Original Paper
Experiences With Wearable Activity Data During Self-Care by
Chronic Heart Patients: Qualitative Study
Tariq Osman Andersen
1*
, DPhil; Henriette Langstrup
2*
, DPhil; Stine Lomborg
3*
, DPhil
1
Department of Computer Science, University of Copenhagen, Copenhagen, Denmark
2
Department of Public Health, University of Copenhagen, Copenhagen, Denmark
3
Department of Communication, University of Copenhagen, Copenhagen, Denmark
*
all authors contributed equally
Corresponding Author:
Tariq Osman Andersen, DPhil
Department of Computer Science
University of Copenhagen
Universitetsparken 5
Copenhagen, 2100
Denmark
Phone: 45 26149169
Email: [email protected]
Abstract
Background: Most commercial activity trackers are developed as consumer devices and not as clinical devices. The aim is to
monitor and motivate sport activities, healthy living, and similar wellness purposes, and the devices are not designed to support
care management in a clinical context. There are great expectations for using wearable sensor devices in health care settings, and
the separate realms of wellness tracking and disease self-monitoring are increasingly becoming blurred. However, patients’
experiences with activity tracking technologies designed for use outside the clinical context have received little academic attention.
Objective: This study aimed to contribute to understanding how patients with a chronic disease experience activity data from
consumer self-tracking devices related to self-care and their chronic illness. Our research question was: “How do patients with
heart disease experience activity data in relation to self-care and chronic illness?”
Methods: We conducted a qualitative interview study with patients with chronic heart disease (n=27) who had an implanted
cardioverter-defibrillator. Patients were invited to wear a FitBit Alta HR wearable activity tracker for 3-12 months and provide
their perspectives on their experiences with step, sleep, and heart rate data. The average age was 57.2 years (25 men and 2 women),
and patients used the tracker for 4-49 weeks (mean 26.1 weeks). Semistructured interviews (n=66) were conducted with patients
2–3 times and were analyzed iteratively in workshops using thematic analysis and abductive reasoning logic.
Results: Of the 27 patients, 18 related the heart rate, sleep, and step count data directly to their heart disease. Wearable activity
trackers actualized patients’ experiences across 3 dimensions with a spectrum of contrasting experiences: (1) knowing, which
spanned gaining insight and evoking doubts; (2) feeling, which spanned being reassured and becoming anxious; and (3) evaluating,
which spanned promoting improvements and exposing failure.
Conclusions: Patients’experiences could reside more on one end of the spectrum, could reside across all 3 dimensions, or could
combine contrasting positions and even move across the spectrum over time. Activity data from wearable devices may be a
resource for self-care; however, the data may simultaneously constrain and create uncertainty, fear, and anxiety. By showing how
patients experience self-tracking data across dimensions of knowing, feeling, and evaluating, we point toward the richness and
complexity of these data experiences in the context of chronic illness and self-care.
(J Med Internet Res 2020;22(7):e15873) doi: 10.2196/15873
KEYWORDS
consumer health information; wearable electronic devices; self-care; chronic illness; patient experiences
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 1https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Introduction
Consumer Wearable Activity Trackers in Chronic
Care
Consumer health information technologies such as wearable
activity trackers are increasingly being considered to improve
chronic care management [1-4]. Contrary to traditional health
information technologies, these devices are developed as
consumer devices and not as clinical devices. Most commercial
activity trackers aim to monitor and motivate sport activities,
healthy living, and similar wellness purposes. Wristbands such
as Fitbit and smart watches that track bodily signs (eg, heart
rate) do not provide diagnostic services or disorder-specific
information, and they are regulated less rigorously than are
monitoring devices aimed at specific patient groups and clinical
measures. This makes them readily available to consumers.
Moreover, their designs aim at easy and noninvasive integration
into everyday life, by way of automated tracking. These features
are attractive for application in chronic disease management,
where a healthy lifestyle can be a central part of treatment,
rehabilitation, and prevention [1,5-8].
The separate realms of “wellness tracking” and “disease
self-monitoring” and “activity data” and “medical data” are thus
blurred, which is somewhat mirrored in an increasing
prominence of concepts such as “patient-generated health data”
and “personal health technology” where the focus is on the
individual producer of data, rather than on the specific context
or purpose of use. Applying leisure activity tracking to chronic
care management provides new opportunities but is often based
on assumptions about what characterizes these devices: easy,
applicable, user-friendly, empowering, and motivating
technology that can collect data with relevance for self-care and
treatment. While there are great expectations and promising
results emerging [2,3,9], little attention has been paid to the
embodied and embedded experiences of self-tracking among
patients with a chronic disease using consumer devices, which
are not integrated in the health care system.
In this paper, we explored how patients who use consumer
wearable devices make sense of them and of the data they
produce and display vis-à-vis their embodied disorders. We
recruited 27 patients with chronic heart disease who had an
implantable cardioverter-defibrillator (ICD) to wear a Fitbit
wearable tracker to understand the experiential qualities of how
they relate self-tracking and activity data to their disease to
explore the question: How do the embodied experiences and
self-care practices of dealing with a specific health condition
respond to the introduction of activity trackers?
Background and Significance: Experiences with
Self-Monitoring in Health Care and Leisure Contexts
The existing literature of experiences with self-tracking
comprises two related fields: (1) rich literature on how patients,
as part of their prescribed treatment, engage in and make sense
of clinically validated data related to self-care for chronic
illnesses and (2) emerging literature that primarily explores
users’ experiences with leisurely oriented self-tracking
technologies that are outside of the health care system. Patients’
experiences with consumer activity-tracking technologies
designed for use outside the clinical context have received scant
academic attention. There is an important research gap in
understanding the relation between the rich human-information
interaction and the contexts of activity tracking such as self-care
[1,10]. Consequently, studies that explore the experiences of
people coping with illnesses — while recognizing the specific
ramifications that self-tracking might have for those who have
severe health problems — are necessary.
Self-Care and Chronic Illness in eHealth
Many patients routinely engage with data from digital devices
that are part of the prescribed treatment. These clinically
integrated data-producing devices affect self-care activities as
they enable managing symptoms, taking medicine, dealing with
the emotional impact, and tackling lifestyle changes [11-13].
Fostering self-care has been a central ambition of much telecare
and electronic health (eHealth) development during the last 20
years. Early telecare technologies were typically designed from
a clinical standpoint with measures to support remote decision
making such as blood glucose tracking in diabetes [14,15],
oxygen saturation, pulse rate and respiration rate tracking in
chronic obstructive pulmonary disease [16], and heart
arrhythmia detection through remote monitoring of cardiac
implantable electronic devices such as pacemakers and ICDs
[17]. In recent years, a more participatory agenda frames eHealth
innovation, aiming to enhance independence and enable patients
to become more active participants in managing their own
disorder (eg, through use of wearable activity trackers) [18,19].
While much research has focused on measurable outcomes of
digital self-tracking and self-management on clinical parameters,
emergent studies have also explored the experiential qualities
of patients’ “data work” [20-22]. Central to such data work,
along with the experiential qualities of patients engaging with
self-tracking data in chronic care management, are the affective
aspects. It is known that self-monitoring of blood glucose data
by patients with diabetes and their caretakers is tightly bound
to an emotional struggle ranging between control and freedom,
peace of mind and anxiety, and empowerment and the burden
of managing technology [23]. Similarly, it is found that
self-tracking data in fertility self-monitoring promotes the
achievement of certain positive goals but may accentuate
negative emotions such as feeling burdened or abandoned [24].
Others have studied patient experiences in heart arrhythmia
telemonitoring and found that not having access to data or
feedback from clinicians has an emotional and life-changing
impact, which in turn creates doubt, guilt, and concern [25].
Self-Tracking and Activity Data
While clinically validated self-monitoring technologies have
become the standard in chronic care, the rise of low-cost sensors
has accelerated the consumer market, making wearable activity
trackers and mobile data-logging applications a widespread
commodity. Large corporations like FitBit and Apple are
entering the medical domain with consumer wearable devices,
most prominent through automated activity measures like step
count [1,26] to support disease monitoring and rehabilitation
of cardiac, pulmonary, and cancer patients [8,27-29], among
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 2https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

others, and most recently, through large-scale interventions
using the Apple Watch for screening of atrial fibrillation.
Literature on the so-called Quantified Self over the past decade
has explored users’ (ie, self-trackers) experiences with these
technologies and the data they produce when applied voluntarily
for leisure or wellness activities [30-34]. Lomborg and Frandsen
[35] showed that, while self-tracking is often depicted as an
entirely individual endeavor to retrieve calculable, reliable
knowledge, it is experienced by users as a deeply communicative
activity, with an often playful and pleasurable quality to it.
In their exploration of self-tracking cycling, Lupton et al [36]
presented the concept of “data sense” to describe how people’s
experience with data from sensors is not just a matter of
cognitive “knowing” — as often assumed in data literacy
approaches — but equally involves sensory and affective
dimensions such as alerting cyclists to new bodily sensations
while possibly invoking feelings of frustration or even
embarrassment. Thus, what emerges are accounts of
self-tracking experiences as complex encounters between the
metric and sensuous, between knowing and feeling. This, in
turn, suggests that self-tracking, while often a purposeful and
systematic practice, is not necessarily guided by goals of
improving the self, forming new and healthier habits, or getting
to know oneself better, as suggested by many of the wellness
technologies currently entering the market [37].
The critical question is then: what happens when technologies,
practices, and data from the consumer market for self-tracking
are introduced in the context of chronic self-care? What kinds
of experiences do they offer in a setting where self-tracking is
“pushed” [38] to patient-consumers who live with a chronic
disorder?
Several studies have examined patients’ experiences with
wearable activity trackers, and the focus tends to be on patient
acceptability and feasibility among the elderly [39-41] and in
the chronic care context [1,5-8,28]. Some support positive
outcomes like ease-of-use and willingness among patients to
wear activity trackers and integrating them into clinical care
[5,28]. Rosenberg et al [28] conducted a 3-week study
examining the acceptability of the Fitbit Zip and attitudes
towards integrating fitness tracking into clinical care among
men with prostate cancer. All participants were willing to wear
the device and endorsed its value in ensuring they engaged in
a “minimal amount of activity.” However, several barriers to
use were found, including health-related limitations (like pain
and injuries making it difficult to walk) and practical or technical
problems with syncing devices and experiencing data
inaccuracies (eg, not capturing the activities).
Other studies present negative aspects and challenges with
integrating patient-generated data in clinical settings [6,7]. Zhu
et al [6] found technical challenges (such as security and privacy
issues and the practical work of clinicians transferring
self-tracking data to the electronic medical record), social
challenges (such as health professionals adapting to new forms
of care where patients are collaborative partners), and
organizational challenges (like organizational policies and
workflows that do not include attending to patient-self tracking
data). Ancker et al [7] conducted an interview study to explore
self-tracking among patients with multiple chronic conditions.
They found that patients associated several negative experiences
with self-tracking and self-tracking data can negatively influence
the patient-clinician relationship owing to a lack of trust in the
data. For these patients, self-tracking thus became burdensome,
which contrasts the pleasurable and playful experiences
promoted in wellness self-tracking.
Objective
The objective of this study was to understand how patients with
chronic heart disease, as opposed to healthy individuals,
experience activity data from consumer self-tracking devices
when engaging in self-care. With this study, we contribute to
the emergent literature on how patients’ experiences with
consumer wearable activity trackers are related to their illness
and their self-care activities and the implications that arise for
design and deployment of these devices. There is a need for
going beyond acceptability and feasibility studies and
conducting more fine-grained analysis of the experiential
qualities of interacting with personal health data outside the
context of clinical practice among the increasing number of
people with chronic illnesses.
Methods
Overview
We conducted a qualitative study to understand how patients
with an ICD experience self-tracking of activity data in relation
to their embodied condition and daily practices of dealing with
a chronic heart condition. As we know from the self-tracking
literature, such experiences may change over time [33]. To grasp
this, we followed patients over a 49-week period from January
2018 to December 2018, during which we observed their activity
tracking data and interviewed them repeatedly (2-3 times each)
about their experiences and insights gleaned from the data.
Setting
This study was part of a larger research and development project,
SCAUT (Self-, Collaborative- and AUTo-detection of signs
and symptoms of deterioration), 2014–2018, which aimed to
improve early detection of deterioration and communication
among patients with a cardiac device and health professionals.
The overall project was carried out at a cardiac device clinic at
the Rigshospital, University of Copenhagen, Denmark, which
is one of the largest cardiac device remote monitoring centers
in Europe, following more than 3500 patients.
Recruitment of Participants
The study comprised a sample of 27 patients with chronic heart
disease who had a secondary prevention ICD and were already
part of an (R&D project). Secondary prevention ICDs were
offered to individuals who survived sudden cardiac arrest or
had a history of dangerous and recurrent abnormal heart
rhythms, which is relevant for this study due to the chronicity
of the disease and related self-care activities. While patients
were similar in having an ICD, their underlying cardiac
diagnosis, possible comorbidities, and psychosocial situation
differed substantially. Participants were recruited through a mix
of purposive sampling and self-signup to ensure that patients
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 3https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

were interested and not too ill to participate. Of the 65 ICD
patients we invited to participate, 27 ICD patients provided
written informed consent to this study, which explored the
experiences of activity data as related to being an ICD patient
and the data’s potential for predictive analytics of dangerous
arrhythmias. Of the 27 patients, 25 patients were male (93%),
and 2 patients were female (7%); the average age was 57 years.
The sample largely reflected the demographic profile of ICD
patients in Denmark in 2017, when 18% of patients were female
and the majority of procedures were carried out on patients aged
55-74 years [42].
The participants were provided with and instructed to wear a
Fitbit Alta HR (Fitbit, San Francisco, CA), which is a wristband
activity tracker that can record and visualize heart rate, sleep,
and steps onscreen and in a Fitbit mobile app. They were
informed that wearing the activity tracker was unrelated to their
treatment at the clinic and that our purpose was to explore how
they experience the relationship between activity data and their
heart disease.
Data Collection: Semi-Structured Interviews
Data were collected with 66 semistructured interviews in 3
overall iterations using 3 interview guides. The first iteration
aimed to create a baseline of patients’ expectations concerning
activity-illness relationships and get a sense of their embodied
experience of everyday living with an ICD. The second iteration
aimed to understand the initial 2-week experiences with activity
tracking using concrete examples from their own tracking data
and asking them what they had learned or wondered about when
using the Fitbit and looking at the data. The third iteration aimed
to understand the longer-term experiences and data-sensing
practices and any ambivalences arising from using the Fitbit
(4–49 weeks).
Patients were interviewed individually (sometimes with
relatives) in their homes or in locations convenient to them (eg,
workplace or hospital office space). Each interview lasted
between 25 minutes and 1 hour and 40 minutes. During
interviews, the interviewers took field notes and pictures of
participants showing concrete examples of activity data in the
Fitbit mobile app to support the analysis of the data. All
interviews were audio-recorded and transcribed either in full or
in selected passages.
Data Analysis
The interview transcripts were hand-coded iteratively, following
an abductive reasoning logic [43,44], starting with a joint
analysis workshop after the second interview with all 27 patients
to identify emergent themes regarding data sensing and insights
from the data to be followed up in the final interviews. At this
stage, field notes and pictures from the interviews offered an
interpretive aid, offering contextual guidance for those of us
who had not been present at the interview. Upon the final round
of interviews, another joint workshop solidified and elaborated
the initial insights and ideas against the body of related work,
to develop a joint analytical framework and coding protocol.
This framework combined dimensions of data sensing —
knowing, feeling, and evaluating the self through data with
contextual embedding and experiences of illness in daily chronic
living [45] — to clarify how patients with an ICD make sense
of Fitbit data relating to their heart disease. Finally, we recoded
the complete empirical material manually according to these
dimensions, first individually and then together, to ensure
reliability in producing a thematically organized analysis of
patients’ data sensing [46].
Study Approval and Ethical Considerations
This paper was based on a substudy of the SCAUT research
and development project, which was approved by the Danish
Data Protection Agency and reviewed by the National Board
of Health and Danish National Committee on Health Research
Ethics (H-19029475). We took several measures to respond to
possible ethical concerns. First, we ensured voluntary
participation through an open invitation with self-signup, and
we emphasized in interviews that participants could opt out at
any time. Second, we communicated with all participants
between interviews to ensure they were comfortable with
wearing the wristband. We made sure that the participants
understood that the Fitbit activity tracker was a consumer device
and not a clinical device and that the data did not have diagnostic
validity. Finally, we adjusted the conversation in interviews
accordingly if patients expressed specific health concerns
brought on by their interaction with the Fitbit; specifically, we
urged them to contact their health professional for guidance.
Results
Device Engagement
Our results showed how patients with an implanted ICD device
engaged with and made sense of activity data from the Fitbit in
the context of chronic illness and self-care. Most (18 of the 27
participants) related their real-time heart rate, sleep, and step
count data directly to their heart disease (Table 1). The
remaining participants, however, connected the data to leisure
activities, wellness, and exercise. Some portrayed themselves
as not being a patient, explaining that they mostly did not have
symptoms. Two patients chose to opt out after only wearing the
tracker a few times owing to finding the wristband annoying to
wear or simply losing interest (P6, P7). Patients used the activity
tracker for an average of 26.1 weeks and took breaks from using
it an average of 5.9 weeks.
The patients who did relate the data directly to their illness did
so in 3 overall dimensions (Table 2): as something that generated
new knowledge, as something that raised affective responses,
and as something that could be used to evaluate themselves and
their overall health. Within these 3 dimensions of experience,
patients accounted for a range of positive, negative, and
ambivalent experiences with activity data. For an extended
analysis of the affective dimension and the consequences for
patients’ interpretation of Fitbit data, see [47].
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 4https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Table 1. Overview of participating patients with chronic heart disease and an implantable cardioverter-defibrillator (n=27).
Experienced Fitbit da-
ta relating to heart
disease
Number of weeks
using the Fitbit
(not using)
Symptoms experienced
Year of ICD
a
implant
SexAge
(years)
Patient
number
No18 (0)
No symptom experiences; experienced palpitations
before1998Male67P1
Yes47.5 (0.5)Severe chest pain and shortness of breath2015Male61P2
No41 (6)
No symptom experiences; experienced shortness of
breath before2009Male41P3
Yes13 (5)Dizziness and sometimes fainting2014Male55P4
Yes8 (23)Dizziness and sometimes fainting2010Male66P5
N/A
b
9.5 (1.5)No symptom experiences2015Male67P6
N/A6 (3)No symptom experiences2008Male28P7
Yes36.5 (11)No symptom experiences (primary prophylaxis)2015Male69P8
Yes33,5 (14.5)No symptoms experiences related to his heart disease;
lung disease; difficulties exercising
2008Male47P9
Yes30 (8)Sometimes feeling very tired2010Male61P10
Yes8.5 (0.5)Shortness of breath and sometimes sleep problems;
finds it difficult to feel his heart rate
2006Male59P11
Yes49 (0)Dizziness and sometimes fainting; anxious about get-
ting a shock and experiences depression
2015Male66P12
Yes44.5 (0.5)No symptom experiences; rarely experience fainting;
leg tenderness and muscle fatigue
2017Male67P13
No49 (0)No symptom experiences2008Female52P14
Yes14 (1)No symptom experiences; sometimes being anxious
about the ICD/irregular heartbeats
2004Female61P15
No35.5 (11.5)Dizziness and sometimes fainting2014Male47P16
Yes35 (7)Dizziness and shortness of breath during high activity
levels
2013Male45P17
Yes18 (29)No symptom experiences2009Male67P18
No20.5 (1.5)Palpitations daily; experiences periods of depression
and has restless leg syndrome
2005Male66P19
Yes39.5 (8.5)No symptom experiences, except sometimes shortness
of breath
2014Male69P20
Yes13.5 (0)No symptom experiences; worries daily about having
a cardiac arrest again
2008Male38P21
Yes19.5 (0.5)Shortness of breath and sometimes dizziness when
exercising and running; rapid heartbeats and chest pain
2001Male59P22
Yes47 (2)No symptom experiences2017Male49P23
Yes42.5 (6)No symptom experiences2017Male74P24
No9 (15)No symptom experiences2014Male51P25
No4 (0)No symptom experiences; sometimes feels palpitations
and shortness of breath
2010Male56P26
Yes12 (5)Shortness of breath; sometimes sleep problems; knee-
pain due to osteoarthritis
2014Male58P27
a
ICD: implantable cardioverter-defibrillator.
b
N/A: not available because the patient did not describe a relation between the activity data and his or her heart disease.
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 5https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
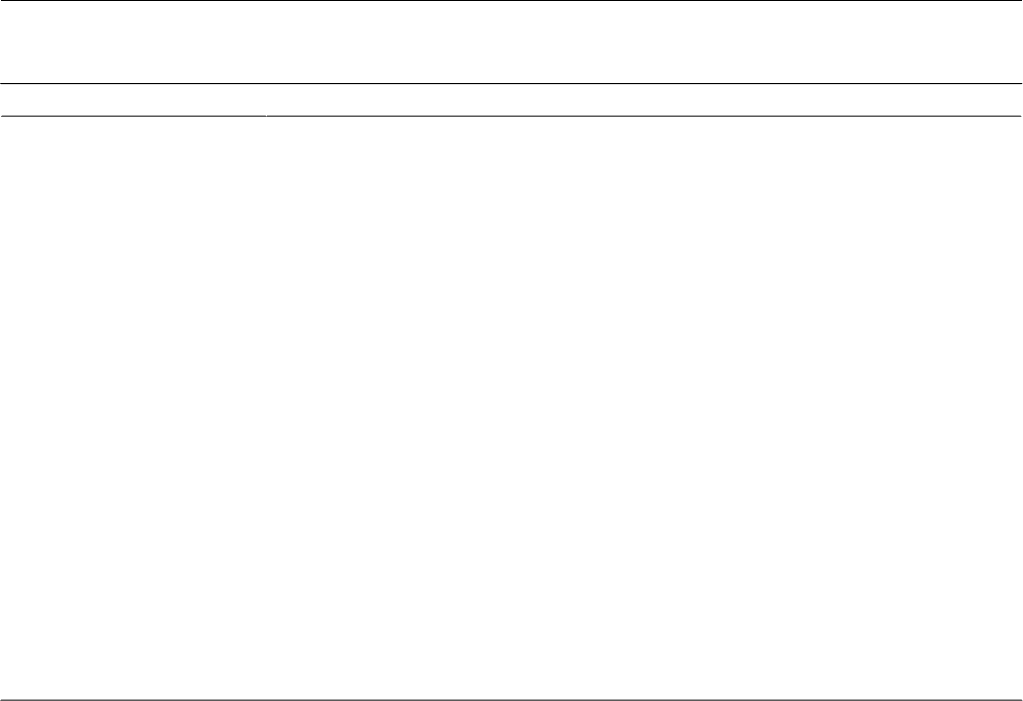
Table 2. Dimensions of how patients experienced activity tracking related to their disease.
ExperienceExperiential dimension
Knowing
Learning that heart disease increases one’s average resting heart rate (P2, P4)Positive: gaining insight
Learning that medication influences the heart rate (P5, P22, P23, P27)
Learning that activity improves one’s average heart rate (P4, P21)
Using activity data to monitor heart pumping ability (P10)
No new learnings: Sensing is more useful than activity data (P1, P5, P16, P22)Negative: evoking doubts
Doubting heart rate data (P2, P22)
When doubt becomes mistrust (P12)
Feeling
Feeling safe through Fitbit reassurance (P11, P12, P17)Positive: being reassured
Reassurance prompts activity (P24)
Both insights and doubts can introduce new anxieties (P12, P13, P15, P23)Negative: becoming anxious
Evaluating
Being nudged and getting praise (P19, P20, P23, P24)Positive: promoting improvement
Recognizing a nudge but not knowing what to do about it (P13)Negative: exposing failure
Not getting the proper reward: the invisibility of “good” activities (P18)
Self-disappointment with poor numbers (P8, P17, P24)
Ignoring or resisting nudges (P18, P19)
Knowing: Gaining Insight and Evoking Doubts
As part of their motivation for participating in the study, many
participants expressed an interest in knowing more about their
health and body and about the possible relationships between
their daily activities and their heart. In our interviews, it became
clear that patients actively sought knowledge about their heart
and health through the readings on the Fitbit and that some
gained insight from the data. For others, the knowledge they
gained from sensing their own bodies was more useful than the
Fitbit data. Finally, some participants experienced that the data
did not align with their activities and sensory experiences; thus,
they doubted the accuracy and trustworthiness of the data.
Learning That Heart Disease Increases One’s Average
Resting Heart Rate
One patient gained insight into his average resting heart rate
and discovered it was higher than he expected:
My resting heart rate ought to be 60, but it is 80, and
as soon as I start moving, it goes up to 100 or 120.
[P2]
It did not concern him since there is not much the clinicians can
do about it, as he said. Another patient had the same kind of
insight:
Then, the pulse is in a zone where I am physically
active. That is what it signals. But I am not physically
active. I am just walking. That has been an
eye-opening experience. [P4]
Learning That Medication Influences the Heart Rate
Several patients knew, speculated, or learned about how
medication can affect the heart rate. By looking at the Fitbit
data, P5 noticed that his average heart rate decreased when on
vacation, and he considered that his medication might have an
effect. P27 also noticed that his medication influenced his heart
rate:
Obviously, the heart rate follows how active you are;
but, for healthy people, it’s not abnormal that it
reaches 116. But I get 13 pills in the morning and 3
in the afternoon.
P23 also noticed that medication influences his heart rate:
My beta blockers have been reduced in dose, which
may also have something to do with the changes in
the resting heart rate.
P22 had always been exercising; however, after an event a few
years ago, he was prescribed a double dosage of beta-blockers,
and it was decided to reduce the dosage again because his heart
rate had dropped too low. Now, he finds it exciting to use Fitbit
to learn about the relationship between medication, exercise,
and heart rate and to confirm his heart rate data:
I still monitor what happens. This morning I saw an
increase in my average heart rate of 12% and my
maximum heart rate of just over 15%. And that's
what’s fun and what I use it for because it has
annoyed me extremely that I couldn't run like I used
to.
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 6https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Learning That Activity Improves One’s Average Heart
Rate
P21 noticed that there is a relationship between exercising and
average heart rate:
After serious ice hockey training, the average heart
rate is higher on the day after, and then it drops in
the weekend.
Similarly, P4 discovered that when he is more active, his average
heart rate drops:
During the 20 days I have been wearing the Fitbit, I
have seen a drop in my heart rate by 10 heartbeats
per minute. I think that’s crazy. I have been more
active, yes, and I have been walking more. I have set
exercise goals.
He experienced this as a positive thing:
I think it’s fine that my resting heart rate goes down
because, if it goes down, the heart does not have to
work as hard. It makes a difference whether my heart
rate is 70 or 60 beats per minute when resting.
Using Activity Data to Monitor Heart Pumping Ability
Several patients had experience with other activity trackers.
One patient (P10) described how he used the data to monitor
his heart condition. He was diagnosed with heart failure, and
his heart had a reduced pumping ability, “Down to around half
of what is normal,” he said. He used the Fitbit to address his
concern that his heart rate will drop even lower:
I use it to keep an eye on my condition. When I go
spinning, I do the same intervals, and I can therefore
see if I have burned the same calories in that hour.
So, I can say, okay, I'm fairly stable, or I can see if it
has gone down. Because then it might be my heart
getting worse in its ability to pump.
No New Learnings: Sensing is More Useful Than
Activity Data
Several patients had learned over the years to sense
developments in their heart condition. One patient explained
that, in the past, when he got rapid and dangerous heartbeats
and the ICD began treatment with antitachycardia pacing to
terminate the arrhythmia, he sat down and waited until it passed.
He used to call the Heart Centre when he felt the symptoms to
get confirmation about the episodes. For him, the Fitbit did not
generate any new insights apart from what he already knew
from sensing and listening to his body when exercising in the
gym:
I know I cannot do physically very demanding
exercises. I have come to terms with that. So, I have
not received any new extra information via Fitbit.
[P1]
P22 found it useful to use the activity tracker to get confirmation
on fast heartbeats, for example, when running in the woods.
However, he trusts his senses more:
If there is a connection between what I feel in my body
and what the tracker shows, then I react. But when
the tracker shows something that I don’t notice in my
body, I consider it an IT error.
Another patient explained that he has tried to look at the Fitbit
data when he gets sudden dizziness; however, it provides no
explanation:
Sometimes I suddenly experience a “dive,” and then
I just have to hold on to something. It’s very different
how often it happens. But when I look at [the Fitbit
activity data], it does not show anything. So, I can’t
find the reason for getting so dizzy. [P5]
Similarly, P16 explained that there is no connection between
symptoms and heart rate:
There is no connection. I've tried to get symptoms
when doing gymnastics where I had the pulse all the
way up, and I've tried to get symptoms while I was
sleeping. I have not found any connection at all.
Doubting Heart Rate Data
Heart rate data also created doubt among some participants.
One patient doubted that the heart rate data presented on his
Fitbit were accurate when walking:
But I have seen sometimes when out for a walk, my
heart rate is really high. Average 166—I think that
is a little high. And, sometimes, I have seen it going
up to 197 beats, and I don’t know why. [P2]
Another patient found the heart rate data “weird” at times. He
experienced chest pain a few times when he was out running;
however, no answers could be found in the activity data:
I felt a little uncomfortable — one might suddenly
think of a blood clot. But I can't see in any of the
trackers that the pulse has been particularly high or
particularly low. [P22]
At other times, when he was just walking, there were sudden
peaks in the heart rate data:
It seems strange. It's just right there — a peak up. It
almost seems like a mistake that it goes from 80 to
almost 170. It seems completely messed up what
happens here. [P22]
Several other patients experienced that their heart rate showed
unusual fluctuations on Fitbit.
When Doubt Becomes Mistrust
One patient had disease-related anxiety and had difficulties
sleeping. Initially, the Fitbit data concerning his sleep revealed
that he slept more than he thought he did when he woke in the
morning after a difficult night. However, when he later saw that
the Fitbit had registered “sleep” while he was calmly watching
a movie, he and his wife started distrusting the measurements
of sleep altogether:
This Saturday, we looked at sleep, for instance, and
we could not make the numbers fit because he had
been awake a lot. The data was wrong, and we do not
know how it works. Then you go on to think, can you
trust this device at all? [wife of P12]
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 7https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Across participants, the sleep tracking measures of Fitbit were
noted as unreliable and not reflective of actual sleep. For some
participants, discovering that sleep data were inaccurate led to
mistrust and a general, critical understanding of the
measurements provided by Fitbit.
Feeling: Being Reassured and Becoming Anxious
Being diagnosed with a heart condition often introduces
profound anxiety into patients’ (and their relatives’) lives. It is
well-known that patients with an ICD are at an increased risk
of being diagnosed with depression and anxiety [48]. In our
interviews, some patients had different levels of anxiety and
used the Fitbit to reassure themselves that their heart was doing
okay. For some, this helped them engage more in physical
activity — something they might have held back on owing to
fear of provoking an attack (ie, kinesiophobia) [49]. However,
the Fitbit — partly owing to the doubts and uncertainties
introduced as described — could also spark new and additional
anxieties and negative feelings regarding participants’ health.
Feeling Safe Through Fitbit Reassurance
For some patients, having a heart condition raised their
embodied attention, making them very alert to bodily signs:
As soon as there is even a little thing in these zones
[in his chest region], and I would even say just one,
like a sprain, I get nervous. [P12]
This patient, who is very affected by anxiety and depression,
used the Fitbit to reassure himself that he is not having a cardiac
arrest:
Then, I was out chopping firewood. I felt like it began
to beat both in a weird way, and it felt like it beat
really fast, but it did not. There was nothing. There
was nothing to be seen [on the Fitbit] anyway. [P12]
For this patient, seeing his heart rate within the normal spectrum
reassured and calmed him down:
Now I get certainty. Is something wrong or not.
Another patient, who experienced getting a shock from his ICD
after walking the stairs, explained that:
Being able to see my heart rate is normal creates a
sense of security because I’m not able to feel when
my heart rate rises. A normal rhythm means that there
is nothing to be afraid of — no danger is underway.
[P11]
Similarly, P17 used the heart rate data as support in vulnerable
situations:
I've tried it so many times, to have those VTs [rapid
heartbeat] and I know what it leads to, and that's
what I fear.
One time he was lying down, and he used the heart rate on the
Fitbit tracker to get confirmation on the duration of rapid
heartbeats:
If it lasted more than 5 minutes, I would have called
112 [emergency services]. Because, sometimes, I think
when I get that feeling of fast heartbeats, it may well
be imagination.
Reassurance Prompts Activity
Holding back during physical activity was something several
of the informants touched upon, as some had had a heart attack
while exercising or had experienced an ICD shock when
climbing a flight of stairs. Checking their heart rate while doing
more physically demanding tasks and sports motivated them to
increase their activity:
I’m not afraid to have a high pulse when we do the
Bikefit exercise at gymnastics … because I can see it
goes down again [his heart rate]. [P24]
Both Insights and Doubts Can Introduce New Anxieties
In the first section, we touched upon some of the doubts
concerning the validity of the Fitbit data. For the anxious patient
in need of reassurance, this uncertainty can be stressful, as noted
by the wife of P12. P15 described it as follows:
There are plenty of worries when you have a heart
disease. You don’t need unnecessary things that make
you worry more.
The Fitbit sleep data, for example, created unwanted attention
to what it meant for her health:
What does it mean for my health? Am I sleeping
enough or too little, and what can I do about it? Such
concerns arise, which I could do well without.
She also experienced getting a high pulse that was “completely
unprovoked” and then seeing it on the Fitbit:
But I can't do anything about it, and I can't use it for
anything—unless they can see it in the clinic.
One patient noticed that Fitbit wants him to sleep 8 hours per
night — a goal he rarely reaches:
I have always had this sleep pattern, and it did not
bother me until I got this Fitbit, which says I should
sleep 8 hours a night … I get worried and start to
question whether I ought to sleep more. [P13]
Finally, the introduction of new concerns and anxieties by Fitbit
goes beyond patients’ individual experiences. P23 explained
that he tried to avoid drawing attention to his Fitbit wristband
when being around his teenage kids:
I think it reminds them a little bit of something bad
… it’s interpreted negatively—like someone has to
keep an eye on me.
Evaluating: Promoting Improvement and Exposing
Failure
As we presented in the previous sections, patients used Fitbit’s
numerical representations to make sense of their bodily
sensations in the context of self-care. The fact that the Fitbit
device allows them to see — as numbers, icons, and graphics
— something that they usually relate to as sensations provides
a new form of motivation. Setting targets for activity, getting
notifications, and seeing achievements represented visually can
be encouraging. Concurrently, however, the Fitbit also exposes
unmet goals, thereby inducing self-disappointment or even
shame. The Fitbit does not register or “see” all the activity that
the patients found to be relevant as fair representations.
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 8https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Being Nudged and Getting Praise
Several of the patients talked about how the Fitbit nudged them
to stay physically active — it is “a kick in the butt,” as one
patient noted (P19). Users can set their activity goals as they
please; however, most went with the default setting of 10,000
steps a day. All patients looked daily to see if they had reached
that goal, and some found it motivating to see the numbers and
get positive feedback (visually provided in the form of stars)
from the user interface. One patient described himself laughingly
as:
Addicted to it because it says you have to walk 10,000
steps a day, and that fits with some of our walks. It
becomes a sport; it gets me going. [P20]
Others noted how the gamification element invoked in the Fitbit
led to small changes in their daily activities. For P23, Fitbit
prompted him to take the stairs instead of the elevator to get
more steps. It also prompted him to get up when it beeped every
hour to take 250 steps by walking up and down the hall during
work breaks. For these patients, Fitbit is “a little push in the
right direction” (P23) and “an inspiration to continue” (P24).
Recognizing a Nudge But Not Knowing What to Do
About It
Whereas simple nudging features such as awarding stars for
accomplishing specific activity benchmarks seemed to motivate
participants, there were also examples of participants becoming
unsure of what constitutes appropriate activity. For example,
Fitbit (by default) beeps once every hour during the day to pace
the wearer to walk 250 steps every hour. For P13, this nudge
suggested that average but regular activity throughout the day
might be preferable to his usual practice of lumping activity
together for more intense periods of exercise. It made him
wonder if he should organize his workout differently, even if
this wondering did not lead him to make any actual change.
Not Getting the Proper Reward: The Invisibility of
“Good” Activities
After wearing the Fitbit for some time and having acquainted
themselves with the collected activity data, some participants
reported being frustrated that the Fitbit did not really measure
all their activity. They did not feel “seen” by the device and
rewarded properly for their efforts. This is particularly the case
for those participants who did cycling, CrossFit, and other
activities beyond walking and running as part of their everyday
life activities. One participant lamented:
It is actually misleading because most of my activity
is on a bike, and it does not register this. But it is also
exercise. [P18]
These experiences added to the doubt in the data and the
accuracy of measurements described previously.
Self-Disappointment With Poor Numbers
If positive feedback is seen as motivating further activity
tracking, conversely, the negative feedback from Fitbit made
some participants feel disappointed with or ashamed of
themselves because it highlighted that they had not been active
enough:
Well, I guess it has to do with that bad conscience
you get the next day, if you cheat. [P8]
This was mentioned by several patients, and, for some, it made
the Fitbit less attractive. P24 talked of self-disappointment when
receiving negative feedback from Fitbit and noted that there
might be someone else looking at their data, surveilling whether
they reached their goals:
Yes, because it gossips all the time, noting that I have
not walked far enough … I think you could always
find an excuse for not walking; like, it’s raining.
For P17, the low step count created negative emotions on “bad
days” and became linked to his heart condition:
It gives me a little guilty conscience that I do not get
much exercise because, I have no doubt, the more
weight I gain, the more fat is generated around my
heart, and the harder it is for the heart to pump.
Ignoring or Resisting Nudges
Some participants tempered their engagement with Fitbit by
actively resisting to do what the device suggests:
I know that it tells me, every once in a while, that it
is time to go for a walk. But I decide when it is time
to go for a walk. And then it says, let’s go, and I’m
like no way because I don’t have the time right now.
[P18]
For some, the Fitbit’s nudging was simply a source of
annoyance:
It can be a little irritating watching all the green
“pling pling.” I don’t want that; I don’t care about
it. I just want the info. [P19]
Yet, for others, such as P18, resisting the nudge to walk every
hour was followed by a deeper reflection about whether activity
tracking is good at all for his health:
I actually think it is a little unhealthy to measure
oneself all the time. It comes to take up a lot, in my
life, and I don’t think it is that important.
Discussion
Principal Findings
Our study contributes to understanding how patients with
chronic heart disease, as opposed to healthy individuals,
experience activity data from consumer self-tracking devices
in self-care. We found that patients with an implanted ICD relate
the activity data to their illness experience and their self-care
activities in 3 overall dimensions: as something that generated
new or destabilized existing knowledge, as something that raised
affective responses, and as something that could be used to
evaluate themselves and their overall health.
The distribution of patients’ experiences on a continuum from
positive to negative suggests that activity data had “dual effects,”
which means that the data created as much as it solved the
problem of chronic illness [50]. The problems that people with
chronic illness have to deal with become mediated in new ways
and what may count as “normal,” “good,” “problematic,” or
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 9https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

“bad” may change accordingly. Positive and negative effects
potentially co-exist and support the experiential ambivalence
that studies of self-tracking and activity data have also found
among leisurely users and quantified self-enthusiasts
[22,45,51,52]. The concept of “ambivalence” unites this stream
of research in which patients’ attitudes towards digital health
devices “neither are consistently negative (implied by the notion
of ‘rejection’) nor consistently positive (implied by the notion
of ‘acceptance’)” [45]. Conflicting or ambivalent experiences
appear constitutive of self-tracking: “doubt, guilt, fear, shame,
dismay, disappointment, and hesitation as well as joy, relief,
excitement, enthusiasm, and pride” [51].
Generating knowledge from interpreting activity data is often
portrayed as the essence of self-tracking. For healthy individuals,
it may comprise discoveries about physical performance in
everyday life and adopting healthier behavior [33,35]. For the
patients in this study, performance-oriented and fitness-oriented
development of self-knowledge also surfaced. Patients obtained
new insights about how exercising improves their average heart
rate and that their heart disease may be the reason for a higher
resting heart rate.
Other more disease-specific reflections surfaced as using Fitbit
data to monitor the status and development of heart failure (heart
pumping ability) and speculating about how heart medication
affects the pulse. Unusually high heart rate data created doubt
when walking or when connected to chest pain while running.
Therefore, Fitbit data became part of generating a type of lay
and personal expertise for, at best, supporting day-to-day
self-care activities and living with a chronic disease and, at
worst, creating uncertainty. This kind of “experiential
knowledge” or “patient knowledge” [53-55] is often considered
distinct from medical and scientific knowledge in that it is a
by-product of bodily sensing and coping with daily practicalities
of the disease as well as it is re-appropriated medical knowledge
used to contribute, but also dispute, the biomedical perspective
[55].
For patients, Fitbit data can provide support for self-care with
informational cues alongside bodily sensations and experiences
in the development of “know-now” [53] (ie, understanding what
is going on or deciding what action to take). As opposed to
healthy individuals’ knowledge-making with Fitbit, the
unsupported lay interpretation of medically unvalidated heart
rate data poses a risk for patients taking inappropriate action,
for example, using Fitbit heart rate numbers to diagnose a
cardiac arrest when running and deciding whether to keep on
running or when getting chest pain and becoming dizzy in the
office and then using Fitbit to decide what to do. The practical
implications of patient knowledge generation from Fitbit suggest
that patients should not be left alone with interpreting activity
data as part of self-care. Deploying self-tracking and activity
data in chronic care should be carefully accompanied by a
purposeful clinical intervention such as rehabilitation and
training programs where clinical staff can support patients in
interpreting activity data, and data visualization should be
designed to support meaningful action in the context of self-care.
The affective dimension of self-tracking when living with a
chronic heart disease also emerged as loaded with ambivalence.
Fitbit numbers may provide numerical reassurance, which can
relieve acute anxiety related to unclear bodily sensations and
provide confidence to exercise. Concurrently, heightened
attention to Fitbit data can also introduce new uncertainties and
anxieties. Moreover, it is important to underline that the
reassurance of the Fitbit data is not based on clinical evidence
and, while reduction of acute anxiety is important to patients’
wellbeing, there is a risk that the numbers provide pseudoproof
not sufficiently reliable to indicate anything clinically relevant
about the patient’s condition. Given the prevalence of mental
health issues, such as anxiety and depression, related to heart
disease and the lack of mental health services for these patients,
it is vital to consider the potential negative interactions between
health tracking and mental health. Patients with chronic mental
health comorbidities should not be left to try to cope with serious
mental health issues alone with consumer devices.
Taken together, we see a tension between Fitbit’s promotion of
success and exposure of failure to comply with set standard
activity levels in the actual experience of using Fitbit. The
ambivalence of knowing, feeling, and evaluating one's chronic
health condition against activity data from consumer devices,
as opposed to clinically validated instruments, poses a concern
for how engagement with data is placed in chronic care contexts
as well as the purposes inscribed in the design of these new
devices. In their analysis of ambivalence in mobile health for
HIV care, Marent and colleagues [45] argued for the need to
consider how the tension implied with ambivalence is embodied
by particular bodily conditions and embedded in particular
relationships and environments. Our study concerns people
who, in clinical terms, have a chronic condition; however, the
embodiment of this condition varies among participants. For
some, the disease has a continuous and very challenging
presence related to managing and coping with severe symptoms
(see Table 1), while others tell us they do not feel sick at all.
We suggest that the ambivalence of Fitbit data is more
problematic when used by people who have a chronic condition
and even more so for people who feel very challenged by their
disease.
This relates to a second point about embeddedness. The
ambivalence of Fitbit data should be understood in relation to
its embeddedness in everyday contexts unrelated to clinical
contexts of treatment. To what extent do people in these contexts
have support from others such as relatives, peers, or health
professionals who choose to engage in handling the
ambivalences they encounter? What resources can they mobilize
to act when experiencing doubt, anxieties, or other concerns
when self-monitoring with Fitbit? With our paper being
specifically concerned with patients who engaged with Fitbit
data outside the established relationships of health care
institutions, these questions become critical. Navigating benefits
and harms of this form of active engagement with personal
health data is, to a large degree, dependent on individual
circumstances, resources, and networks, leaving inequalities
potentially less mitigated by public health systems. We find
that these are very central insights to take into account in
research that focuses on how self-care practices can be furthered
by harnessing the power of data and personal health technology.
Too often, this literature focuses narrowly on individual
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 10https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

information processing and empowerment while disregarding
the relational and situational embeddedness of chronic disease
management. It not only neglects insights from health
information-seeking literature, which has convincingly shown
that patients’ information behavior is more often based on
serendipity, avoidance, blissful ignorance, indolence,
bewilderment, and indolence than on rational choices and
reflections [56], but also neglects that self-care practices are
always collective in the sense that they are embedded in complex
sociomaterial relationships [57,58]. What our study further adds
to the literature on self-care and technology, then, is that
technology and data mobilized outside the established
arrangements of health care with consumer tracking devices
may introduce new ambivalences that some patients may have
difficulties managing without professional assistance.
For human-computer interaction research and the design of
activity-data visualization for patients, our study aligns with
the understanding that data supports different levels of reflection
and serves multiple purposes [59]. The implications of our study,
we suggest, is that consumer activity-tracking devices deployed
in health care contexts should be designed to also support
collaborative reflection (ie, “co-reflection”) with health
professionals, rather than focusing mainly on individual
reflection [60]. Research on ways to support the shared work
of tracking [61], co-reflection, and “co-care” [62] might be
necessary to consider for personal informatics research in
chronic self-care [25,47,63].
As an increasing number of people are generating and interacting
with digital and individual health data outside the context of
clinical practice, issues of inequality in health must be
considered. Thus, there are important implications to consider
in this typically optimistic, yet blurred, realm of “personal health
data” (actualized for wellness purposes) and “patient-generated
data” (actualized for clinical purposes).
Conclusions
We presented the findings from an explorative intervention
study of how patients with a heart arrhythmia who have an
implanted ICD experience activity data from Fitbit concerning
their self-care and chronic illness. The aim was to further
emergent literature and offer crucial empirical insight into the
introduction of wellness tracking devices to various forms of
chronic care management and the associated user experiences.
Through repeated semistructured interviews with 27 patients
equipped with a Fitbit wristband, we offer support and further
elaboration on existing work on patients’ ambivalent
experiences. We found that wearable activity trackers actualize
patients’ experiences across 3 dimensions on a spectrum: (1)
gaining new knowledge versus evoking doubt, (2) feeling
reassured versus becoming anxious, and (3) evaluating one’s
health by celebrating improvements and exposing failure. The
experiences of individual patients can reside more on one end
of the spectrum, can reside across all 3 dimensions, or can
combine contrasting positions and even move across the
spectrum over time. While activity data from wearable devices
may be a resource for self-care through reassurance and
motivation, they may also constrain patients and create increased
uncertainty, fear, and anxiety.
The ramifications of knowing, feeling, and evaluating one’s
chronic health condition against activity data from consumer
devices, as opposed to clinically validated instruments, are
largely unexplored. Our study suggests that we need critical
attention in scholarship and health care practice concerning how
engagement with such data is practiced in chronic care contexts,
not least to assess how the purposes inscribed in the design of
these new devices may be molded and twisted in self-care when
meeting the logics, needs, and abilities of patients in different
health care circumstances. Designers and health authorities
should consider this complexity and ambiguity when
determining the usefulness of self-tracking data in chronic
illness.
Acknowledgments
The authors wish to thank all the patient participants and their relatives who shared their experiences with us for this study. We
also thank Tanja Munk Warmdahl, Amalie Lund Bendtsen, Oliver Carl Clemmensen, Andreas Millarch, and Anders Riis
Vestergaard for their collaboration in interviewing, transcribing, and analysis workshops. This study is cofunded by the Innovation
Fund Denmark #72-2014-1 and the University of Copenhagen, Vital Beats, and Medtronic. This study was also, in part, supported
by a grant from the Danish Velux Foundations #33295.
Conflicts of Interest
None declared.
References
1. Shin G, Jarrahi MH, Fei Y, Karami A, Gafinowitz N, Byun A, et al. Wearable activity trackers, accuracy, adoption,
acceptance and health impact: A systematic literature review. J Biomed Inform 2019 May;93:103153. [doi:
10.1016/j.jbi.2019.103153] [Medline: 30910623]
2. Piwek L, Ellis DA, Andrews S, Joinson A. The Rise of Consumer Health Wearables: Promises and Barriers. PLoS Med
2016 Feb;13(2):e1001953 [FREE Full text] [doi: 10.1371/journal.pmed.1001953] [Medline: 26836780]
3. Garge GK, Balakrishna C, Datta SK. Consumer Health Care: Current Trends in Consumer Health Monitoring. IEEE
Consumer Electron. Mag 2018 Jan;7(1):38-46. [doi: 10.1109/mce.2017.2743238]
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 11https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
4. Weiner K, Will C. Thinking with care infrastructures: people, devices and the home in home blood pressure monitoring.
Sociol Health Illn 2018 Feb;40(2):270-282. [doi: 10.1111/1467-9566.12590] [Medline: 29464773]
5. Mercer K, Giangregorio L, Schneider E, Chilana P, Li M, Grindrod K. Acceptance of Commercially Available Wearable
Activity Trackers Among Adults Aged Over 50 and With Chronic Illness: A Mixed-Methods Evaluation. JMIR Mhealth
Uhealth 2016 Jan 27;4(1):e7 [FREE Full text] [doi: 10.2196/mhealth.4225] [Medline: 26818775]
6. Zhu H, Colgan J, Reddy M, Choe E. Sharing Patient-Generated Data in Clinical Practices: An Interview Study. 2016
Presented at: AMIA Annual Symposium Proceedings; 2016; Chicago, IL p. 1303-1312.
7. Ancker JS, Witteman HO, Hafeez B, Provencher T, Van de Graaf M, Wei E. "You Get Reminded You're a Sick Person":
Personal Data Tracking and Patients With Multiple Chronic Conditions. J Med Internet Res 2015 Aug 19;17(8):e202 [FREE
Full text] [doi: 10.2196/jmir.4209] [Medline: 26290186]
8. Cook DJ, Thompson JE, Prinsen SK, Dearani JA, Deschamps C. Functional recovery in the elderly after major surgery:
assessment of mobility recovery using wireless technology. Ann Thorac Surg 2013 Sep;96(3):1057-1061. [doi:
10.1016/j.athoracsur.2013.05.092] [Medline: 23992697]
9. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-Scale Assessment of a Smartwatch to
Identify Atrial Fibrillation. N Engl J Med 2019 Nov 14;381(20):1909-1917. [doi: 10.1056/NEJMoa1901183] [Medline:
31722151]
10. Liu YH, Scifleet P, Given LM. Contexts of Information Seeking in Self-tracking and the Design of Lifelogging Systems.
2014 Presented at: MindTheGap@ iConference; 2014; Berlin, Germany p. 44-50.
11. Nunes F, Verdezoto N, Fitzpatrick G, Kyng M, Grönvall E, Storni C. Self-Care Technologies in HCI. ACM Trans.
Comput.-Hum. Interact 2015 Dec 14;22(6):1-45. [doi: 10.1145/2803173]
12. Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions:
a review. Patient Education and Counseling 2002 Oct;48(2):177-187. [doi: 10.1016/S0738-3991(02)00032-0]
13. Schermer M. Telecare and self-management: opportunity to change the paradigm? J Med Ethics 2009 Nov 30;35(11):688-691.
[doi: 10.1136/jme.2009.030973] [Medline: 19880706]
14. Greenwood DA, Young HM, Quinn CC. Telehealth Remote Monitoring Systematic Review: Structured Self-monitoring
of Blood Glucose and Impact on A1C. J Diabetes Sci Technol 2014 Mar 21;8(2):378-389 [FREE Full text] [doi:
10.1177/1932296813519311] [Medline: 24876591]
15. Piras EM, Miele F. Clinical self-tracking and monitoring technologies: negotiations in the ICT-mediated patient–provider
relationship. Health Sociology Review 2016 Aug 05;26(1):38-53. [doi: 10.1080/14461242.2016.1212316]
16. Chau JP, Lee DT, Yu DS, Chow AY, Yu W, Chair S, et al. A feasibility study to investigate the acceptability and potential
effectiveness of a telecare service for older people with chronic obstructive pulmonary disease. Int J Med Inform 2012
Oct;81(10):674-682. [doi: 10.1016/j.ijmedinf.2012.06.003] [Medline: 22789911]
17. Burri H, Senouf D. Remote monitoring and follow-up of pacemakers and implantable cardioverter defibrillators. Europace
2009 Jun 26;11(6):701-709 [FREE Full text] [doi: 10.1093/europace/eup110] [Medline: 19470595]
18. Eysenbach G. Medicine 2.0: social networking, collaboration, participation, apomediation, and openness. J Med Internet
Res 2008 Aug 25;10(3):e22 [FREE Full text] [doi: 10.2196/jmir.1030] [Medline: 18725354]
19. Swan M. Emerging patient-driven health care models: an examination of health social networks, consumer personalized
medicine and quantified self-tracking. Int J Environ Res Public Health 2009 Feb;6(2):492-525 [FREE Full text] [doi:
10.3390/ijerph6020492] [Medline: 19440396]
20. Fiske A, Prainsack B, Buyx A. Data Work: Meaning-Making in the Era of Data-Rich Medicine. J Med Internet Res 2019
Jul 09;21(7):e11672 [FREE Full text] [doi: 10.2196/11672] [Medline: 31290397]
21. Piras EM. Beyond self-tracking: Exploring and unpacking four emerging labels of patient data work. Health Informatics J
2019 Sep;25(3):598-607. [doi: 10.1177/1460458219833121] [Medline: 30848690]
22. Ruckenstein M, Schüll ND. The Datafication of Health. Annu. Rev. Anthropol 2017 Oct 23;46(1):261-278. [doi:
10.1146/annurev-anthro-102116-041244]
23. Kaziunas E, Ackerman M, Lindtner S, Lee J. Caring through Data: Attending to the Social and Emotional Experiences of
Health Datafication. 2017 Presented at: CSCW '17: Proceedings of the 2017 ACM Conference on Computer Supported
Cooperative Work and Social Computing; Feb 2017; Portland, Oregon p. 2260-2272. [doi: 10.1145/2998181.2998303]
24. Figueiredo MC, Caldeira C, Reynolds TL, Victory S, Zheng K, Chen Y. Self-Tracking for Fertility Care: Collaborative
Support for a Highly Personalized Problem. 2017 Dec 06 Presented at: Proceedings of the ACM on Human-Computer
Interaction (CSCW); 2017; Portland, Oregon p. 1-21. [doi: 10.1145/3134671]
25. Andersen TO, Andersen PRD, Kornum AC, Larsen TM. Understanding Patient Experience: A Deployment Study in Cardiac
Remote Monitoring. 2017 May Presented at: the 11th EAI International Conference on Pervasive Computing Technologies
for Healthcare; 2017; Barcelona, Spain p. 221-230. [doi: 10.1145/3154862.3154868]
26. Treacy D, Hassett L, Schurr K, Chagpar S, Paul SS, Sherrington C. Validity of Different Activity Monitors to Count Steps
in an Inpatient Rehabilitation Setting. Phys Ther 2017 May 01;97(5):581-588. [doi: 10.1093/ptj/pzx010] [Medline: 28339904]
27. Benzo R. Activity monitoring in chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev 2009;29(6):341-347
[FREE Full text] [doi: 10.1097/HCR.0b013e3181be7a3c] [Medline: 19940638]
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 12https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
28. Rosenberg D, Kadokura EA, Bouldin ED, Miyawaki CE, Higano CS, Hartzler AL. Acceptability of Fitbit for physical
activity tracking within clinical care among men with prostate cancer. 2016 Presented at: AMIA Annual Symposium
Proceedings; 2016; Chicago, IL p. 1050-1059.
29. Thorup CB, Grønkjær M, Spindler H, Andreasen JJ, Hansen J, Dinesen BI, et al. Pedometer use and self-determined
motivation for walking in a cardiac telerehabilitation program: a qualitative study. BMC Sports Sci Med Rehabil 2016;8:24
[FREE Full text] [doi: 10.1186/s13102-016-0048-7] [Medline: 27547404]
30. Ruckenstein M, Pantzar M. Datafied Life: Techno-Anthropology as a Site for Exploration and Experimentation. Techné:
Research in Philosophy and Technology 2015;19(2):191-210. [doi: 10.5840/techne20159935]
31. Didžiokaitė G, Saukko P, Greiffenhagen C. The mundane experience of everyday calorie trackers: Beyond the metaphor
of Quantified Self. New Media & Society 2017 Mar 24;20(4):1470-1487. [doi: 10.1177/1461444817698478]
32. Sharon T, Zandbergen D. From data fetishism to quantifying selves: Self-tracking practices and the other values of data.
New Media & Society 2016 Mar 09;19(11):1695-1709. [doi: 10.1177/1461444816636090]
33. Kristensen DB, Ruckenstein M. Co-evolving with self-tracking technologies. New Media & Society 2018 Feb
21;20(10):3624-3640. [doi: 10.1177/1461444818755650]
34. Lomborg S, Thylstrup NB, Schwartz J. The temporal flows of self-tracking: Checking in, moving on, staying hooked. New
Media & Society 2018 May 31;20(12):4590-4607. [doi: 10.1177/1461444818778542]
35. Lomborg S, Frandsen K. Self-tracking as communication. Information, Communication & Society 2015 Aug
03;19(7):1015-1027. [doi: 10.1080/1369118X.2015.1067710]
36. Lupton D, Pink S, LaBond C, Sumartojo S. Digital Traces in Context: Personal Data Contexts, Data Sense, and Self-Tracking
Cycling. International Journal of Communication 2018;12:647-665.
37. Schüll ND. Data for life: Wearable technology and the design of self-care. BioSocieties 2016 Oct 13;11(3):317-333. [doi:
10.1057/biosoc.2015.47]
38. Lupton D. Self-Tracking Modes: Reflexive Self-Monitoring and Data Practices. SSRN Journal 2014 Aug:2483549 [FREE
Full text] [doi: 10.2139/ssrn.2483549]
39. Ehn M, Eriksson LC, Åkerberg N, Johansson A. Activity Monitors as Support for Older Persons' Physical Activity in Daily
Life: Qualitative Study of the Users' Experiences. JMIR Mhealth Uhealth 2018 Feb 01;6(2):e34 [FREE Full text] [doi:
10.2196/mhealth.8345] [Medline: 29391342]
40. Puri A, Kim B, Nguyen O, Stolee P, Tung J, Lee J. User Acceptance of Wrist-Worn Activity Trackers Among
Community-Dwelling Older Adults: Mixed Method Study. JMIR Mhealth Uhealth 2017 Nov 15;5(11):e173 [FREE Full
text] [doi: 10.2196/mhealth.8211] [Medline: 29141837]
41. Lyons EJ, Swartz MC, Lewis ZH, Martinez E, Jennings K. Feasibility and Acceptability of a Wearable Technology Physical
Activity Intervention With Telephone Counseling for Mid-Aged and Older Adults: A Randomized Controlled Pilot Trial.
JMIR Mhealth Uhealth 2017 Mar 06;5(3):e28 [FREE Full text] [doi: 10.2196/mhealth.6967] [Medline: 28264796]
42. Hjerteforeningen (Danish Heart Association). ICD (Implantable Cardioverter Defibrillator). HjerteTal. 2018. URL: https:/
/hjerteforeningen.dk/alt-om-dit-hjerte/hjertetal/hjertetaldk [accessed 2020-03-20]
43. Timmermans S, Tavory I. Theory Construction in Qualitative Research. Sociological Theory 2012 Sep 10;30(3):167-186.
[doi: 10.1177/0735275112457914]
44. Blaikie N. Abduction. In: Lewis-Beck MS, Bryman A, Liao TF, editors. The SAGE Encyclopedia of Social Science Research
Methods. Thousand Oaks, CA: SAGE Publications; 2004.
45. Marent B, Henwood F, Darking M, EmERGE Consortium. Ambivalence in digital health: Co-designing an mHealth platform
for HIV care. Soc Sci Med 2018 Oct;215:133-141. [doi: 10.1016/j.socscimed.2018.09.003] [Medline: 30232053]
46. Boyatzis RE. Transforming qualitative information: Thematic analysis and code development. Thousand Oaks, CA: SAGE
Publications; 1998:1-169.
47. Lomborg S, Langstrup H, Andersen TO. Interpretation as luxury: Heart patients living with data doubt, hope, and anxiety.
Big Data & Society 2020 May 08;7(1):205395172092443-205395172092413. [doi: 10.1177/2053951720924436]
48. Pedersen SS, von Känel R, Tully PJ, Denollet J. Psychosocial perspectives in cardiovascular disease. Eur J Prev Cardiol
2017 Jun 16;24(3_suppl):108-115. [doi: 10.1177/2047487317703827] [Medline: 28618908]
49. Hoffmann JM, Hellwig S, Brandenburg VM, Spaderna H. Measuring Fear of Physical Activity in Patients with Heart
Failure. Int.J. Behav. Med 2017 Dec 11;25(3):294-303. [doi: 10.1007/s12529-017-9704-x]
50. Lehoux P. The duality of health technology in chronic illness: how designers envision our future. Chronic Illn 2008
Jun;4(2):85-97. [doi: 10.1177/1742395308092475] [Medline: 18583444]
51. Salmela T, Valtonen A, Lupton D. The Affective Circle of Harassment and Enchantment: Reflections on the ŌURA Ring
as an Intimate Research Device. Qualitative Inquiry 2018 Sep 22;25(3):260-270. [doi: 10.1177/1077800418801376]
52. Fotopoulou A, O’Riordan K. Training to self-care: fitness tracking, biopedagogy and the healthy consumer. Health Sociology
Review 2016 Jun 02;26(1):54-68. [doi: 10.1080/14461242.2016.1184582]
53. Pols J. Knowing Patients: Turning Patient Knowledge into Science.. Science, Technology, & Human Values 2013 Sep
13;39(1):73-97. [doi: 10.1177/0162243913504306]
54. Hartzler A, Pratt W. Managing the personal side of health: how patient expertise differs from the expertise of clinicians. J
Med Internet Res 2011 Aug;13(3):e62 [FREE Full text] [doi: 10.2196/jmir.1728] [Medline: 21846635]
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 13https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

55. Storni C. Patients' lay expertise in chronic self-care: a case study in type 1 diabetes. Health Expect 2013 Aug
28;18(5):1439-1450. [doi: 10.1111/hex.12124]
56. Johnson DJ. Health-related information seeking: Is it worth it? Information Processing & Management 2014
Sep;50(5):708-717 [FREE Full text] [doi: 10.1016/j.ipm.2014.06.001]
57. Pols J. Care at a Distance. On the Closeness of Technology. Amsterdam: Amsterdam University Press; 2012:1-163.
58. Mol A. The logic of care: Health and the problem of patient choice. London and New York: Routledge; 2008:1-95.
59. Fleck R, Fitzpatrick G. Reflecting on reflection: framing a design landscape. 2010 Presented at: OZCHI '10: Proceedings
of the 22th conference of the computer-human interaction special interest group of Australia on Computer-human interaction;
Nov 22, 2010; Brisbane Australia p. 216-223. [doi: 10.1145/1952222.1952269]
60. Choe EK, Lee B. Toward supporting personalized tracking experiences in healthcare. Interactions 2019 Dec 26;27(1):84-87.
[doi: 10.1145/3371287]
61. Vizer LM, Eschler J, Koo BM, Ralston J, Pratt W, Munson S. "It's Not Just Technology, It's People": Constructing a
Conceptual Model of Shared Health Informatics for Tracking in Chronic Illness Management. J Med Internet Res 2019
Apr 29;21(4):e10830 [FREE Full text] [doi: 10.2196/10830] [Medline: 31033452]
62. von Thiele Schwarz U. Co-care: Producing better health outcome through interactions between patients, care providers and
information and communication technology. Health Serv Manage Res 2016 Apr 04;29(1-2):10-15. [doi:
10.1177/0951484816637746]
63. Nunes F, Fitzpatrick G. Self-Care Technologies and Collaboration. International Journal of Human-Computer Interaction
2015 Jul 30;31(12):869-881. [doi: 10.1080/10447318.2015.1067498]
Abbreviations
eHealth: electronic health
ICD: implantable cardioverter-defibrillator
Edited by G Eysenbach, S Nelson; submitted 15.08.19; peer-reviewed by YH Liu, B Dinesen, D Amiri, GE Iyawa; comments to author
24.01.20; revised version received 20.03.20; accepted 14.05.20; published 20.07.20
Please cite as:
Andersen TO, Langstrup H, Lomborg S
Experiences With Wearable Activity Data During Self-Care by Chronic Heart Patients: Qualitative Study
J Med Internet Res 2020;22(7):e15873
URL: https://www.jmir.org/2020/7/e15873
doi: 10.2196/15873
PMID: 32706663
©Tariq Osman Andersen, Henriette Langstrup, Stine Lomborg. Originally published in the Journal of Medical Internet Research
(http://www.jmir.org), 20.07.2020. This is an open-access article distributed under the terms of the Creative Commons Attribution
License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete
bibliographic information, a link to the original publication on http://www.jmir.org/, as well as this copyright and license information
must be included.
J Med Internet Res 2020 | vol. 22 | iss. 7 | e15873 | p. 14https://www.jmir.org/2020/7/e15873
(page number not for citation purposes)
Andersen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
