collaborations
• Summary
2

Food and Drug Regulations (FDR)
Section C.08.001.1 of the FDR provides a definition for the Canadian
Reference Product (CRP):
• (a) a drug in respect of which a notice of compliance is issued pursuant
to section C.08.004 and which is marketed in Canada by the innovator
of the drug,
• (b) a drug, acceptable to the Minister, that can be used for the purpose
of demonstrating bioequivalence on the basis of pharmaceutical and,
where applicable, bioavailability characteristics, where a drug in
respect of which a notice of compliance has been issued pursuant to
section C.08.004 cannot be used for that purpose because it is no
longer marketed in Canada, or
• c) a drug, acceptable to the Minister, that can be used for the purpose
of demonstrating bioequivalence on the basis of pharmaceutical and,
where applicable, bioavailability characteristics, in comparison to a
drug referred to in paragraph a).
3

C.08.001.1 Paragraph (c)
Guidance documents regarding the use of foreign reference
product (FRPs) establish acceptance criteria to justify acceptable
FRPs, pursuant to paragraph (c) of the regulation:
• c) a drug, acceptable to the Minister, that can be used for the
purpose of demonstrating bioequivalence on the basis of
pharmaceutical and, where applicable, bioavailability
characteristics, in comparison to a drug referred to in paragraph
a).
4

Earlier Development of HC’s Approach to FRPs
• 1995: published first guidance document
– Canada first to accept FRPs for BE studies
– only a subset of products based on lower risk/complexity.
• 2000 – 2013: HC sought advice from Scientific Advisory
Committees (SACs) on expansion of scope.
– SAC did not support the expansion of the scope.
– The tests to establish identicality to CRP were not reliable.
5

Risk Factors
• Low solubility drugs
– Other chemicals (e.g., surfactants) required to solubilize the drug
– Testing not reliable to determine FRP is identical to CRP
• Modified-release drugs
– Number of unknown factors that contribute to drug release in the body
(e.g., modified-release mesalamine)
– Complex technology (e.g., OROS technology; complicated PK release
profiles)
• Drugs with NTI/CDD
– Very high risk (e.g., graft rejection)
6

Limitations in Establishing Identicality
• It is recognized that it cannot be established unequivocally that
the FRP is identical to CRP
HOWEVER …
• HC is of the opinion that, for the products in scope of the 1995
guidance, even if slight differences should exist between
products which meet the criteria, the differences would be of no
therapeutic consequence.
7

Recent Development of HC’s Approach to FRPs
• 2017: publication of updated draft guidance
– Solubility definition expanded for when drug is highly soluble (dose-
solubility volume < 250 mL over the pH range)
– Notice of guidance publication also made request for proposals to
industry on expansion of scope – No responses received to date.
• 2018: Guidance finalized following receipt of stakeholder
comments.
– No stakeholder comments provided any scientific rationale for expansion
of scope.
8

Expansion of Scope
• Dosage forms added to the guidance:
– immediate release inhaled dosage forms (e.g. orally inhaled solutions,
suspension and dry powders)
– immediate release nasal suspensions
• comparative tests should be conducted with regard to formulation,
physicochemical properties, and device attributes.
See the Health Canada Guidance Document: Use of a Foreign-sourced Reference Product as a Canadian Reference
Product (2018) (https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-
products/applications-submissions/guidance-documents/canadian-reference-product-guidance.html) for additional
information.
9
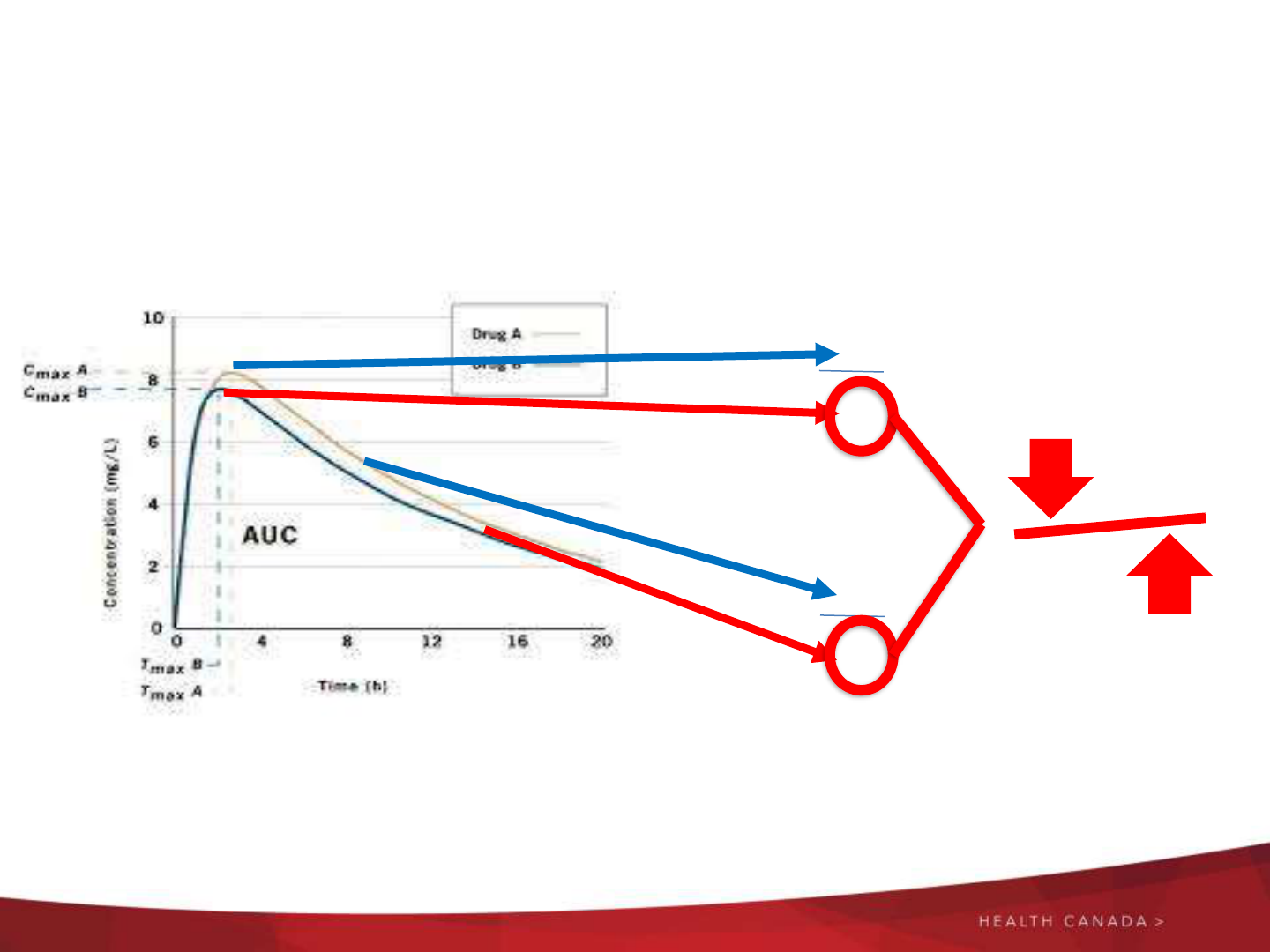
Risk of Using a Foreign Comparator
A
B
AUC
Cmax
A
B
lower
higher
Foreign vs.
Local
Comparator
Absorption
10

Case Study
• Prolonged-release suspension dissolves slowly after IM injection due
to low aqueous solubility
• Hydrolysed to active metabolite, which is absorbed into systemic
circulation.
• Dissolution rate at injection site is rate-limiting step for absorption.
• Complexity wrt MR not related to formulation but rather inherent
properties of API.
– Long Acting Injectable (IM) for treatment of schizophrenia
– Long elimination half-life
– MR and low solubility
– BE study conducted at large number of clinical sites and large number of
patients enrolled – CI’s were narrow
11

Case Study (continued)
Applicant provided:
• results of analytical studies comparing the CRP and biobatch of FRP demonstrating
their similarity including physiochemical, dissolution release, particle size distribution,
and morphology.
• a letter from the local regulatory authority stating that proposed generic product was
found, in a preliminary determination, to be Q1 and Q2 to the FRP.
Additional considerations:
• ethical and practical challenges of conducting an additional study in patients with
schizophrenia.
• Factors influencing the risk vs. benefit of requiring an additional bioequivalence study
include:
– large number of patients required
– Nature of the treated disorder
12

International Collaborations
• International Pharmaceutical Regulators Programme (IPRP)
– Publication: Survey of the Regulatory Requirements for the Acceptance of Foreign
Comparator Products by Participating Regulators and Organizations of IGDRP J
Pharm Pharm Sci (www.cspsCanada.org) 22, 28 - 36, 2019
– Publication: Survey of the Criteria Used for the Selection of Alternative Comparator
Products J Pharm Pharm Sci (www.cspsCanada.org) 25, 323 - 339, 2022
• ACCESS Generic Medicines Work Sharing Initiative
– Operational procedures with an appendix on FRP acceptance
13

Summary
• Use of foreign comparators allow generic pharmaceutical
companies to leverage BE studies conducted for other
jurisdictions.
– Reduce development costs
– Avoid unnecessary exposure of subjects in BE studies
• Applicable criteria used to identify eligible foreign comparators
where the risk of undetected differences are not likely to be
therapeutically significant.
• Flexibilities may be applied on a case-by-case basis.
14

Questions
• Bureau of Pharmaceutical Sciences
bpsenquiries@hc-sc.gc.ca
15

16
