1
INTRODUCTION 2
Promoting an Ethical Industry 2
Key Legislation 3
Aims and Principles of the Code 4
Interpreting the Code 6
Administering the Code 6
Implementation and Transition Period 7
Transition Period to phase out direct support for HCP
attendance at Third Party Organised Educational Events
and for HCP speakers at satellite symposia 7
PART 1: Guidelines on the Interactions with
Healthcare Professionals and Healthcare
Organisations 8
Chapter 1: General Criteria for Events 9
1. Event Programme 9
2. Event Location and Venue 10
3. Guests 11
4. Reasonable Hospitality 11
5. Travel 12
6. Transparency 13
Chapter 2: Third Party Organised Educational Events 14
1. Third Party Organised Educational Conferences 14
2. Third Party Organised Procedure Training 16
3. Transition Period: Support of Individual Healthcare
Professionals to Third Party Organised
Educational Events 17
Chapter 3: Company Events 18
1. General Principles 18
2. Product and Procedure Training and
Education Events 19
3. Sales, Promotional and Other Business Meetings 22
Chapter 4: Grants and Charitable Donations 23
1. General Principles 23
2. Charitable Donations 25
3. Educational Grants 27
4. Research Grants 31
Chapter 5: Sponsored Posts 32
Chapter 6: Arrangements with Consultants 33
1. General Principles 33
2. Criteria for Genuine Consulting Arrangements 34
3. Remuneration and Fair Market Value 35
4. Disclosure and Transparency 35
Chapter 7: Research 36
1. Member Company-Initiated Research 36
2. Member Company Post-Market Product Evaluation 37
3. Third Party-Initiated Research 38
Chapter 8: Royalties 39
Chapter 9: Educational Items and Gifts 41
Chapter 10: Demonstration Products and Samples 43
1. General Principles 43
2. Demonstration Products (Demos) 44
3. Samples 45
PART 2: Disclosure Guidelines 46
Preamble 47
Chapter 1: Applicability of these Guidelines 48
Chapter 2: Disclosure Obligation 50
Chapter 3: Form of Disclosure 51
PART 3: Guidelines on Advertisements and
Promotions addressed solely or primarily to
Healthcare Professionals 53
Preamble 54
Guidelines 55
1. Scope of Guidelines 55
2. Accuracy and Substantiation of Claims
and Information 55
3. Comparative Advertising 57
4. Requests for Substantiating Data 57
5. Use of Published Data to Support Advertising 58
6. No Disparagement 58
7. Non-Referreed Articles 58
8. Off-Label Use 59
9. Quotations 59
10. Material Commissioned by the Advertiser 59
11. Testimonials and Endorsements 59
12. Reimbursement of Expenses for Providers of
Testimonials or Endorsements 60
13. Effect of Background Collaboration or
Sponsorship on Testimonials and Endorsements 61
14. Annex 1: General Advertising Law and Codes 62
PART 4: Complaints Principles, Procedure & Panel
Constitution 63
Introduction 64
Dispute Resolution Principles 65
Structure & Responsibilities 65
Complaints Procedure 66
General Provisions 66
PART 5: Glossary and Definitions 70
PART 6: Annexes 75
Annex I: CVS scope: When are CVS assessments
required? 76
Annex II: Disclosure Guidelines Template Example 77
Annex III: MedTech Europe Geographical Area 78
Annex IV: Verification Of The Use Of Funds 79
Annex V: Example of Disclosure Guidelines
Methodology Note 80
Annex VI: Direct support to HCP Participation
in Events as of 1st January 2019 81
CONTENTS

2
CODE
PROMOTING AN ETHICAL INDUSTRY
ABHI is the industry
association for the health
technology sector in the UK.
INTRODUCTION

3
The Association of British HealthTech Industries (ABHI) supports the health technology
community to provide products and services that help people live healthier lives.
With over 275 members, ABHI’s work is focused on showing the value of healthtech
and encouraging a healthy environment for economic growth. ABHI help Members
understand healthtech regulation and our work is underpinned by our Code of Ethical
Business Practice, which all Members adhere to.
The Code sets out the minimum standards appropriate to the various types of activities
carried out by the Members. The Code is not intended to supplant or supersede national
laws or regulations or professional codes (including company codes) that may impose
more stringent requirements upon Members and all Members should independently
ascertain that their activities comply with all current national and local laws, regulations
and professional codes.
Furthermore, Member Companies must be mindful of the fact that they may be
liable for the activities of third party intermediaries who interact with Healthcare
Professionals or Healthcare Organisations in connection with the sale, promotion or
other activity involving Member Companies’ products. Accordingly, it is recommended
that where such arrangements are entered into, the relevant contractual documentation
impose obligations upon the third party (for example, third party sales & marketing
intermediaries (SMIs), consultants, distributors, sales agents, marketing agents,
brokers, commissionaire commercial agents and independent sales representatives)
to comply with provisions set out in the Code or equivalent guidelines
1
.
KEY LEGISLATION
The health technology industry in the UK and Europe, in common with other
industries, is subject to national and supranational laws which govern many aspects
of their business operations. ABHI underlines compliance with the following laws and
regulations as having particular relevance to the health technology industry:
• Safety, Quality and Performance Laws;
•
Advertising and Promotion Laws;
•
Data Protection Laws;
•
Anti-corruption Laws;
•
Environmental Health and Safety Laws;
•
Competition Laws.
National and European Union (EU) competition legislation applies not only to Members
in their business operations, but also to ABHI, each of the association’s working groups
and any sub-group within the association, irrespective of size and name. Liability under
competition laws may be strict and a Member may become liable for the infringement
of such laws by other Members of an association group in which it participates.
Accordingly, Members must make every effort to observe UK and EU competition laws
in all their interactions.
1
. For further details, please refer to the Eucomed/AdvaMed Third Party SMIs guidance

4
CODE QUESTIONS AND ANSWERS
AIMS AND PRINCIPLES
OF THE CODE
The interaction between Members and Healthcare
Professionals and Healthcare Organisations is an important
feature in achieving ABHI’s mission to make safe, innovative
and reliable technology and related services available to
more people. For example:
• Advancement of
Health Technologies
The development of innovative medical devices,
technologies and in vitro diagnostics and the
improvement of existing products require collaboration
between Member Companies and Healthcare
Professionals and Healthcare Organisations. Innovation
and creativity are essential to the development and
evolution of health technologies and/or related services.
•
Safe and Effective Use
of Health Technology
The safe and effective use of health technology and
related services requires Member Companies to offer
Healthcare Professionals and Healthcare Organisations
appropriate instruction, education, training, service and
technical support.
•
Research and Education
Member Companies’ support of bona fide medical
research and education, serves to enhance Healthcare
Professionals’ clinical skills and thereby contribute to
patient safety and increase access to new technologies
and/or related services.
In each such interaction Member Companies must continue
to respect the obligation of Healthcare Professionals to
make independent decisions regarding treatment and
safeguard the environment in which the interaction takes
place to ensure the integrity of the industry. To achieve
this aim, the Code provides guidance on the interactions
of Member Companies with both Healthcare Professionals
and Healthcare Organisations, based upon the following
underlying principles:
Q1: Does the definition of Healthcare Professional
include purchasing professionals employed in the retail
sector, such as a purchasing professional employed by a
supermarket chain?
A1: No, the definition of Healthcare Professional does not include
a purchasing professional employed in the retail sector unless
that individual purchaser arranges for the purchase of Member
Companies’ medical devices for or on behalf of medical or clinical
personnel. For example, if a Member Company’s medical devices
are sold as part of the common merchandise of the retail outlet,
interactions between the Member Company and the purchasing pro-
fessional do not fall under the Code. However, where the Member
Company’s medical devices are sold in a retail pharmacy (even if
this is located within a supermarket unit), interactions between the
Member Company and the responsible purchasing professional will
fall under the Code.

5
CODE QUESTIONS AND ANSWERS
•
The Principle of Image
and Perception
Member Companies should, at all times, consider the
image and perception of the health technology industry
that will be projected to the public when interacting with
Healthcare Professionals and Healthcare Organisations.
•
The Principle of Separation
Interaction between industry and Healthcare
Professionals / Healthcare Organisations must not
be misused to influence through undue or improper
advantages, purchasing decisions, nor should such
interaction be contingent upon sales transactions or use
or recommendation of Member Companies’ products.
•
The Principle of Transparency
Interaction between industry and Healthcare
Professionals / Healthcare Organisations must be
transparent and comply with national and local laws,
regulations or professional codes of conduct. In
countries where specific provision is not made, Member
Companies shall nevertheless maintain appropriate
transparency by requiring prior written notification to the
hospital administration, the Healthcare Professional’s
superior or other locally-designated competent authority,
fully disclosing the purpose and scope of the interaction.
•
The Principle of Equivalence
Where Healthcare Professionals are engaged by a
Member Company to perform a service for or on behalf
of a Member Company, the remuneration paid by the
Member Company must be commensurate with, and
represent a fair market value for, the services performed
by the Healthcare Professional.
Q2: Must a Member Company require Employer
Notification to be given whenever Company personnel
meet HCPs at an HCO? (added in May 2017)
A2: No. Unless the Member Company’s interaction with an HCP
entails a transfer of value or raises a potential conflict of interest
there is no requirement for Employer Notification. However, Member
Companies must comply with any access requirements imposed by
HCOs to visiting Member Company personnel.
6
CODE QUESTIONS AND ANSWERS
•
The Principle of Documentation
For interactions between a Member Company and
a Healthcare Professional, such as where services
are performed by a Healthcare Professional for or on
behalf of a Member Company, there must be a written
agreement setting out, inter alia, the purpose of the
interaction, the services to be performed, the method for
reimbursement of expenses as well as the remuneration
to be paid by the Member Company. The activities
envisaged by the agreement must be substantiated
and evidenced by activity reports and the like. Adequate
documentation such as the agreement, related reports,
invoices etc. must be retained by the Member Company
for a reasonable period of time to support the need
for, and materiality of, the services as well as the
reasonableness of the remuneration paid.
INTERPRETING THE CODE
The use of capital letters indicates that a word or expression
is a defined term, the meaning of which is set out in the
Glossary
.
Any phrase introduced by the terms: including, include, in
particular, or any similar expression shall be interpreted
as illustrative and shall not limit the sense of the words
preceding those terms.
ADMINISTERING THE CODE
The Code operates within a procedural framework which
includes procedures designed to provide an effective and
efficient complaint-handling process, to ensure compliance
with the Code. ABHI’s complaints handling system is based
on the principle that disputes are best resolved through
mediation.
The Conference Vetting System
1
is an independently-
managed system which reviews the compliance of Third
Party Organised Educational Events with the Code.
1.
For scope of application of CVS please refer to:
www.ethicalmedtech.eu
Q3: What is the Conference Vetting System (CVS) and,
is CVS approval required for all Third Party Organised
Educational Events before a Member Company can
provide support to these events? (added in May 2017)
A3: The Conference Vetting System (see the Glossary) has been
established as the online, binding and centralised decision-making
process to help Member Companies review the compliance of
relevant Third Party Organised Educational Events with the Code.
It is managed independently of the MedTech Europe Secretariat
and Members and is under the supervision of the MedTech
Europe Compliance Panel. CVS approval is only required for Third
Party Organised Educational Events which fall within its scope, as
provided here. Where there is a CVS decision in relation to a specific
Third Party Organised Educational Event, this decision is binding
upon all Member Companies.
7
CODE QUESTIONS AND ANSWERS
IMPLEMENTATION AND
TRANSITION PERIOD
This edition of the Code comes into force as follows:
• PART 4: The Complaints Procedure & Panel
Constitution shall enter into force on 1 January 2017;
and
• The balance of the Code i.e. Introduction, PART 1 and
PART 2, shall enter into force on 1 January 2018.
For the avoidance of doubt, during the transposition period
1 January 2017 to 31 December 2017, no material or activity
will be regarded as being in breach of the Code if it fails to
comply with its provisions only because of requirements
which this edition of the Code newly introduces.
TRANSITION PERIOD TO PHASE
OUT DIRECT SUPPORT FOR HCP
ATTENDANCE AT THIRD PARTY
ORGANISED EDUCATIONAL EVENTS
AND FOR HCP SPEAKERS
AT SATELLITE SYMPOSIA
After the end of the Transition Period (see the Glossary)
on 31 December 2018, Member Companies shall no
longer provide financial or in-kind support directly to
individual Healthcare Professionals to cover costs of their
attendance at Third Party Organised Educational Events
with the exception of Third Party Organised Procedure
Training meetings or pursuant to a consulting agreement
with a Healthcare Professional speaker engaged by a
Member Company to speak at a satellite symposium. This
means that support of individual Healthcare Professionals
to attend Third Party Organised Educational Events (as
provided for at Chapter 2, Section 3) shall no longer be
permitted under the Code.
After the Transition Period, Member Companies may
provide financial or in-kind support to Third Party Organised
Educational Events only through Educational Grants or
other types of funding in accordance with the rules of
Chapter 2: Third Party Organised Educational Events and
Chapter 4: Grants and Charitable Donations.
Q4: What is the difference between the Transposition
period and the Transition Period as defined in the
Glossary? (added in May 2017)
A4: Transposition means the process of incorporating the Code
within the Member Company’s own policy and procedures. This
process must be completed by 1 January 2018.
Transition Period means the period between 1 January 2017 and
31 December 2018 by the end of which Member Companies must
have ceased all financial or in-kind direct support to Healthcare
Professionals to attend Third Party Organised Educational
Conferences. Any exceptions to this rule are outlined in the Code

GUIDELINES ON THE INTERACTIONS
WITH HEALTHCARE PROFESSIONALS
AND HEALTHCARE ORGANISATIONS
PART 1

9
CODE QUESTIONS AND ANSWERS
GENERAL
CRITERIA
FOR EVENTS
1
Member Companies may invite Healthcare Professionals
to Company Events and Third Party Organised
Educational Events. The principles and criteria set out in
this Chapter 1 shall apply to all such Events supported
in any way by Member Companies, irrespective of who
organises the Event.
1. EVENT PROGRAMME
The Event programme should directly relate to the specialty
and/or medical practice of the Healthcare Professionals
who will attend the Event or be sufficiently relevant to justify
the attendance of the Healthcare Professionals. For Third
Party Organised Educational Events, the agenda should be
under the sole control and responsibility of the third party
organiser.
A Member Company shall not organise Events which
include social, sporting and/or leisure activities or other
forms of Entertainment, nor support such elements where
Q5: What is meant by “legitimate” or “genuine” as used
in the definitions of ‘Company Event’ and ‘Third Party
Organised Educational Conferences’?
A5: Any Event should be relevant to the Healthcare Professional
attendees; the detailed programme should be available sufficient
time prior to the Event; present a clear schedule with no gaps
during the sessions, (e.g., the minimum duration for a full day
Event should be 6 hours or 3 hours for a half day Event including
refreshment breaks). If it is a Third Party Organised Educational
Event the Faculty must be identified. It is also important that
all supporting materials (e.g. flyers, brochures and website)
are consistent with the scientific or promotional nature of the
programme content, as the case may be.
CODE QUESTIONS AND ANSWERS

10
CODE QUESTIONS AND ANSWERS
part of Third Party Organised Educational Events. For
Third Party Organised Educational Events, Entertainment
must be outside of the educational programme schedule
and paid for separately by the Healthcare Professionals.
Entertainment should not dominate or interfere with the
overall scientific content of the programme and must
be held during times that do not overlap with a scientific
session. The Entertainment should not be the main
attraction of the Third Party Organised Educational Event.
2. EVENT LOCATION AND VENUE
The Event location and venue should not become the main
attraction of the Event. For the location and the venue,
Member Companies must take into account at all times the
following considerations:
a. Potential adverse public perceptions of the location and
venue for the Event. The perceived image of the location
and venue must not be luxury, or tourist/holiday-oriented,
or that of an Entertainment venue.
b.
The Event location and venue should be centrally located
when regard is given to the place of residence of the
majority of invited participants.
c.
The need for ease of access for attendees.
d.
The Event location and venue should be in or near a city or
town which is a recognised scientific or business centre,
suitable for hosting an Event which is conducive to the
exchange of ideas and the transmission of knowledge.
e.
Member Companies must take into account the season
during which the Event is held. The selected time of year
must not be associated with a touristic season for the
selected geographic location.
Q6: Can a Member Company organise or support an
Event at a hotel or resort that offers significant leisure
facilities such as golf, casino or ski/water sports?
(Amended in July 2018)
A6: In principle, no. It is not appropriate for a Member Company to
organise or support Events at hotels or resorts renowned for their
entertainment facilities or centred around recreational or sporting
activities such as golf, private beach or ski/water sports.
Exceptions might be considered for venues well adapted to
business meetings in an otherwise compliant geographic location
where there is a compelling need to use the chosen venue, for
example, a lack of alternative venues or genuine safety or security
issues. In certain circumstances, hotel accommodation separate
from the Third-Party Organised Event venue might be required for
compliance.
Q7: Under the Code, what is meant by “ease of access”
in relation to Event location and venue?
A7:When originating location of the majority of attendees is
considered, Event location and venue need to be in close proximity
to an airport and / or train station with appropriate connections,
with associated reliable ground transportation infrastructure to
the venue.
Q8: Under the Code, how does the “season” impact
evaluation of Event location? (Amended in July 2018)
A8: Even assuming a location or venue meets all other applicable
requirements under the Code, geographic locations renowned
primarily as seasonal vacation or holiday destinations (for example,
ski-, island-, or beach resorts) are still not appropriate locations
during the season in question. For this purpose, in Europe, the ski
season is considered to run from 20th December to 31st March
and the summer season from 1st June to 15th September.
Equivalent, seasonally adjusted dates apply in other regions of the
world. Member Companies must not support or organise Events
at these locations if they take place during those seasons, even if
only in part.
11
CODE QUESTIONS AND ANSWERS
3. GUESTS
Member Companies are not permitted to facilitate or pay
for meals, travel, accommodation or other expenses for
Guests of Healthcare Professionals, or for any other person
who does not have a bona fide professional interest in the
information being shared at the Event.
4. REASONABLE HOSPITALITY
Member Companies may provide reasonable hospitality to
Healthcare Professionals in the context of Company Events
and Third Party Organised Educational Events but any
hospitality offered must be subordinate in time and focus
to the Event purpose. Member Companies must in any
event meet the requirements governing hospitality in the
country where the Healthcare Professional carries on their
profession and give due consideration to the requirements
in the country where the Event is being hosted
1
.
The Code seeks to find a balance between the courteous
and professional treatment of Healthcare Professionals
by Member Companies, with the desire to avoid
even the appearance that hospitality may be used by
Member Companies as a means to induce Healthcare
Professionals to purchase, prescribe or recommend
Member Companies’ products.
Q9: What does the term “facilitate” mean where used in
connection with the Guest expenses?
A9: The term “facilitate” refers to the prior arrangement,
organisation or booking of meals, travel or accommodation
by or on behalf of a Member Company on behalf of the Guest
of a Healthcare Professional participant. Such organisation
or booking is not permitted unless the individual qualifies as a
participant in his/her own right, irrespective of who pays. Such
actions are open to misinterpretation. If Healthcare Professionals
attending the Event wish to be accompanied by a Guest who does
not have a professional interest in the information being shared,
the Healthcare Professional must take sole responsibility for the
payment and organisation of the Guest’s expenses.
Q10: In the event that a Healthcare Professional is
accompanied by a Guest at the Event, may this Guest be
admitted to any Company Event, or Third Party Organised
Educational Events?
A10: It is not appropriate for a Guest of a Healthcare Professional to
attend either Company Events (including Satellite Symposia) or Third
Party Organised Educational Events (unless the individual qualifies as
a participant in their own right), nor is it appropriate, in the interest of
maintaining the scientific exchange, for a Guest to participate in related
hospitality during such Events (for example, lunches and coffee breaks)
even when the Healthcare Professional pays for the Guest’s expenses.
Member Companies, however, may financially support Third
Party Organised Educational Events which offer extra-curricular
programmes/activities beyond the scientific, educational or training
sessions for Guests of Healthcare Professionals (such as touristic
activities and hospitality), always provided that such an extra -curricular
programme/activity (including attendance of the conference dinner or
a cocktail reception) is subject to a separate charge which must not
be paid for, facilitated or reimbursed by, a Member Company.
Q11: Is it acceptable to offer a cash advance by way
of a cheque or bank transfer payable to a Healthcare
Professional for a specific amount to cover all or part of
the Healthcare Professionals’ travel or accommodation
expenses for attendance at the Event?
A11: It is not acceptable to make an advance payment to a
Healthcare Professional to cover prospective expenses. Payments
should generally be made to the supplier/vendor or intermediary
agency. Alternatively Member Companies may reimburse
individual Healthcare Professional expenses retrospectively
against original invoices or receipts.
1.
In the UK, the NHS England document Managing Conflicts of Interest in the NHS
(Publications Gateway Reference: 06419) sets the upper limit for meals and refresh-
ments that may be accepted by NHS HCPs at £75. While this only directly applies to
the NHS in England it should be taken as indicative of what is acceptable in the rest
of the UK, including in relation to HCPs working in the non-NHS sector. Different lim-
its may apply in other territories and Member Companies should comply with these
as appropriate. Where no such limits apply, the UK figure may be taken as indicative
of what is acceptable under the Code although in exceptional circumstances local
considerations may allow the £75 limit to be exceeded.
12
CODE QUESTIONS AND ANSWERS
Accordingly, Member Companies must assess what
is “reasonable” in any given situation and regional
variations will apply. As a general guideline, “reasonable”
should be interpreted as the appropriate standard for the
given location and must comply with the national laws,
regulations and professional codes of conduct. The term
“hospitality” includes meals and accommodation and it is
important that Member Companies differentiate between
“hospitality” which is permitted and Entertainment which
is not. Please refer to the Glossary for the definition of
Entertainment.
Member Companies may not pay for or reimburse
Healthcare Professionals’ lodging expenses at top category
or luxury hotels. For the avoidance of doubt, if the Event
venue is a hotel which complies with the requirements of
the Code, it would be acceptable for Member Companies to
offer participants meals and accommodation at the same
hotel. However, accommodation and/or other services
provided to Healthcare Professionals should not cover a
period of stay beyond the official duration of the Event.
5. TRAVEL
Member Companies may only pay or reimburse for
reasonable and actual travel. Travel provided to Healthcare
Professionals should not cover a period of stay beyond the
official duration of the Event.
For air travel, in principle, this means that Member
Companies can only pay or reimburse economy or standard
class unless the flight time is of a duration of greater than 5
hours including connection flights, in which case business
class can be considered. First class is never appropriate.
Q12: May Member Companies offer to cover the
travel and accommodation expenses of Healthcare
Professionals for periods that extend beyond the
duration of the Event programme attended?
A12: Generally, travel and accommodation support offered
by Member Companies to Healthcare Professionals should be
tailored to the duration of the Event. Member Companies must
always keep in mind the impression which may be created by the
arrangements for any meeting.
13
CODE QUESTIONS AND ANSWERS
6. TRANSPARENCY
Member Companies must ensure full compliance with
national laws with regard to the disclosure or approval
requirements associated with such financial support
and where no such requirements are prescribed, shall
nevertheless maintain appropriate transparency, as a
minimum, by requiring Employer Notification (as defined in
the Glossary) is made prior to the Event.
.
Q13: Under the Code, is Employer Notification required
for each interaction with a Member Company? For
example, is such notification required each time a
Member Company pays for a reasonably priced meal or
gives a Healthcare Professional a gift, which is otherwise
in line with the requirements of the Code?
A13: Employer Notification is required whenever a Member
Company engages a Healthcare Professional or whenever
a member makes a financial contribution to the Healthcare
Professional’s medical education. Incidental interactions arising
in the normal course of business such as meals associated with
educational or business meetings or the receipt of modest gifts
related to the Healthcare Professional’s practice, do not require
Employer Notification.
Q14: Are members required to provide additional
written notification under the Code to the hospital
administration, Healthcare Professional’s superior (or
other locally-designated body) for Member Company/
Healthcare Professional interactions in countries where
there are compulsory notification systems already in
place?
A14: No. Only the compulsory notification is required. Additional
notification under the Code is not required in countries where
specific notification requirements of law or regulation govern the
transparency of interactions between industry and Healthcare
Professionals. The transparency provisions of the Code apply only
in countries where there is an absence of national transparency
laws and regulations.
Q15: When making Employer Notification, are Member
Companies required to provide details of the proposed
financial contribution Member Companies will make to
the Healthcare Professional in exchange for the services
rendered?
A15: The written notification must comply with national laws,
regulations and professional codes of conduct. In countries
where specific provision is not made, there is no requirement
to notify employers of the amounts involved. Under the Code,
Member Companies must ensure that the level of remuneration
is commensurate with the services provided and not greater
than a fair market value. However, the purpose of the Employer
Notification is to provide transparency on the nature of the
interaction between the Member Company and the Healthcare
Professional and to enable the employer to raise objections if they
perceive a potential conflict or have other issues concerning the
interaction.

14
CODE QUESTIONS AND ANSWERS
Member Companies may provide financial and/or in-kind
support (e.g. Member Company products) to Third Party
Organised Educational Events in accordance with the rules
of this Code. Such Events include:
•
Third Party Organised Educational Conferences;
•
and Third Party Organised Procedure Training meetings.
1. THIRD PARTY ORGANISED
EDUCATIONAL CONFERENCES
Member Companies may support in cash and/or in-kind
Third Party Organised Educational Conferences (see the
Glossary) which comply with:
•
Chapter 1: General Criteria for Events
;
•
and where applicable, has approval via the
Conference
Vetting System
1
(see the
Glossary
).
Q16: What is meant by “in-kind support” as used in
Chapter 2, Section 1 of the Code in connection with
“Third Party Organised Educational Conferences”?
(Amended in July 2018)
A16: “In-kind support” can be provided to the Healthcare
Organisation (HCO) and Member Companies should ensure that
any such in-kind support does not, nor is perceived to, circumvent
the prohibition of Member Companies providing direct financial
support to identifiable Healthcare Professionals to attend Third
Party Organised Educational Conferences. For example, after
the Transition Period, it would not be appropriate for Member
Companies to directly handle the conference registration, travel,
or accommodation arrangements for individual (and identifiable)
HCP delegates at a Third Party Organised Educational Conference.
Examples of “in-kind support” which Member Companies may
provide could include modest secretarial and/or logistical support
to assist with meeting arrangements.
1.
For scope of application of CVS please refer to:
www.ethicalmedtech.eu
THIRD PARTY
ORGANISED
EDUCATIONAL
EVENTS
2
CODE QUESTIONS AND ANSWERS

15
CODE QUESTIONS AND ANSWERS
Where permitted under national laws, regulations and
professional codes of conduct, Member Companies may
provide financial and/or in-kind support to Third Party
Organised Educational Conferences (always provided that
the Third Party Organised Educational Conference has
been approved via the Conference Vetting System, where
appropriate) through grants and other types of funding,
such as:
a. Educational Grants
Please refer to
Chapter 4: Grants and Charitable Donations
for guidance on Educational Grants.
b. Promotional Activity
Member Companies may purchase packages that may
include promotional and advertising services, for example,
advertisement space and booth space for company
displays. Member Companies should ensure that the
overall image projected by the promotional activity at Third
Party Organised Educational Conferences is perceived as
professional at all times. It should never bring discredit
upon or reduce confidence in the health technology
industry.
c. Satellite Symposia
Member Companies may purchase satellite symposia
packages at Third Party Organised Educational
Conferences and provide presentations on subjects that
are consistent with the overall content of the Third Party
Organised Educational Conference. Member Companies
may determine the content of these satellite symposia and
be responsible for speaker selection.
Q17: Please provide examples of appropriate booth
activities which will be perceived as professional?
A17: Booth activities at Third Party Organised Educational
Conferences should aim primarily at displaying Member
Companies’ products and services and related literature.
Therefore, other activities should be limited and reasonable and
in principle, only soft drinks and snacks should be served.
Q18: Can a Member Company, for example, be present
via a satellite symposium or rent booth space at a Third
Party Organised Educational Conference which was
assessed as non-compliant by the Conference Vetting
System (CVS)? (Amended in May 2017)
A18: Please refer to Annex I for a detailed visualisation of the
scope CVS and its impact on commercial activities.
Q19: Can Member Companies directly support
attendance by Healthcare Professionals engaged to
speak only at satellite symposia at Third Party Organised
Educational Conferences, e.g. registration fee, travel
and/or accommodation? (Amended in July 2018)
A19: Member Companies must ensure all aspects of the
arrangement comply with the Code, including entering into a
consulting agreement with Healthcare Professionals engaged
to speak at satellite symposia. The consulting agreements
may provide for payments to be made in respect of travel and/
or accommodation for the purpose of delivering the speaker
services. Where payment of a registration fee is required in order
for speakers to access satellite symposia, Member Companies
may also pay for the registration fee.

16
CODE QUESTIONS AND ANSWERS
2. THIRD PARTY ORGANISED
PROCEDURE TRAINING
Member Companies may support Third Party Organised
Procedure Training either via Educational Grants (in
accordance with
Chapter 4: Grants and Charitable Donations
)
or by providing financial support directly to individual
Healthcare Professionals to cover the cost of attendance
at Third Party Organised Procedure Training sessions in
accordance with the following rules:
a.
Financial support must comply with the criteria provided
in
Chapter 1: General Criteria for Events
. Member
Companies may therefore pay travel, hospitality and the
registration fee.
b.
Where applicable, the Third Party Organised Procedure
Training has approval via the Conference Vetting System
(see the
Glossary
)
1
.
c.
For financial support to Third Party Organised Procedure
Training meetings Member Companies must apply the
requirements governing conduct and attendance at
such meetings in the country where the Healthcare
Professional carries on their profession and give due
consideration to the requirements in the country where
the meeting is being hosted.
Q20: What are the main differences between Third Party
Organised Educational Conferences and Procedure
Trainings? (added in May 2017)
A20: Both Third-Party Organised Educational Conferences (see the
Glossary) and Third-Party Procedure Trainings (see the Glossary)
are a type of Third Party Organised Educational Events. Therefore,
they must comply with Chapter 1. General Criteria for Events; and,
where applicable, are subject to the Conference Vetting System (see
the Glossary). However, unlike Third Party Organised Educational
Conferences, Third Party Organised Procedure Training is not
subject to the phase out of direct support for the attendance of
Healthcare Professionals. Nonetheless, for Third Party Organised
Procedure Training the following three criteria shall apply:
• Programme: Unlike Third Party Organised Educational Conferences
which are theoretical in nature, Third Party Organised Procedure
Training consists of practical, hands-on training, generally involving
more than one provider/ manufacturer/sponsor. This must be
evident by the programme of the Event.
The programme, which is often referred to as a “course”, rather than
a conference or seminar, must be focused on acquiring specific
medical skills relevant to certain medical procedures (rather than
products, or health technologies). Examples may include courses
aimed at acquiring or improving the Healthcare Professional’s skills
in minimally invasive surgery; orthopaedic trauma surgery; or the
implantation of cardiac rhythm devices; etc. The programme must
also include practical demonstrations (and/or actual live surgeries,
where allowed). Examples of practical demonstrations may include
surgery simulations where technologies are used on cadavers; skin
models; synthetic bones; cath labs; etc.
• Venue: Third Party Organised Procedure Training is typically
organised in a clinical environment, as opposed to, e.g., a classroom
setting.
For the avoidance of doubt, the adjective “clinical” includes places
suitable for the simulation of medical procedures, rather than just
the medical treatment of real patients.
Examples of clinical environment include hospitals or clinics,
where medical treatment on real patients may be given; as well as
conference rooms which are appropriately set up to simulate medical
procedures, for example with the presence of health technologies to
be used on cadavers; skin models; synthetic bones; etc.
• Stand-alone event: Third Party Organised Procedure Training must
stand alone. Where the majority of the Training is not given in a
clinical environment, for example, where the Training is organised
in connection, adjacent to or at the same time as a larger Third
Party Organised Educational Conferences, that Training will not
qualify as a Third Party Organised Procedure Training, as defined
in the Code.
1.
For scope of application of CVS please refer to:
www.ethicalmedtech.eu
17
CODE QUESTIONS AND ANSWERS
Q21: In the definition of Third Party Organised
Procedure Training, what is meant by “Proctorship”
and “Preceptorship”? Further, do Proctorships and
Preceptorships require CVS approval before they can
be provided and/or supported by a Member Company?
(added in May 2017)
A21: For the purpose of the Code both Proctorship and
Preceptorship are types of clinician-to-clinician trainings funded
by a Member Company.
Proctorship is where the trainee clinician performs a procedure
under the supervision of another clinician and where the trainee
clinician has primary responsibility for the patient undergoing the
procedure.
Preceptorship is where the supervising clinician oversees the
procedural training of the trainee clinician and the trainee does
not have primary responsibility for the patient undergoing the
procedure.
Such Proctorships and Preceptorships normally take place on
Healthcare Organisation premises and are not subject to CVS
approval as it is not considered to be either a Third Party Organised
Educational Event or a Third Party Organised Procedural Training.
3. TRANSITION PERIOD:
SUPPORT OF INDIVIDUAL
HEALTHCARE PROFESSIONALS
TO THIRD PARTY ORGANISED
EDUCATIONAL EVENTS
Member Companies may provide financial support directly
to individual Healthcare Professionals to cover the costs of
attendance at Third Party Organised Educational Events
where this is permitted under national laws, regulations
and professional codes of conduct. Such support shall be
in accordance with the following rules:
a.
Financial support must comply with the criteria provided
in
Chapter 1: General Criteria for Events
. In addition
Member Companies may pay the registration fee.
b.
Where applicable, the Third Party Organised Educational
Event has approval via the Conference Vetting System
1
(see the
Glossary
).
c. For financial support to Third Party Organised
Educational Events Member Companies must apply
the requirements governing conduct and attendance
at such Third Party Organised Educational Event in
the country where the Healthcare Professional carries
on their profession and give due consideration to the
requirements in the country where the meeting is being
hosted.
1.
For scope of application of CVS please refer to:
www.ethicalmedtech.eu

18
CODE QUESTIONS AND ANSWERS
1. GENERAL PRINCIPLES
Member Companies may invite Healthcare Professionals
to Company Events. Such events include, as defined in the
Glossary
:
•
Product and Procedure Training and Education Events
•
Sales, Promotional and Other Business Meetings
Company Events should comply with the principles
mentioned in
Chapter 1: General Criteria for Events
.
Where there is a legitimate business purpose, Company
Events may include or take place in Member Company’s
manufacturing plant or Healthcare Organisations, used by
the Member Company as reference centres.
Q22: Is it appropriate for Member Companies to invite
Healthcare Professionals on company plant or factory
tours where the Healthcare Professionals reside outside
the country of location of the plant or factory?
A22: Yes, it is appropriate for Member Companies to invite
Healthcare Professionals to plant or factory tours in countries
outside their country of residence if there is a legitimate business
purpose and the tour complies with the Code in all respects.
CODE QUESTIONS AND ANSWERS
COMPANY
EVENTS
3

19
CODE QUESTIONS AND ANSWERS
2. PRODUCT AND PROCEDURE
TRAINING AND COMPANY
ORGANISED EDUCATION EVENTS
Where appropriate, in order to facilitate the safe and
effective use of health technologies, therapies and/or
services, Member Companies should make product and
procedure training and education available to relevant
Healthcare Professionals.
Member Companies shall ensure that personnel conducting
the Product and Procedure Training and Education Events
have the appropriate expertise to conduct such training.
Q23: Can Member Companies directly support travel
and/or accommodation or other expenses of individual
Healthcare Professionals for attendance as delegates at
Company Organised Events happening during or around
a Third-Party Organised Educational Event? (Added July
2018)
A23: No, as from 1st January 2019, Member Companies cannot
directly support travel and/or accommodation or other expenses
of individual Healthcare Professionals participating as delegates
in Company Organised Events which take place during, around, or
at the same time and in the same approximate location as a Third-
Party Organised Event.
Company Organised Events, including fee-for-service arrangements
like Advisory Boards and Clinical Investigator meetings, may be
organised at or around a Third-Party Organised Educational Event
for reasons of convenience and efficiency, given the attendance
of Healthcare Professionals at that Third-Party Organised
Educational Event. In order for Member Companies to be able to
directly support travel and/or accommodation or other expenses
at such Company Organised Events the Healthcare Professionals
must have an active role at such a Company Organised Event,
rather than being mere passive attendees. [For example, no
support shall be provided by Member Companies to Healthcare
Professionals attending a Company Organised Educational Event
as a delegate or trainee where this is organised at or around a
Third-Party Organised Educational Event.] Where there is active
participation by the attending Healthcare Professional, the Member
Company may still only pay for the contractual remuneration
and expenses agreed for the provision of the services by the
healthcare Professional at the Company Organised Educational
Event itself. Under no circumstances may a Member Company
pay for incremental costs relating to the Healthcare Professional’s
attendance at the Third-Party Organised Educational Event, such as
registration costs, hospitality, additional travel or accommodation.
Specific examples of support which can and cannot be provide
include the following:
For an advisory board or a clinical investigator meeting organised
at or around a Third-Party Organised Educational Event:
– the registration fee of the Healthcare Professional to the Third-
Party Organised Educational Event shall in no circumstances be
covered by the Member Company as this would not be related to
the services to be provided.
– the flight and accommodation costs can be covered where these
are tailored to the services to be provided for the advisory board
or clinical investigator meeting. However, any possible additional
costs, e.g. arising in consequence of changes to travel plans
must be borne by the Healthcare Professional.
For a satellite symposium or a booth speaker engagement taking
place during the Third-Party Organised Educational Event (i.e. as
part of that Third-Party Organised Educational Event):
– the Healthcare Professional’s registration fee for the Third-
Party Organised Educational Event can be covered but only if
the Healthcare Professional’s access to the satellite symposium

20
CODE QUESTIONS AND ANSWERS
or booth at the Third-Party Organised Educational Event is
conditional upon the payment of the registration fee. Where
this does apply, the registration fee must, where possible, be
pro rata’d to the actual attendance required in order to deliver
the required services. E.g. if the satellite symposium is held on a
single day of the three-day event, and it is possible to choose a
one-day registration, that option should be selected.
– the flight and accommodation costs can only be covered if
the Healthcare Professional is not already benefiting from an
educational grant covering his/her attendance at the event.
The costs shall be tailored to the services to be provide and any
possible additional costs, e.g. arising from changes to travel
plans must be borne by the Healthcare Professional.
Q24: Under the Code, Chapter 3, Point 2, what is meant by
“Company Organised Education Events”? (added in May
2017)
A24: A “Company Organised Education Event” is a Company
Event as defined in the Glossary, whose objective is genuine and
bona fide medical education, and the enhancement of professional
skills. “Educational” or “education” means communicating
information directly concerning or associated with the use of
Member Companies’ health technologies, e.g., information about
disease states and the benefits of health technologies to certain
patient populations. In all cases the information and/ or training
must directly concern a Member Company’s health technologies,
therapies and/or related services.
This means that a Member Company must meet the following tests
when organizing such an Event in order to be compliant with the Code:
a) The entire Event must comply with the criteria of Chapters 1 and 3;
b) The programme must be rigorous from a scientific and/or
educational point of view. This means that its content must
include current scientific information of a nature and quality
which is appropriate to the Healthcare Professionals who are
attendees at the Event.
c) The programme must be genuine and bona fide educational, and
therefore cannot have a primary sales and marketing objective. This
means that the education part must fill most of the programme.
d) Information on the programme, clearly indicating the name
of the Company organising the Event, should be made
available sufficiently in advance in order for invited Healthcare
Professionals to be able to make a reasoned judgment as to
the rigor and quality of the programme, provided however that
subsequent changes, deletions and additions to the programme
are acceptable to the extent that such changes, deletions and
additions are reasonable and do not significantly modify the
quality or nature of the programme.
e) The programme should in principle involve full days, with the
majority of the morning and afternoon parts dedicated to
scientific and/or educational sessions, unless the Event is a half
day event, commences or ends on a midday or lasts less than
half a day. Such half-day or less sessions are permissible, but
there should not be any non-scientific or non-educational events
or activities organised for the other part of the day. Furthermore,

21
CODE QUESTIONS AND ANSWERS
there should be no significant gaps in the programme which
would permit Healthcare Professionals to engage in non-
scientific or non-educational activities. For example, early
morning sessions should not be followed by late afternoon or
evening sessions with large blocks of free time in between.
Q25: Are cruise ships or golf clubs appropriate venues for
Product and Procedure Training and Education Events?
A25: No. Cruise ships, golf clubs or health spas and venues
renowned for their entertainment facilities are not appropriate
venues and should not be used. Appropriate examples include
hospital, clinic or surgical centre laboratory, educational,
conference, or other appropriate settings, including Member
Companies’ own premises or commercially available meeting
facilities, that are conducive to effective transmission of
knowledge and any required “hands on” training.
Q26: What criteria should a Member Company apply
when considering the country location of Product and
Procedure Training and Education Events?
A26: If the participants are primarily of one country, the venue
should be in the specific country involved. If the participants
are from multiple countries in Europe, then a European country
affording ease of access for participants should be chosen. It
is expected that the country selected is the residence of at least
some of the participants of the Product and Procedure Training
and Education Event.
Q27: Can a Member Company use a meeting venue
outside Europe?
A27: Yes, provided the participants are from multiple countries
outside Europe. If the participants are primarily from within
Europe, the venue should be in Europe. It is expected that the
country selected (and the state, if the location is in the United
States) is the residence of at least some of the participants of the
Product and Procedure Training and Education Event.
Q28: Can Member Companies directly support travel and/
or accommodation of individual Healthcare Professionals
at Company Events, which include new product launches,
even if only portable equipment or solutions are being
demonstrated. (Added July 2018)
A28: Member Companies can pay for travel and/or
accommodation of individual Healthcare Professionals to attend
Company events which include product launches provided that
such Events fall within the scope of Chapter 3, Section 2, of the
Code (“Product and Procedure Training and Educational Events”).

22
CODE QUESTIONS AND ANSWERS
3. SALES, PROMOTIONAL AND
OTHER BUSINESS MEETINGS
Where it is appropriate, Member Companies may organise
Sales, Promotional and Other Business Meetings where
the objective is to discuss product and related services
features and benefits, conduct contract negotiations, or
discuss sales terms.
In addition to the principles laid down in the
Chapter 3,
Section 1
, Sales, Promotional and Other Business Meetings
should also comply with the following more stringent
requirements:
a.
Such meetings should, as a general rule, occur at or
close to the Healthcare Professional’s place of business;
b.
It is not appropriate for travel or accommodation
support to be provided to Healthcare Professionals by
Member Companies, except where demonstrations of
non-portable equipment are necessary.

23
CODE QUESTIONS AND ANSWERS
1. GENERAL PRINCIPLES
a. Grants and Charitable Donations (see the
Glossary
)
shall not be contingent in any way on past, present
or potential future purchase, lease, recommendation,
prescription, use, supply or procurement of the Member
Company’s products or services. It is important that
support of charitable and/or philanthropic programmes
and activities by Member Companies is not viewed
as a price concession, reward to favoured customers
or as an inducement to purchase, lease, recommend,
prescribe, use, supply or procure Member Companies’
products or services.
b. A Member Company shall not provide Grants or
Charitable Donations to individual Healthcare
Professionals. Grants and Charitable Donations must be
provided directly to the qualifying organisation or entity,
CODE QUESTIONS AND ANSWERS
GRANTS AND
CHARITABLE
DONATIONS
4

24
CODE QUESTIONS AND ANSWERS
as the case may be. Grants and Charitable Donations
shall not be provided in response to requests made
by Healthcare Professionals unless the Healthcare
Professional is an employee or officer of the qualifying
organisation or entity and submits the request in writing
on behalf of the qualifying organisation or entity.
c. The payment (or provision of other support) by way of
any Grant or Charitable Donation shall always be made
out in the name of the recipient organisation and shall
be paid directly to the organisation. A Member Company
shall not provide Grants or Charitable Donations in
the name of any Healthcare Professional. In addition,
all Grants and Charitable Donations shall identify the
Member Company as the provider of the Grant or
Charitable Donation.
d. It must in all cases be lawful under applicable national
laws and regulations for the Grant or Charitable
Donation recipient to receive and benefit from the
particular type of Grant/Charitable Donation.
e. Member Companies shall implement an independent
decision-making/review process to identify, prevent
and mitigate against potential bribery and corruption
risks arising in connection with the provision of a Grant
or a Charitable Donation to a specific prospective
recipient. This process shall include a documented,
prior evaluation of any such associated risks and of the
relevant information concerning the intended recipient
organisation or entity.
Q29: Under the General Principles in Chapter 4.
Grants and Charitable Donations, what is meant by an
“independent decision-making/review process”?
A29: In accordance with the Principle of Separation, an
“independent decision-making/review process”, is a process
where the decision-making criteria are not primarily sales-
driven and where the Member Company’s sales function does
not decide upon and/or approve a decision to provide a Grant or
Charitable Donation. For example, such a process could be led
by a Member Company’s legal, finance or compliance functions,
operating within a robust governance framework and according to
clear, consistent and transparent criteria for review and decision-
making.
Q30: Under the Code, what is meant by “prior evaluation
of any associated risks and of the relevant information”
relating to a Grant or a Charitable Donation?
A30: Prior to deciding to provide a Grant or a Charitable Donation,
the Member Company must evaluate the appropriateness of
the award of the proposed Grant or Charitable Donation to the
proposed recipient. Such an evaluation shall consider all the
circumstances including, but not limited to, consideration of
the legal status and structure of the requesting ( i.e. prospective
recipient) organisation as well as of the nature and scope of
its activities and the terms and conditions to which the Grant
or Charitable Donation will be subject. The evaluation shall be
documented and shall be based on information available to
the Member Company, such as information or documentation
available from public sources.
For Educational Grants provided in relation to Third Party
Organised Educational Events, this may also include information
of how the funds have been applied by the recipient in relation to
previous equivalent Events and whether funds have been spent in
accordance with the terms and conditions of any previous Grant.
25
CODE QUESTIONS AND ANSWERS
f. All Grants and Charitable Donations must be
appropriately documented by the Member Company.
Moreover, Grants and Charitable Donations shall only be
provided in response to a written request submitted by
the requesting organisation or documented initiative from
a Member Company containing sufficient information to
permit an objective evaluation of the request to be carried
out by the Member Company. No Grant or Charitable
Donation shall be provided until a written agreement
documenting the terms of this is signed by both parties.
g. This section of the Code (Chapter 4: Grants and
Charitable Donations) is not intended to address the
legitimate practice by Member Companies of providing
appropriate rebates, additional product and/or service
offerings, including free of charge, or other comparable
pricing incentive mechanisms (“value adds”) which are
included in competitive and transparent centralised
purchasing arrangements, such as, for example, tenders.
2. CHARITABLE DONATIONS
Member Companies may make unrestricted Charitable
Donations for genuinely charitable or other philanthropic
purposes. “Unrestricted” in this context means that
Member Companies shall have no control over the final
use of funds (or other support) they provide as Charitable
Donations beyond general restrictions to ensure that the
funds (or other support) are applied for charitable and/or
philanthropic purposes.
Charitable Donations may be made only to charitable
organisations or other non-profit entities which have
charitable and/or philanthropic purposes as their main
purposes and which are objectively engaged in genuine
charitable or philanthropic activities. Charitable Donations
shall always be made in accordance with the general
principles set out in
Chapter 4: Grants and Charitable
Donations
.
Restricted Charitable Donations to non-profit hospitals may
be permissible in case of demonstrated Financial Hardship
(see
Glossary
), when Charitable Donations serve exclusively
the benefit of the patient, are limited in value, or explicitly
permitted by applicable national laws.
This section of the Code (Chapter 4: Grants and Charitable
Donations – Charitable Donations) is not intended to
address legitimate commercial transactions by Member
Companies in the form of leasing of stands or booth space
at Third Party Organised Educational Events and/or at
any conference or event organised by a charity or other
philanthropic organisation. Such activity is considered to
be part of Member Companies’ normal marketing activity.
Member Companies should, however, always consider
the appropriateness of the location, venue and the general
arrangements for any such events and the impression that
may be created by the arrangements in order not to bring the
industry into disrepute.
Q31: What does “sufficient information” mean where
used in connection with documentation of Grants and
Charitable Donations?
A31: The written request by a requesting organisation should
include as a minimum a detailed description of the scope and
purpose of the programme, activity or other project, which is the
object of the Grant or Charitable Donation. It shall also contain
a description of the proposed recipient, its legal status and
structure, and where relevant, a budget.
Q32: Under the Code, can a Member Company make a
Charitable Donation to support the general running of
hospital or other Healthcare Organisation?
A32: No, a Member Company cannot make available a
Charitable Donation to support the general running of a hospital
or other Healthcare Organisation. A Charitable Donation shall
only be given to a legal entity or body which has charitable and/
or philanthropic purposes as its main purposes. For the purpose
of the Code and irrespective of their legal status, hospitals and
Healthcare Organisations are considered to generally have
health functions as their main purposes and accordingly are
not generally considered to have charitable and/or philanthropic
functions as their main purposes. It is not therefore appropriate
to provide Charitable Donations to support their general running.
Q33: Is it permissible for a Member Company to specify
restrictions in relation to the final use of a Charitable
Donation where a Member Company wishes its
Charitable Donation to be applied as part of a specific
aid programme or as part of the relief effort following a
specific natural disaster, such as a major earthquake in
a particular country? (added in May 2017)
A33: Under the Code it is not appropriate for a Member Company
to apply conditions or restrictions to the final use of a Charitable
Donation which go beyond general restrictions to ensure that
the funds (or other support) are applied for charitable and/or
philanthropic purposes. Member Companies may therefore impose
general restrictions concerning the final use, such as the relief
of a specific disaster in a particular country (e.g. for use to aid
reconstruction and/or re-equipping of healthcare facilities following
the earthquake in that country). However, Member Companies must
take care that such general restrictions are not so specific that
the Charitable Donation is no longer unrestricted. The Charitable
Donation must not be misused or be perceived to influence through
undue or improper advantages, purchasing decisions, nor should
such Donation be contingent upon sales transactions or use or
recommendation of Member Companies’ products.
26
CODE QUESTIONS AND ANSWERS
3. EDUCATIONAL GRANTS
Member Companies may provide restricted Educational
Grants (see the
Glossary
) for the advancement of genuine
medical education. “Restricted” in this context means that
Member Companies shall specify the intended purpose of
the Educational Grant in the Grant agreement. A Member
Company shall also ensure that the Educational Grant
agreement with the recipient organisation includes rights
to enable it to verify that the Grant is in fact used for the
agreed intended purpose.
Member Companies shall document and publicly disclose
all Educational Grants in accordance with the Code’s
Disclosure Guidelines, and publication shall commence no
later than the end of the Transition Period.
Member Companies may provide Educational Grants for
the following (non-exhaustive) purposes:
Q34: Is it permissible for a Member Company to make
a Charitable Donation to a Healthcare Professional’s
designated charity in instances where the Healthcare
Professional has requested the Member Company to do
so in lieu of receiving a professional fee for the provision
of consultancy or speaking services to the Member
Company?
A34: No. Under the Code it is not appropriate for a Member
Company to support the favourite charity of a Healthcare
Professional in response to a request by that Healthcare
Professional irrespective of the underlying reasons. No exception
can be made for sport events, such as payment of the registration
charge to participate in a charity run.
Q35: Under the Code, may a Member Company make a
Charitable Donation such as the purchase of a table of
dinner invitations at a fundraising dinner or entries to
participate in, or attend at, a fundraising sports or other
event?
A35: Yes, Charitable Donations made by Member Companies
may take the form of dinner invitations for a fundraising dinner
or participating in other recreational events such as a fundraising
golf tournament, if arranged by a charitable or other non-profit
philanthropic organisation. The Member Company may use some
or all of its ticket allotment for its own employees and return
any unused portion to the sponsoring charitable or non-profit
philanthropic organisation for use as the sponsoring organisation
sees fit. However, the Member Company should not invite
Healthcare Professionals to attend such an event at the Member
Company’s expense. Furthermore, the Member Company is not
permitted to suggest to the sponsoring organisation, the names
of Healthcare Professionals who could be invited to attend the
event, irrespective of whether or not the specified Healthcare
Professionals will be seated at the Member Company’s table.
Q36: Can a small sized Healthcare Organisation receive
Educational Grants to support Healthcare Professionals
participation at Third Party Organised Educational Events?
(added in May 2017)
A36: Yes, in principle. There are no size limits for Healthcare
Organisations to receive Educational Grants; however, Member
Companies must ensure that the final beneficiaries of the Educational
Grant cannot be identified beforehand. For example, Healthcare
Organisations composed of a single Healthcare Professional will
in practice not be allowed to receive Educational Grants to support
Healthcare Professionals participation at Third Party Organised
Educational Events, as the final beneficiary is known upfront.

27
CODE QUESTIONS AND ANSWERS
1.
For scope of application of CVS please refer to: www.ethicalmedtech.eu
a. Support for Third Party
Organised Educational Events:
As a general principle, any Third Party Organised
Educational Event supported by way of an Educational
Grant from a Member Company to a Healthcare
Organisation must:
•
Comply with Chapter 1. General Criteria for Events; and
•
Where applicable, have approval via the Conference
Vetting System
1
(see the
Glossary
)
a.1. Support for HCP Participation at Third
Party Organised Educational Events:
Where the Educational Grant is provided for the purpose
of supporting Healthcare Professionals’ attendance at
Third Party Organised Educational Events, the Healthcare
Organisation receiving the Grant shall be solely
responsible for selection of participants and this shall be
expressly reflected in the written Grant agreement.
Q36-bis: Can an Educational Grant or funds earmarked
for education be provided to a specific hospital or
department or specify individual hospital or department
as criteria for HCOs and/or PCOs? (Added July 2018).
A36-bis: One of the guiding principles in the Code is that
companies should not receive or be able to determine the names
of the ultimate HCP beneficiaries and the inclusion of a criterion
specifying an individual hospital or hospital department is not
prohibited under the Code. However, companies should bear in
mind that the smaller the hospital or department, the greater will be
the risk that companies will be able to make such a determination
making the use of such a criterion inappropriate under the Code. In
addition, companies should be mindful of any proximate or ongoing
tender proceedings with that specific hospital, as such tenders may
raise additional red flags.
Q37: How can Member Companies in practice ensure
that Educational Grants made available for Third Party
Organised Educational Events which are subject to the
Conference Vetting System, are positively reviewed by
CVS?
A37: It is the responsibility of Member Companies to individually
ensure compliance with this Code obligation. For example,
Member Companies may themselves consider submitting
relevant Third Party Organised Educational Events for CVS review
or they may decide to include appropriate contractual obligations
making it a pre-condition for an Educational Grant that the Third
Party Organised Educational Event be submitted and positively
assessed via the CVS, for example by the prospective Grant
recipient or by a third party.
Q37-bis: Can Member companies give criteria for HCOs
and/or PCOs to allocate their Educational Funds? (Added
July 2018)
A37-bis: Yes, objective criteria for HCOs or PCOs to select HCPs
to benefit from Educational funds may be given as long as such
selection criteria are relevant to the HCPs’ educational needs and
are not so specific that it would effectively select individual HCPs.
Examples of criteria for selecting Educational Grant Recipients are
Healthcare Professionals’ speciality, years of practice, country,
city/region of practice and/or academic criteria such as number
of publications, participation in clinical trials in a given pathology.
Q38: Is it appropriate for a Member Company that has
provided an Educational Grant to support Healthcare
Professional attendance at a Third-Party Organised
Educational Event to receive the names of the Healthcare
Professionals benefiting from the Educational Grant?
(Added July 2018).
A38: A Member Company should not proactively seek to receive
the names of the healthcare Professionals benefiting from its
Educational Grant. Generally, when a Third-Party Organised
Educational Event is supported by more than one company, all
28
CODE QUESTIONS AND ANSWERS
a.2. Support for Third Party Organised
Educational Events:
Where the prospective beneficiary of an Educational
Grant is the organiser of the Third Party Organised
Educational Event and is also a Healthcare Organisation,
the recipient Healthcare Organisation shall be solely
responsible for:
•
The programme content;
•
The selection of Faculty; and
•
The payment of Faculty honoraria, if any.
Member Companies shall not have any detailed
involvement in determining the content of the
educational programme for selection of Faculty (see
Glossary
) and this shall be reflected in the written Grant
agreement. If expressly requested to do so, Member
Companies may recommend speakers or comment on
the programme.
companies should receive the same attendance list, from which it
should not be possible to identify which Healthcare Professionals
have benefited from a particular Member Company’s Educational
Grant.
However, where required by law, a Member company may, in
accordance with the applicable legal requirements, request and
obtain the names of the Healthcare Professionals participating in
the Event, who are benefiting from the Company’s Educational Grant.
For purposes of auditing, compliance and monitoring by relevant
Company functions, it may be necessary for a Member Company
to request and receive the names of the Healthcare Professionals
who have benefited from the Educational Grant provided by the
Member Company after the Event has taken place.
In either of the above cases, unless required by law, such
Healthcare Professional names should never be received by the
Member Company until the Educational Grant agreement has been
signed and the independent selection process of the Healthcare
Professionals has been completed.
Q38-bis: Under the Code, can a Member Company
provide an Educational Grant or Educational funds directly
to a Third Party Travel Agency? (Added in July 2018)
A38-bis: No, under the Code, Member Companies cannot
directly take care of the travel, [registration] or accommodation
arrangements of Healthcare Professionals attending Third-Party
Organised Educational Events so it is not permitted under the
code to make an Educational Grant available to a third party travel
agency for this purpose.
However, a Member Company may provide an Educational Grant to
a Healthcare Organisation (HCO) or funds earmarked for education
to a Professional Conference Organiser (PCO) which is structured
so that payments for travel, accommodation [and registration] are
remitted directly by the Member Company to a third party travel
agency on behalf of the HCO/PCO, which is the recipient of the
Educational Grant or the funds earmarked for education. In these
circumstances, the Member Company may choose to establish
a tri-partite contract, with the HCO/PCO and the third party travel
agency. Such a third party travel agency could in principle include
a third party travel agency also used by the Member Company for
its own internal travel arrangements provide this is not a Company-
internal function or Company-owned entity. In any event, where a
Member Company decides to use any such arrangement involving
funding for, or payments to a third party travel agency to arrange
travel, accommodation (and/or registration) it is important that the
Member Company carries out appropriate, prior due diligence on a
country-by country and case-by-case basis in order to evaluate and
mitigate the particular compliance risks and practicalities where
such an arrangement is considered. Additionally, the Member
Company must include in all of the contractual arrangements
appropriate and specific compliance-related criteria and conditions
for the HCO/PCO to outsource travel arrangements to a third party
travel agency, which should include appropriate provisions to allow
the monitoring and controlling the activity of the third party travel
agency.

29
CODE QUESTIONS AND ANSWERS
Q39: Does Chapter 4: Donations and Grants –
Educational Grants of the Code apply to requests
received by Member Companies in the context of public
procurement processes for educational support for Third
Party Organised Educational Events from Healthcare
Organisations and purchasing bodies?
A39: No. Such requests and any subsequent financial or other
support provided by a Member Company are not considered
to be Educational Grants for the purpose of the Code. Such
arrangements are commercial in nature and not philanthropic
and should be documented in a written commercial agreement
in accordance with normal business practice.
Q40: In the event that a commercial organisation, such
as a Professional Conference Organiser, organises a
Third Party Organised Educational Event independently
of any Healthcare Organisation, is it appropriate for
Member Companies to sponsor such events and what
rules shall apply? (modified in May 2017)
A40: Member Companies may enter into a commercial sponsorship
arrangement with a Professional Conference Organiser organising
a Third Party Organised Educational Event independently of any
Healthcare Organisation. However, such arrangements do not fall
within the definition of Educational Grant as Professional Conference
Organisers are for-profit organisations. Sponsorship arrangements
are therefore commercial in nature and Member Companies should
consequently document these in a written commercial agreement in
accordance with normal business practice and the requirements of
the Code (Chapter 2: Third Party Organised Educational Events). In
addition, where a Member Company provides funds earmarked for
the advancement of genuine educational purposes to a Professional
Conference Organiser, acting independently of any Healthcare
Organisation, all the Code provisions governing Educational Grants
shall also apply. For example, if a Member Company provides
funding to a Professional Conference Organiser to fund Healthcare
Professional delegate places and expenses at a Third Party Organised
Educational Conference, such Events, where applicable, must have
CVS approval and the Member Company shall publicly disclose such
funding in accordance with the Code’s Disclosure Guidelines.
Q41: Can a Member Company pay for or reimburse travel
costs to a Third Party Organised Educational Event for a
Scholar or Fellow?
A41: No, a Member Company cannot additionally pay for, or
reimburse, the travel or other participation costs incurred by a
Scholar or Fellow attending a Third Party Organised Educational
Event. Such costs shall be included in the Educational Grant
supporting the Scholarship or Fellowship if it is intended that the
Grant should extend to such attendance.
Q42: Can Member Companies support professional
training of Healthcare Professionals on general medical
education topics, such as courses on health economics/
b. Scholarships and Fellowships
Member Companies may provide Educational Grants on
a restricted basis in the form of Grants for Scholarships
and Fellowships to support advancement of genuine
medical education of Healthcare Professionals (see the
Glossary
). Only Healthcare Organisations where Healthcare
Professionals are in training shall be eligible to request
and/or receive such Educational Grants. A Member
Company shall not provide Educational Grants to support
Scholarships and Fellowships upon request of individual
Healthcare Professionals. Similarly, the Member Company
shall not have any involvement in any way in the selection
of the HCPs who will benefit from the Educational Grant
and this shall be reflected in the written Grant agreement
between the Member Company and the recipient HCO.

30
CODE QUESTIONS AND ANSWERS
health technology appraisal, good laboratory practices
or similar topics that promote patient care? (Added in
July 2018).
A42: Member Companies can support genuine medical
education for Healthcare Professionals on general healthcare-
related topics through Educational Grants in accordance with
Chapter 4 of the Code.
Member Companies can also support genuine medical training
on general healthcare-related topics through company-organised
“Product and Procedure Training and Education Events” as long as
the information directly concerns the Member Company’s health
technologies, therapies, and/or related services. The Event must
be conducted in accordance with and meet the other requirements
of Chapter 3, Section 2 of the Code.
Q43: What are examples of relevant disease awareness
and health education for patients, carers and the general
public for which a Member Company may legitimately
provide an Educational Grant?
A43: A Member Company may provide an Educational Grant to
support the provision of high quality information to patients and the
public about health and disease provided there is an objective patient
or public need for such information and the topics covered are linked
to the therapeutic areas in which the Member Company is interested
and/or involved. Such disease awareness campaigns must not,
however, promote the use of particular therapies, services or promote
specific HCOs, nor may they aim to stimulate demand by the public
for specific therapies or for specific Healthcare Organisations.
c. Grants for Public Awareness
Campaigns
Member Companies may also provide Educational Grants
on a restricted basis to Healthcare Organisations for the
legitimate purpose of providing information, promoting
awareness and/or educating patients, carers or the
general public about relevant healthcare topics or medical
conditions or diseases in therapeutic areas in which the
Member Company is interested and/or involved.
4. RESEARCH GRANTS
Where permitted by national laws, regulations, national
guidelines and professional codes of conduct, Member
Companies may provide restricted Research Grants (see
the
Glossary
) to support clearly defined third party-initiated
research studies for clinical or non-clinical research
programmes in therapeutic areas in which the Member
Company is interested and/or involved. Research Grants
may include in-kind or financial support for legitimate,
study-related, documented expenses or services, and/or
reasonable quantities of single-use and/or multiple-use
free of charge product(s) for the limited duration of the
research.
Member Companies providing Research Grants shall
ensure that they do not influence the research. However,
in order to ensure that Research Grants are provided
on a “restricted” basis, Member Companies shall clarify
the intended research scope and purposes for which
the Grant is requested and shall ensure that the written
Grant agreement with the recipient organisation includes
rights for the Member Company to verify that the Grant

31
CODE QUESTIONS AND ANSWERS
is applied solely for the agreed intended research use.
Such verification may include a request for study-related
documentation, such as a copy of the research protocol, a
copy of the ethics committee and/or regulatory approvals
or a copy of the study report upon completion or earlier
termination of the research.
All requests for Research Grants from prospective Grant
beneficiaries must be in writing and must detail, as a
minimum, the type, nature and objectives of the research
activity, the milestones and budget, the approximate
duration of the research, and where applicable, the
requirements for ethics committee, regulatory and/or
other authorisations or approvals. A Member Company
may give consideration to a request for a Research Grant
prior to ethics committee approval for the specific research
project but shall not take any final decision regarding the
Grant request unless and until the research receives formal
ethics committee approval.
Research Grant agreements shall include provisions
relating to adverse event reporting where appropriate, and
shall require full disclosure of the Member Company and
of the Grant by the Grant recipient organisation and the
lead- investigator in all oral or written presentations of the
results.
For guidance on how Member Companies may undertake
Member Company-initiated research please refer to
Chapter
6: Research: Member Company-Initiated Research
.

32
CODE QUESTIONS AND ANSWERS
Safeguards are required to ensure that the deployment
of sponsored posts does not cause a conflict of interest
between the aims of the sponsoring Member Company
and the aims of the Healthcare Organisation, particularly in
relation to procurement and competition.
Where a Member Company sponsors part or all of the salary
of a Healthcare Professional employed by a Healthcare
Organisation, they must have due regard to national rules
or relevant codes of practice that may apply. Sponsorship
must be with the Healthcare Organisation not the HCP and
must be in response to a request from the HCO through
a formal and transparent procurement process. Written
agreements should detail the circumstances under which
the HCO may exit from the sponsorship arrangement if
conflicts of interest which cannot be managed arise.
There should be written affirmation that the arrangements
will have no effect on purchasing decisions or prescribing
and dispensing habits and no pressure must be exerted
on the sponsored HCP to favour the Member Company’s
products over any other. For the duration of the Sponsored
Post, auditing arrangements should be established to
ensure independence of product or service choice.
Q44: If part of the funds provided to a Healthcare
Organisation for a Sponsored Post are intended to cover
training and education of the sponsored Healthcare
Professionals, is it necessary to declare these monies
as part of the annual declaration of indirect sponsorship
of HCP attendance at Third Party Organised Educational
Events or Conferences?
A44: If the funds are specifically linked to attendance at particular
Third Party Educational Events or Conferences then this should be
declared in the normal way as set out under the Part 2 Disclosure
Guidelines in this document. If the funds are specified as being for
general training and education needs of the sponsored HCP(s) and
the decisions on how those funds are spent in this regard are under
control of the Healthcare Organisation rather than the HCP(s) then
it is not necessary to disclose under Part 2.
CODE QUESTIONS AND ANSWERS
SPONSORED
POSTS
5
(Added in May 2017)

33
CODE QUESTIONS AND ANSWERS
1. GENERAL PRINCIPLES
Member Companies may engage Healthcare Professionals
as consultants and advisors to provide bona fide consulting
and other services, including but not limited to research,
participation on advisory boards, presentations at Company
Events and product development. Member Companies may
pay Healthcare Professionals reasonable remuneration
for performing these services. In all cases, consulting
arrangements must be permitted under the laws and
regulations of the country where the Healthcare Professional
is licensed to practise and be consistent with applicable
professional codes of conduct in that country.
The principles in this chapter are applicable to all consulting
arrangements between Healthcare Professionals and
Member Companies including where a consultant Healthcare
Professional declines a fee for provision of their services.
Consulting arrangements shall not be contingent in any way
on the prospective consultant’s past, present or potential
future purchase, lease, recommendation, prescription, use,
supply or procurement of the Member Company’s products
or services.
CODE QUESTIONS AND ANSWERS
ARRANGEMENTS
WITH CONSULTANTS
6
34
CODE QUESTIONS AND ANSWERS
When selecting consultants, Member Companies shall
implement an independent decision-making/review process
to identify, prevent and mitigate against potential bribery and
corruption risks arising in connection with use of consultants.
This process shall include a documented, prior evaluation of
any such associated risks and of the relevant background
information concerning each prospective consultant.
2. CRITERIA FOR GENUINE
CONSULTING ARRANGEMENTS
In addition to the general principles above, the arrangements
which cover genuine consultancy or other services must, to
the extent relevant to the particular arrangement, fulfil all
the following criteria:
a. Consulting arrangements must be entered into only
where a legitimate business need for the services is
identified in advance.
b. The number of consultants retained must not be greater
than the number reasonably necessary to achieve the
identified need.
c. Selection of consultants must be based on criteria
directly related to the identified business need and the
relevance of the consultant’s qualifications, expertise and
experience to address the identified need. The volume or
value of business generated by a prospective consultant
or the Healthcare Organisation where s/he performs her/
his professional activity is not a relevant criterion.
d. Consulting arrangements with Healthcare Professionals
must be documented in a written agreement, signed
by the parties in advance of the commencement of the
services, which must specify the nature of the services to
be provided and the basis for payment for those services.
e. The hiring of the consultant must not be an inducement
to purchase, lease, recommend, prescribe, use, supply or
procure the Member Company’s products or services.
f. The remuneration for the services rendered must be
reasonable and reflect the fair market value of the
services provided.
g. Member Companies must maintain records of the
services, and associated work products, provided by
the consultant Healthcare Professionals and of the use
made of those services by the Member Company.
h. The venue and other arrangements (e.g. hospitality,
travel etc.) for Member Company meetings with
consultants shall follow the rules for Events set out in
Chapter 1: General Criteria for Events.

35
CODE QUESTIONS AND ANSWERS
3. REMUNERATION
AND FAIR MARKET VALUE
The remuneration paid to Healthcare Professionals
engaged as consultants by Member Companies shall reflect
fair-market-value for the services provided. It shall not be in
any way contingent upon the value of products or services
which consultants may purchase, lease, recommend,
prescribe, use, supply or procure in the course of their own
professional practice or that may be purchased, leased,
recommended, prescribed, used, supplied or procured by
HCOs where they carry on their professional activities.
All payments made for services must comply with all
applicable tax and other legal requirements. Member
Companies may pay for expenses reasonably incurred
by consultants in providing the services which are the
subject of the consulting agreement including reasonable
travel, meals and accommodation expenses incurred by
consultants if attending meetings with, or on behalf of
Member Companies. The written consulting agreement
must detail which expenses can be claimed by the
consultant in relation to the provision of the services and
the basis for payment of these by the Member Company.
4. DISCLOSURE
AND TRANSPARENCY
Member Companies shall ensure they fully comply with
all applicable national laws, regulations and professional
codes of conduct requiring any publication, disclosure or
approval in connection with the use by Member Companies
of Healthcare Professionals as consultants. All required
consents and approvals shall be obtained, including from
the hospital or other Healthcare Organisation administration
or from the Healthcare Professional’s superior (or locally-
designated competent authority), as applicable. Where no
such national requirements apply, Member Companies
shall nevertheless maintain appropriate transparency
by requiring the relevant Employer Notification which
shall disclose the purpose and scope of the consultancy
arrangement.
Member Companies shall also include appropriate
obligations on the consultant to ensure that the consultant’s
status as a consultant for the Member Company and his/
her involvement in the research for, or the preparation of,
material for scientific publication is disclosed at the time of
any publication or presentation.
Q45: What is meant by fair market value (FMV) in the
context of consulting arrangements?
A45: Fair market value is the value of the specified consultancy
services which would be paid by the Member Company to
the consultant, each dealing at arm’s length in an open and
unrestricted market, and when neither party is under any
compulsion to buy or sell, and both parties have reasonable
knowledge of the relevant facts.
Q46: How should Member Companies determine FMV
for a service?
A46: A Member Company must be able to demonstrate internal
methodology to determine fair market value. Amongst other
matters this shall take account of the consultant’s qualifications,
expertise and experience as well as the actual services to be
provided to the Member Company.

36
CODE QUESTIONS AND ANSWERS
1. MEMBER COMPANY-
INITIATED RESEARCH
Where there is a legitimate business need to do so, Member
Companies may initiate, conduct, manage and finance
scientifically valid research to generate data, whether
pre- or post-market. In this context, legitimate business
needs for data include medical needs, including patient
safety; research and development; scientific purposes (e.g.
performance indicators, comparing objective scientific
parameters); regulatory, including post-market surveillance
(PMS) and post-market clinical follow up (PMCF), vigilance,
safety, or reimbursement and health economic, including
clinical and cost-effectiveness and outcomes data
relevant to health technology assessments (HTA) and
reimbursement decision-making.
RESEARCH
7
CODE QUESTIONS AND ANSWERS

37
CODE QUESTIONS AND ANSWERS
Where a Member Company uses a Healthcare Professional
as a consultant, for example to lead a study on the Member
Company’s behalf (i.e. act as Principal Investigator), the
Member Company shall ensure that such consulting
arrangements comply fully with
Chapter 5: Arrangements
with Consultants
.
In accordance with the Documentation Principle, any
arrangements made by a Member Company to procure
research-related services shall be set out in a written
agreement which shall reference a written research
protocol, written schedule of work and provide for all
required consents, approvals and authorisations to be
obtained prior to the commencement of the study.
Member Companies must ensure that their research
activities comply with all applicable national laws,
regulations and researchers’ own professional codes of
conduct, as well as with applicable Good Clinical Practice
guidelines, if relevant.
In accordance with the Principles set out in the
Introduction:
Aims and Principles of the Code
, Member Companies shall
also ensure appropriate clinical trial transparency in relation
to their research activities and results. This shall include
appropriate disclosure of information about Member
Companies’ clinical trials, for example in external public
registries and peer-reviewed journals.
Where Member Companies engage third party
intermediaries for research (e.g. contract research
organisations (CROs)), they shall ensure that the research
conducted by these third parties on behalf of the Member
Company is carried out in accordance with all applicable
legal and ethical requirements, including the applicable
requirements of the Code.
2. MEMBER COMPANY POST-
MARKET PRODUCT EVALUATION
Where there is a legitimate business need to do so, Member
Companies may initiate, post-market third party evaluation
of their products, therapies and/or related services and
may therefore provide Evaluation Products under a written
contract for services in order to obtain defined user
evaluation by Healthcare Organisations in relation to the
Evaluation Products. Evaluation Products may be provided
on a no charge basis in return for the requested user
feedback from Healthcare Professionals at the Healthcare
Organisation, which shall be formally described in a written
protocol or questionnaire forming part of the contract.
Q47: What is an example of an external public registry
for clinical trial transparency?
A47: Examples of an external public register for clinical trial
transparency are www.clinicaltrials.gov or www.who.org

38
CODE QUESTIONS AND ANSWERS
Where the Evaluation Products are multiple-use Evaluation
Products the defined period of time necessary for the
evaluation and feedback to occur will depend on the
frequency of anticipated use; the nature of the user
evaluation feedback requested; the duration of any required
training and similar considerations. Member Companies
shall in all cases ensure that they retain title to multiple-
use Evaluation Products and that they have a process in
place for promptly removing such multiple use Evaluation
Products and/or any unused single-use Evaluation
Products from the Healthcare Organisation’s location at
the conclusion of the evaluation period unless these are
purchased by the Healthcare Organisation.
Provision of Evaluation Products and/or related services
must not improperly reward, induce and/or encourage
Healthcare Professionals and/or Healthcare Organisations
to purchase, lease, recommend, prescribe, use, supply or
procure Member Companies’ products or services. Any
offer and/or supply of Evaluation Products shall always
be done in full compliance with applicable national laws,
regulations and industry and professional codes of
conduct.
3. THIRD PARTY-INITIATED
RESEARCH
Please refer to
Chapter 4: Grants and Charitable Donations:
Research Grants.

39
CODE QUESTIONS AND ANSWERS
Healthcare Professionals, acting individually or as part of
a group in which they are an active participant, often make
valuable contributions that improve products or health
technologies. They may develop intellectual property, for
example, patents, trade secrets, or know-how, under a
product or technology development or intellectual property
licensing agreement.
A royalty arrangement between a Member Company and
a Healthcare Professional should be entered into only
where the Healthcare Professional is expected to make or
has made a novel, significant, or innovative contribution
to, for example, the development of a product, technology,
process, or method, such that the Healthcare Professional
would be considered to be the sole or joint owner of such
intellectual property under applicable laws and regulations.
The foregoing is without prejudice to Member Companies’
CODE QUESTIONS AND ANSWERS
ROYALTIES
8

40
CODE QUESTIONS AND ANSWERS
obligations to comply with any applicable obligations to
pay royalties which may arise under applicable laws and
regulations in some countries.
Arrangements involving the payment of royalties by or on
behalf of Member Companies to a Healthcare Professional
must be set out in a written agreement providing
appropriate and reasonable remuneration in accordance
with applicable laws and regulations. For example, royalties
paid in exchange for intellectual property should not be
conditional on:
a.
A requirement that the Healthcare Professional purchase,
order or recommend any product, services or health
technology of the Member Company or any product or
technology produced as a result of the development
project; or
b.
A requirement to market the product or health technology
upon commercialisation.
Subject to national regulations and requirements, Member
Companies should exclude from the calculation of royalties
the number of units purchased, prescribed, used, or
ordered by the Healthcare Professional and/or members
of the Healthcare Professional’s practice or Healthcare
Organisation.

41
CODE QUESTIONS AND ANSWERS
Member Companies exceptionally may provide inexpensive
educational items and/or gifts, in accordance with national
laws, regulations and industry and professional codes of
conduct of the country where the Healthcare Professional
is licensed to practise
1
. Member Companies may only
provide such educational items and/or gifts in accordance
of the following principles:
a. Educational items and/or gifts may be provided but these
must relate to the Healthcare Professional’s practice, or
benefit patients, or serve a genuine educational function.
b. No educational items and/or gifts should be provided in
response to requests made by Healthcare Professionals.
c. Educational items and/or gifts must not be given in the
form of cash or cash equivalents.
d. Educational items and/or gifts must be modest in value,
and can be branded or non-branded items.
Q48: What are examples of items of modest value that
are “related to the Healthcare Professional’s practice or
for the benefit of patients”.
A48: Stationery items, calendars, diaries, computer accessories
for business use and clinical items such as wipes, nail brushes,
surgical gloves and tourniquets are examples of modest value
items that could be appropriately provided as gifts to Healthcare
Professionals provided their value falls within the maximum value
prescribed under national laws, regulations and industry and
professional codes of conduct. Food, alcohol and items which are
primarily for use in the home or car are not appropriate as they are
not related to the Healthcare Professional’s practice nor are they
for the benefit of patients.
CODE QUESTIONS AND ANSWERS
EDUCATIONAL
ITEMS AND GIFTS
9
1.
In the UK, the NHS England document Managing Conflicts of Interest in the NHS
(Publications Gateway Reference: 06419) sets the limit for such items at £6. While
this only directly applies to the NHS in England it should be taken as indicative of
what is acceptable in the rest of the UK, including in relation to HCPs working in the
non-NHS sector. Different limits may apply in other territories and Member Compa-
nies should comply with these as appropriate. Where no such limits apply, the UK
figure may be taken as indicative of what is acceptable under the Code.
42
CODE QUESTIONS AND ANSWERS
e. A Member Company may occasionally provide
educational items of greater value to a Healthcare
Organisation always provided that the item serves
a genuine educational function for the Healthcare
Professionals at that Healthcare Organisation and is
of benefit to patients. Such items shall not be provided
to Healthcare Professionals for their personal use.
The item shall also be related to the therapeutic areas
in which the Member Company is interested and/or
involved. For higher value educational items, Member
Companies must maintain appropriate records of their
provision of such educational items to Healthcare
Organisations. Such items should not be part of the
Healthcare Organisation’s normal overheads or routine
costs of operation.
f. Provision of educational items and/or gifts must not
improperly reward, incentivise and/or encourage
Healthcare Professionals to purchase, lease,
recommend, prescribe, use, supply or procure the
Member Company’s products or services.
Prize draws and other competitions at Events are
permissible if the prize awarded complies with
Chapter 8.
Educational Items and Gifts.
In addition, it must comply with
national laws, regulations and industry and professional
codes of conduct.
This Chapter is not intended to address the legitimate
practice of providing appropriate Evaluation Products,
Demonstration products or Samples. For guidance on how
Member Companies may provide Evaluation Products,
Demonstration products or Samples, please refer to
Chapter 6: Research
and
Chapter 9: Demonstration Products
and Samples
, as applicable.
Q49: May a Member Company provide a small gift to a
Healthcare Professional to mark significant life events
such as a marriage, birth, birthday or death?
A49: The Code restricts the types of gift that may be given to a
Healthcare Professional and it would not be appropriate to give
gifts to mark significant life events such as a marriage, birth or
birthday. However, in the case of death, it is for each Member
Company to determine the appropriateness of making a tasteful
gift as a mark of respect.
Q50: Where Healthcare Professionals engaged by
Member Companies as consultants or speakers decline a
professional fee for their services, would it be appropriate
for the Member Company to show its appreciation by
giving the Healthcare Professional a small gift such as
a bottle of wine or a bouquet of flowers?
A50: No, it would not be acceptable for the Member Company
to make such a gift because to do so could be open to
misinterpretation and would be likely to breach the Principle of
Image and Perception. Moreover such gifts would not comply
with Chapter 8. Educational Items and Gifts as they neither relate
to a Healthcare Professional’s practice nor serve an educational
function.
Q51: Please provide examples of educational items
of greater value that can be provided to Healthcare
Organisations under the Code?
A51: Examples of educational items of greater value that can be
provided may include medical textbooks or anatomical models,
but only if those relate to the therapeutic areas in which the
Member Company is interested and/or involved.

43
CODE QUESTIONS AND ANSWERS
1. GENERAL PRINCIPLES
Member Companies may provide their own products as
Demonstration Products and/or Samples (see the
Glossary
)
at no charge in order to enable Healthcare Professionals
and/or Healthcare Organisations (as applicable) to evaluate
and/or familiarise themselves with the safe, effective and
appropriate use and functionality of the product and/or
related service and to determine whether, or when, to use,
order, purchase, prescribe or recommend the product and/
or service in the future.
Demonstration Products and/or Samples may be either
single- or multiple-use products. Member Companies may
also provide products from another company in conjunction
with the Member Company’s own Demonstration Products
CODE QUESTIONS AND ANSWERS
DEMONSTRATION
PRODUCTS
AND SAMPLES
10

44
CODE QUESTIONS AND ANSWERS
and/or Samples on an exceptional basis if those other
company’s products are required in order to properly and
effectively demonstrate, evaluate or use the Member
Company’s products, e.g. computer hardware and software
produced by a company other than the Member Company.
Provision of Demonstration Products and/or Samples
must not improperly reward, induce and/or encourage
Healthcare Professionals and/or Healthcare Organisations
to purchase, lease, recommend, prescribe, use, supply or
procure Member Companies’ products or services. Any
offer and/or supply of such products shall always be done
in full compliance with applicable national laws, regulations
and industry and professional codes of conduct.
Member Companies shall in all cases maintain appropriate
records in relation to the provision of Demonstration
Products and/or Samples to Healthcare Professionals
and/or Healthcare Organisations, for example recording
proof of delivery for any Demonstration Products and/
or Samples provided and receipt of return for multiple-
use Demonstration Products and/or Samples. Member
Companies shall clearly record in the Member Company’s
records as well as clearly disclose to Healthcare
Professionals and/or Healthcare Organisations the
no-charge basis and other conditions applicable for the
supply of such Demonstration Products and/or Samples
no later than the time of the supply. The disclosure to
Healthcare Professionals and Healthcare Organisations
shall be in writing.
This Chapter is limited to the provision of Demonstration
Products and/or Samples and related services at no
charge and is not intended to apply to provision of products
or related services under any other arrangements, for
example (but not limited to) provision within the framework
for clinical trials and/or other research or commercial
supplies by way of rebates or pricing incentives in a public
procurement context.
2. DEMONSTRATION
PRODUCTS (DEMOS)
Member Companies may provide examples of their
products to Healthcare Professionals and/or Healthcare
Organisations in the form of mock-ups (such as unsterilised
single use products) that are used for Healthcare
Professionals and patient awareness, education and
training. For example, a Healthcare Professional may use
a Demonstration Product to show a patient the type of
technology which will be implanted in the patient or may
use the Demo to train other Healthcare Professionals in the
use of the product.
45
CODE QUESTIONS AND ANSWERS
Demonstration Products are not intended for clinical use in
any patient care nor are they intended for on-sale or other
transfer. Member Companies shall clearly record in the
Member Company’s records as well as clearly disclose to
Healthcare Professionals and/or Healthcare Organisations
the no-charge basis and other conditions applicable for the
supply of such Demonstration Products no later than the
time of the supply. It is recommended that the disclosure
to Healthcare Professionals and Healthcare Organisations
be in writing.
3. SAMPLES
Member Companies may provide a reasonable number of
Samples at no charge to allow Healthcare Professionals
and/or Healthcare Organisations to familiarise themselves
with the products and/or related services, to acquire
experience in dealing with them safely and effectively in
clinical use and to determine whether, or when, to use,
order, purchase, prescribe or recommend the product and/
or service in the future.
For Samples, which are single-use products, the quantity
provided for purposes of familiarisation must not exceed
the amount reasonably necessary for the Healthcare
Professionals/Healthcare Organisation to acquire adequate
experience in dealing with the products.
For Samples, which are multiple-use products, the specific
length of time necessary for a Healthcare Professional
to familiarise him/herself with the product will depend
on the frequency of anticipated use; the duration of
required training; the number of Healthcare Professionals
who will need to acquire experience in dealing with the
product and similar considerations. Member Companies
shall in all cases ensure that they retain title to multiple-
use Samples and that they have a process in place for
promptly removing such multiple use Samples from the
Healthcare Professional’s location at the conclusion of the
familiarisation period.

46
CODE QUESTIONS AND ANSWERS
DISCLOSURE GUIDELINES
PART 2
Adopted by the ABHI in May 2017

47
PREAMBLE
Under the ABHI Code of Ethical Business Practice (the “Code”), Member Companies are not permitted to
pay registration fees, travel or hospitality expenses directly to individual Healthcare Professionals for their
participation in educational conferences organised by third-parties as of 1st January, 2019.
Medical Education may be supported through the provision of Educational Grants to Healthcare
Organisations in compliance with the rules set out in the Code. To prevent abuses, specific safeguards
when providing Educational Grants have been developed, including the obligation to disclose these
Educational Grants.
Section 3 of Chapter 4 of the Code states that Member Companies shall document and publicly disclose
all Educational Grants in accordance with these Disclosure Guidelines. These Disclosure Guidelines are
therefore an integral part of the Code, and need to be interpreted as such.
For the avoidance of doubt, all funds provided by a Member Company for the advancement of genuine
educational purposes to a Professional Conference Organiser (“PCO”), acting independently of any
Healthcare Organisation, fall under the scope of these Disclosure Guidelines and are subject to the same
conditions as Educational Grants. Whenever these Disclosure Guidelines refer to Healthcare Organisations,
these shall also include Professional Conference Organisers. All capitalised concepts used in the Guidelines
are concepts defined in the Code.

48
CODE QUESTIONS AND ANSWERS
CHAPTER 1:
APPLICABILITY OF THESE
GUIDELINES
1. Scope
These Disclosure Guidelines apply to Member Companies
in their interactions with Healthcare Organisations based or
registered in the ABHI Europe Geographic Area.
Separate entities belonging to the same multinational
company (“Affiliates”) – which could be the parent company
(e.g. the headquarters, principal office, or controlling
company of a commercial enterprise), subsidiary company
or any other form of enterprise or organisation – shall be
deemed to constitute a single company, and are as such
committed to compliance with these Disclosure Guidelines.
Transfers of value that are not included in the definition of
Educational Grants (as described in Chapter 4, Section 3 of
the Code) and that consequently cannot be allocated to any
of the categories set forth in Chapter 2, Section 2 Aggregate
Disclosure are not within the scope of these Disclosure
Guidelines.
2. Applicability of these Disclosure
Guidelines
Member Companies need not report the same information
twice due to being bound by national laws, regulations
or professional codes imposing disclosure obligations
regarding Educational Grants (as described in Chapter 4,
section 3 of the Code) equivalent to the ones imposed by
these Disclosure Guidelines.
Q1: Does the Disclosure Guideline’s definition of
“Affiliate” include legal entities belonging to the same
parent Member Company but registered outside Europe?
A1: Yes. Educational Grants made by Affiliates (parent companies
are included in the definition of Affiliates to the effect of the
Disclosure Guidelines) incorporated outside of MedTech Europe
Geographical Area to Healthcare Organisations registered in
Europe are within the scope of these Disclosure Guidelines. Any of
the Affiliates registered in Europe can disclose these Educational
Grants. Each Member Company can choose which Affiliate will
report these Educational Grants made by Affiliates from outside
the MedTech Europe Geographical Area.
Q2: Are these Disclosure Guidelines applicable to
third party intermediaries who interact with Healthcare
Organisations in connection with the sale, promotion or
other activity involving Member Companies’ products?
A2: No, these Disclosure Guidelines are not applicable to third
parties such as third party sales & marketing intermediaries
(SMIs), consultants, distributors, sales agents, marketing agents,
brokers, commissionaire commercial agents and independent
sales representatives (list not exhaustive). Nevertheless, it is
recommended to document arrangements concluded between
Member Companies and third party intermediaries in order to
comply with the provisions set out in the Code.
Q3: Where a national code already imposes disclosure
obligations in a given country, where may Corporate
Members disclose the Educational Grants?
A3: Where a national code imposes disclosure obligations
regarding Educational Grants (as regulated in Chapter 4, Section 3
of the Code) to the same extent as regulated by these Guidelines,
Corporate Members, who are not a member of the National
Association responsible for that national code, may choose either:
− To disclose only on the MedTech Europe platform; or
− To disclose on the national platform, if that possibility is
provided for.
Corporate Members who are bound by this national code may
choose either:
− To disclose only on the national platform or

49
CODE QUESTIONS AND ANSWERS
− To disclose both on the MedTech Europe platform and the
national platform.
This selected option shall be included in the Methodology Note.
Q4: Who will decide if a national law, regulation or code
imposes disclosure obligations regarding Educational
Grants equivalent to the ones imposed by the Disclosure
Guidelines?
A4: The MedTech Europe Secretariat shall conduct a yearly
assessment of the equivalence of national laws, regulations
and/or professional codes imposing disclosure obligations with
the MedTech Europe Transparency Obligations (as regulated in
Chapter 4, Section 3 of the Code).
Members can at any time submit any information or documentation
they possess that could be relevant for this assessment to the
Secretariat.
The MedTech Europe Secretariat shall submit its assessment
to the MedTech Europe Transparency Task Force, who will
analyse the proposal. If the MedTech Europe Transparency Task
Force agrees with the proposal, it will be submitted for approval
to the MedTech Europe Compliance Network. If the disclosure
obligations imposed by a national law, regulation or professional
code are deemed equivalent to the ones imposed by the Disclosure
Guidelines, the assessment will be made public on the MedTech
Europe Transparency website. This selected option shall be
included in the Methodology Note.
3. Applicability to Non-Member Companies
Non-member companies may implement these Disclosure
Guidelines provided they are committed to ethical standards
equivalent to those enshrined in the Code. Non-member
companies may prove this commitment by signing up to
the ABHI Code of Ethical Business Practice
1
.
1.
Non-members can sign up to the ABHI Code of Ethical Business Practice at
abhicodeofpractice.org.uk

50
CODE QUESTIONS AND ANSWERS
Q5: Which Affiliate should disclose a particular
Educational Grant?
A5: To facilitate the tracking of Educational Grants made to
individual Healthcare Organisations, it is recommended that the
Affiliate making the payment in relation to a particular Educational
Grant is the one disclosing the Educational Grant, but this is an
internal decision of each Member Company.
A Member Company may choose to use internal arrangements of
its choice to report the aggregated sum in relation to Educational
Grants made by each legal entity composing the company
(Affiliates) to a particular Healthcare Organisation during a
disclosure period.
CHAPTER 2:
DISCLOSURE OBLIGATION
1. General Obligation
Subject to the terms of these Disclosure Guidelines,
each Member Company shall document and disclose all
payments related to Educational Grants (as described
in Chapter 4, section 3 of the Code) that it makes to a
Healthcare Organisation based or registered in Europe,
without limitation of value. The disclosure of Educational
Grants provided by Affiliates of the Member Company
described above, but which are not registered in the
MedTech Europe Geographic Area shall be made by any of
the Affiliates comprising said Member Company that are
registered in the MedTech Europe Geographic Area.
2. Aggregate Disclosure
Educational Grants shall be disclosed on an aggregate
basis. Each Affiliate of a Member Company shall disclose,
for each clearly identifiable and separate recipient, the
amounts paid as Educational Grants to such recipient in
each Reporting Period
1
which can be reasonably allocated
to one of the categories set out below. Such amounts
will be aggregated on a category-by-category basis, but
itemised disclosure shall be made available upon request
by the Member Company, as deemed necessary, to (i) the
relevant recipient, and/or (ii) the relevant authorities.
Member Companies shall disclose an aggregate amount
related to any of the categories set forth below:
a. Educational Grants to support Third Party Organised
Events (including Support for HCP Participation at Third
Party Organised Educational Events) and,
b. Other Educational Grants to Healthcare Organisations
(including Scholarships, Fellowships and/or Grants for
Public Awareness Campaigns).
3. Optional Object Specification
If desired, Member Companies may indicate the object of
the Educational Grants disclosed for one or both categories
set forth in Chapter 2, Section 2 Aggregate Disclosure.
1.
Reporting Period means a full calendar year (starting on the 1st of January and
ending in the 31st of December).
51
CODE QUESTIONS AND ANSWERS
Q6: When should a Methodology Note be made available?
A6: Member Companies should create a comprehensive
Methodology Note that would allow any Healthcare Organisation
directly affected by a disclosure to understand how the amount
disclosed was aggregated. The Methodology Note should therefore
be made available upon specific request to Healthcare Organisations
concerned about particular disclosure that directly affects them.
Q7: When will the first Reporting Period start?
A7: Chapter 4, Section 3 of the ABHI Code of Ethical Business
Practice establishes that the first disclosure shall occur no later than
the end of the Transition Period. The Transition Period ends on the
31st December 2018. As a consequence, the first Reporting Period is
the calendar year 2018, starting on the 1st January 2018, and ending
on 31st December 2018.
Q8: In what currency should the amounts payed be
disclosed?
A8: Disclosed amounts should be in the currency used in the
payment. In the event the aggregate amount includes Educational
Grants made in different currencies, the reporting company may
choose in which currency they wish to disclose the aggregate
amount, and keep appropriate record of the exchange rate used
to convert the different currencies. This information will then be
included in their Methodology Note.
4. Methodology
Each Member Company shall create a note summarising
the methodologies used by it in preparing the disclosures
and identifying Educational Grants for each category
described in Chapter 2, Section 2 Aggregate Disclosure.
The note, including a general summary and/or country
specific considerations, shall describe the recognition
methodologies applied, and should include the treatment
of VAT and other tax aspects, currency aspects and other
issues related to the timing and amount of Educational
Grants for purposes of these Disclosure Guidelines, as
applicable. This Methodology Note shall be made available
upon request by an interested party.
CHAPTER 3:
FORM OF DISCLOSURE
1. Reporting Period
Disclosures shall be made on an annual basis and each
Reporting Period shall cover a full calendar year.
2. Time of Disclosure
Disclosures shall be made by each Member Company
within 6 months after the end of the relevant Reporting
Period
3. Time of Publication
The disclosures shall be made public at the time of
publication. The time of publication is the 31st August of
the year of the relevant time of disclosure.
4. Template and Language of Disclosure
For consistency purposes, disclosures made pursuant to
these Disclosure Guidelines shall be made in English using
the template set forth in the Annex.

52
CODE QUESTIONS AND ANSWERS
5. Disclosure Platform
Disclosures shall be made on the EthicalMedTech website
1
unless the Member Company is already bound by national
laws, regulations or professional codes as regulated in
Chapter 1, Section 2 Applicability of these Disclosure
Guidelines. Member Companies will remain liable for the
accuracy of the disclosed data. For the avoidance of doubt,
MedTech Europe shall not be held liable for (i) maintaining,
correcting, deleting the published data nor (ii) for the
storage of data after the three years period of disclosure in
the public domain.
6. Disclosures Retention and Modification
Member Companies shall be able to modify, delete or in any
way alter their disclosures at any time before or after the
time of publication.
The information disclosed shall remain in the public domain
for 3 years after the time such information is first published.
7. Enquiries Regarding Reported Disclosures
Member Companies shall be able to modify, delete or in any
way alter their disclosures at any time before or after the
time of publication.
Member Companies shall make available to Healthcare
Organizations upon request any data concerning their
common contractual relations published in accordance
with these Disclosure Guidelines at any time while the
disclosed information remains in the public domain as
stated in Chapter 3, Section 3 Time of Publication.
1.
www.ethicalmedtech.org

53
GUIDELINES ON ADVERTISEMENTS
AND PROMOTIONS ADDRESSED
SOLELY OR PRIMARILY TO
HEALTHCARE PROFESSIONALS
PART 3

54
PREAMBLE
Introduction
These Guidelines are published by ABHI because under
current UK law and other advertising codes it is difficult to
take legal action or to complain against a person or company
who publishes misleading promotional material directed
solely or primarily at Healthcare Professionals.
Consumer advertising is governed both by legislation (the
main legal instruments being listed in Section 1) and by
the codes of advertising practice issued by the Committee
of Advertising Practice and the Broadcast Committee of
Advertising Practice and administered by the Advertising
Standards Authority. However, Advertising directed at
Healthcare Professionals is not clearly caught by these
provisions.
The intention of these Guidelines is to set out principles
to be applied to Advertising directed solely or primarily at
Healthcare Professionals. These principles will be part of
the ABHI Code of Ethical Business Practice with which all
ABHI Members agree to comply. The principles apply to all
such Advertising issued by or on behalf of ABHI Members
where it is directed at Healthcare Professionals in the UK.
The principles set out in these Guidelines are, however, based
upon the general principles contained in existing laws and
codes of practice and are therefore generally applicable to
all medical devices advertising. ABHI therefore encourages
all persons advertising medical devices, not just ABHI
Members, to ensure that Advertising published by them or
on their behalf complies with these Guidelines.
Complaints that any ABHI Member has failed to comply
with these Guidelines will be handled in accordance with the
established Complaints Procedure set out in the ABHI Code
of Ethical Business Practice Complaints Procedure and Panel
Constitution, as amended from time to time.
Interpretation
The singular includes the plural.
Reference to any “commissioned” article, study or material
is a reference to work done at the request or on behalf of
an Advertiser, often in return for payment or some reward
or other support. It may include the work of a journalist or
opinion leader carried out directly or indirectly as a result of
such request. However, reports on collected medical device
clinical data (including reports on clinical investigations, as
the expression is used in the Medical Devices Directives/
Regulation) that are written by or at the direction of the
clinical investigator (“investigator-initiated reports”) shall
not be considered to be “commissioned” whether or not
payments have been made in respect of the investigators’
services or expenses reimbursed or other in-kind support
has been provided if they meet the conditions below. Equally,
reports on collected medical device clinical data (including
clinical investigations, as the expression is used in the
Medical Devices Directives/Regulation) that are written by or
at the direction of the Advertiser pursuant to an agreement
to conduct the clinical data collection (“Advertiser-initiated
reports”) shall not be regarded as “commissioned”, always
provided that such investigator-initiated or Advertiser-initiated
reports relate to clinical data collection and evaluation
processes which are:
a. performed according to scientifically valid standards;
b. subjected to ethical review independent of the Advertiser,
e.g. hospital ethics committee; and
c. initiated and conducted for scientifically and/or medically
legitimate purposes.

55
GUIDELINES
1. SCOPE OF GUIDELINES
1.1 These Guidelines apply to all Advertisements
produced by or on behalf of Advertisers.
Advertising directed wholly or mainly at consumers,
patients or others who are not Healthcare
Professionals is not covered by these Guidelines.
However, such advertising is subject to general UK
advertising law as well as to the industry regulatory
codes administered by the Advertising Standards
Authority, and it should consequently comply with
the law and with those rules.
ABHI and its Members encourage all persons
advertising medical devices, not just ABHI Members,
to ensure that advertising directed at non-Healthcare
Professionals which is published by them or on their
behalf complies with these Guidelines.
1.2 The following is a non-exhaustive list of the forms
of Advertising that may be captured by these
Guidelines:
• Advertisements in Healthcare Professional
journals, brochures, leaflets, circulars, mailings,
e-mails, text transmissions (including SMS and
MMS), social media sites, fax transmissions,
catalogues, follow-up literature and other
electronic or printed material and/or verbal
communications;
• detail aids and other printed material used by
representatives;
• posters and other promotional media in public
places at Healthcare Professional events,
including moving images;
• video and DVD Advertisements intended solely
or primarily for release or use at Healthcare
Professional events;
• audio-cassettes, films, records, tapes, video
recordings intended solely or primarily for
release or use at Healthcare Professional events;
• Advertisements in non-broadcast electronic
media, including but not limited to: online
Advertisements (including banner or
pop-up Advertisements and online video
Advertisements); search listings; commercial
classified Advertisements;
• Advertisements transmitted by Bluetooth;
Advertisements distributed through web widgets
and online sales promotions and prize promotions
• web-based data services;
• Advertisements in marketing databases containing
Healthcare Professionals’ contact information;
• Advertorials.
1.3 To comply with these Guidelines, Advertising
must also comply with all other applicable laws
and regulations. For example, Advertising that
is in breach of the requirements of the Business
Protection from Misleading Marketing Regulations
2008 will also be a breach of these Guidelines and
therefore of the ABHI Code.
1.4 Advertising must be suitable for the intended
audience and must conform to generally acceptable
standards of good taste. It should respect the
principles of fair competition generally accepted in
business.
1.5 An Advertisement should be readily recognisable
by the intended audience as an Advertisement and
its commercial intent must be made clear if that is
not obvious from the context.
2. ACCURACY AND SUBSTANTIATION
OF CLAIMS AND INFORMATION
2.1 Information, claims and comparisons included in
or as part of any Advertisement must be accurate,
balanced, fair, objective and unambiguous and must
be based on a fair evaluation of appropriate evidence
and reflect that evidence clearly. They must not
mislead the intended audience either directly or by
implication, by distortion, exaggeration or undue
emphasis. All reasonable efforts must be used to
ensure that the substantiation for all information,
claims and/or comparisons in an Advertisement is
in accordance with an up to date evaluation of all the
relevant clinical and scientific evidence.

56
Material used in or as part of any Advertisement must be
sufficiently complete to enable the intended audience to form
their own opinion of the therapeutic value of the device.
2.2 Different types of evidence are permissible to
support claims in Advertisements. The evidence
may include clinical data (which could be pre- or
post-market data, including registry data); the
results of a clinical investigation; laboratory data
and testing, including in vitro test data; engineering
data; and historical post-market experience.
All evidence must be relevant, balanced,
comprehensive and credible and must in all
cases be consistent with any specified device’s
CE marked Intended Purpose, as must the overall
impression created by the Advertisement, including
any graphics or artwork. Advertisers must in
all cases hold documentary evidence (which
includes equivalent recorded evidence, e.g. video)
to substantiate all claims (direct or implied). This
documentary evidence must be in existence
before or at the time of the publication of the
Advertisement.
2.3 Claims or comparisons made, or information
included, in Advertisements must accurately reflect
the balance of all relevant evidence. If justification
for the content of an Advertisement relies on any
selection from the available evidence, that selection
must be fair and balanced so that the Advertisement
does not mislead or give a false impression.
Evidence should be scientifically robust. If there
is a significant division of scientific, medical or
other expert opinion about any claims made in an
Advertisement, those claims must not be presented
as being generally agreed and it should be clear
from the Advertisement that there is a division of
opinion on the relevant matter.
Advertisers must make clear whether the evidence
relied upon to substantiate claims used in an
Advertisement is clinical or some other type of
evidence or a combination. If the Advertisement
includes claims that rely on a particular clinical
investigation, that investigation must have been
carried out to a standard equivalent to that required
for clinical evaluation of a device under Annex X of
Directive 93/42/EEC or Annex 7 of Directive 90/385/
EEC or Annex XV of Regulation (EU) 2017/745, as
applicable, as at the date of the Advertisement.
Advertisers must not imply that claims are based
upon peer-reviewed clinical investigation evidence
where this is not the case as this will create a
misleading impression.
2.4 Testimonial evidence on its own is not sufficient
substantiation for objective claims. However,
provided the overall effect is not misleading, it
may be sufficient to use testimonial evidence to
justify subjective claims (for example based upon
subjective perception) or opinions. Testimonial
evidence must not be misleading and must illustrate
typical examples only. (See also section 11.1 below.)
2.5 If engineering data or in vitro or other laboratory
test data is used to substantiate claims made in
Advertisements it must be directly relevant to,
and significant for, the product being advertised.
Particular care must be taken in extrapolating from
such data to avoid any misleading impression as to
the significance of the data.
2.6 Information and claims about side-effects used
in or as part of any Advertisement must reflect
available evidence or be capable of substantiation
by clinical experience. It must not be stated that a
product has no side-effects. The word ‘safe’ must
not be used without qualification. Medical devices
are all CE marked on a risk-benefit balance. No
medical device is 100% safe and advertising claims
should not create such an impression i.e. that the
medical device is absolutely or completely safe as
this is likely to be misleading.
2.7 Advertisements must encourage the appropriate
use of a device (or Related Service) by presenting it
objectively and without exaggerating its properties.
Exaggerated or all-embracing claims must not be
made, and superlatives must not be used except for
those limited circumstances where they relate to a
clear fact about a device.
Any claim that a device (or a particular material,
component comprised in a device or an active
ingredient forming an integral part of that device)
has some special merit, quality or property must be
substantiated.
2.8 The word ‘new’ must not be used for more than
twelve months from the date on which a device or
an Intended Purpose of that device or any Related
Service has been generally available in the UK in the
form referred to in the Advertisement.
The claim that the medical device or Related Service
(or new indication or feature) is “new” is no longer
acceptable after the device or Related Service (or

57
new indication or feature) has been available for
more than a year as this is no longer up-to-date
and therefore misleading. While Advertising already
in circulation need not be actively withdrawn,
Advertisers should not continue to use these once
the new device or Related Service (or new indication
or feature) has been placed on the market in the UK
for more than 12 months.
Where a device or Related Service (or new indication
or feature) has been available in the UK in only one
sector but subsequently becomes available in
additional sectors (e.g. private healthcare sector
availability is expanded to include NHS availability)
it is still not possible to claim the device or Related
Service (or new indication or feature) is “new”
though it is allowable to refer to the fact that the
device or Related Service (or new indication or
feature) is “new to the NHS” provided the overall
impression this creates is not misleading.
3. COMPARATIVE ADVERTISING
3.1 A comparison used in or as part of any
Advertisement is only permitted if:
• it is not misleading;
• devices or services for the same needs or for
the same Intended Purpose are compared;
• one or more material, relevant, substantiable
and representative features are compared;
• no confusion is created between the device or
service advertised and that of a competitor or
between the Advertiser’s trade marks, trade
names, other distinguishing marks and those
of a competitor;
• the trade marks, trade names, other
distinguishing marks, products, services,
activities or circumstances of a competitor are
not discredited or denigrated;
• no unfair advantage is taken of the reputation of
a trade mark, trade name or other distinguishing
marks of a competitor; and
• the Advertiser’s devices or services are not
presented as imitations or replicas of goods or
services bearing a competitor’s trade mark or
trade name.
3.2 Where comparative claims are made there should
be clear evidence to support the claim bearing
in mind the potential commercial impact of
comparative claims. The intent of any comparison
should be that it provides valuable, objective and
accurate information comparing products and/or
associated services for the benefit of Healthcare
Professionals and their patients. It should not simply
be a means of denigrating a competitor’s product or
Related Service.
3.3 Care must be taken not to mislead when expressing
data as percentages. Patient numbers should be
included whenever possible. Differences which
do not reach statistical significance must not be
presented in such a way as to mislead.
3.4 It is acceptable for a member to report on the
outcomes of comparative testing of medical
devices in an Advertisement provided:
a. the devices have been subjected to the same
and appropriate testing; and
b. the outcomes are reported in a fair and balanced
manner; and each outcome is referenced and
consistent with the body of evidence.
3.5 It is acceptable to report on the outcomes
of separate testing of medical devices in an
Advertisement provided a qualifying statement is
included to make clear that the substantiating data
comes from separate studies.
3.6 Hanging comparisons whereby a device or Related
Service is described as being better or stronger or
such like without stating that with which the device
is compared must not be made.
4. REQUESTS FOR SUBSTANTIATING
DATA
4.1 If a bona fide request is made to an Advertiser to
substantiate any information, claim or comparison
used in or as part of any Advertisement, the enquiry
must be acknowledged within ten working days of the
date when the Advertiser has sufficient information
to understand the nature of the enquiry or complaint.
The initial response should where relevant indicate
when a full response will be provided.

58
Unless section 4.2 applies, a full response together
with relevant substantiating data must be provided
within thirty working days of an adequately clear
request being received.
A bona fide request means one received from an
independent Healthcare Professional or from
another person (including from companies) having
a legitimate interest in the substantiation requested.
However, there is no requirement to respond
to fishing expeditions by competitors or others
that are simply designed to obtain confidential
or commercially sensitive information about the
Advertiser’s products or business.
There is no need to supply information relating
to the validation of a device’s explicit CE marked
Intended Purpose or for claims that are expressly
covered by the Intended Purpose of the device.
4.2 In justifying an Advertisement, the Advertiser
must be prepared to give relevant technical, clinical
and scientific data. The Advertiser may be justified
in requiring the person requesting substantiation
of the Advertisement to enter into a confidentiality
agreement in relation to information disclosed.
An Advertiser shall not be obliged to disclose
confidential or commercially sensitive data or
material directly to the person requesting it where
such disclosure might cause financial harm or
otherwise damage the business of the Advertiser.
In such cases confidential disclosure to an
independent mediator or expert or, (if a formal
complaint is to be lodged under the Code) to
the Panel, for consideration may be appropriate.
Confidential disclosure of this type shall not be
appropriate in cases where it is primarily the
insufficiency of the Advertiser’s substantiating
data, which is likely to result in the financial harm or
damage to the Advertiser’s business.
5. USE OF PUBLISHED DATA TO
SUPPORT ADVERTISING
5.1 When promotional material used in or as part of
any Advertisement refers to published studies,
including clinical investigations, clear references
must be given.
5.2 All artwork used in or as part of any Advertisement
including illustrations, graphs and tables must
conform to the letter and spirit of these Guidelines
and of the CoBP. If artwork or data is taken from
published studies a reference must be given. Graphs
and tables must be presented in such a way as to
give a clear, fair, balanced view of the matters with
which they deal, and must not be included unless
they are relevant to the claims or comparisons being
made.
If a graph or table is reproduced from a published
study, it should not be altered unnecessarily. In any
event, the way in which the material is used must
not distort or give a false impression of the evidence
published in that study and the Advertiser must
clearly state that the material has been modified.
5.3 Advertisers may use scientific peer-reviewed
journals, clinical or otherwise, to substantiate
claims made in an Advertisement. There may
also be relevant data to be found in publications
of patient data and case studies, whether or not it
is published in a scientific journal. The key issues
are the quality, relevance and overall credibility of
the data when relied upon to support claims made
in an Advertisement. (See also section 7.1 of these
Guidelines).
6. NO DISPARAGEMENT
6.1 The products and activities of other medical
device companies must not be disparaged in an
Advertisement.
6.2 Healthcare Professionals and the clinical and
scientific opinions of Healthcare Professionals
must not be disparaged in any Advertisement.
7. NON-REFEREED ARTICLES
7.1 Journal or other articles which have not been
refereed are unlikely to be sufficiently robust to
justify science-based claims about a device’s safety
or performance on their own. It also follows that
reprints of such material must not be provided to
Healthcare Professionals unsolicited.
However, if claims in an Advertisement are based
upon subjective patient or consumer perceptions,
it may be acceptable to use non-refereed articles to
promote those specific aspects of a product.

59
8. OFF-LABEL USE
8.1 If Journal articles discuss off-label use of a
particular device, such discussion must not be
used with the intention of promoting such off-label
use. For example, the unsolicited supply of such
materials to Healthcare Professionals would be
an infringement of these Guidelines. However, it is
permissible to supply such articles upon receipt
of a bona fide unsolicited, written request from a
Healthcare Professional. Care should be taken to
record the written request and the response sent.
9. QUOTATIONS
9.1 Quotations from medical and scientific literature or
from personal communications must be accurate
and must reflect the meaning of the author. The
precise source of the quotation must be identified.
9.2 Quotations relating to devices taken from public
broadcasts, for example on radio and television,
and from private occasions, such as medical
conferences or symposia, must not be used without
the formal permission of the speaker.
9.3 Where references are made to medical and
scientific literature or to personal communications
these must accurately reflect the author’s meaning.
9.4 All reasonable care must be taken to avoid
ascribing claims or views to authors when these no
longer represent the current views of the authors
concerned.
10. MATERIAL COMMISSIONED BY
THE ADVERTISER
10.1 An article or piece of information that is
commissioned by or on behalf of the Advertiser
must be clearly identified as such on its face and
the Advertiser must also be clearly identified.
In line with the Introduction, Section 1.2 of these
Guidelines, “commissioning” of an article in this
context means the provision of any financial or other
support or reward to the author for the purpose of
producing the article. This includes the provision
of any in-kind support, e.g. products, facilities,
secretarial or administrative support by Advertisers.
However, reports on collected medical device clinical data
(including reports on clinical investigations, as the expression
is used in the Medical Devices Directives / Regulation) that
are written by or at the direction of the clinical investigator
(“investigator-initiated reports”) shall not be considered to be
“commissioned” whether or not payments have been made in
respect of the investigators’ services or expenses reimbursed
or other in-kind support has been provided if they meet the
conditions below. Equally, reports on collected medical device
clinical data (including clinical investigations, as the expression
is used in the Medical Devices Directives / Regulation) that are
written by or at the direction of the Advertiser pursuant to an
agreement to conduct the clinical data collection (“Advertiser-
initiated reports”) shall not be regarded as “commissioned”,
always provided that such investigator-initiated or Advertiser-
initiated reports relate to clinical data collection and evaluation
processes which are:
a. performed according to scientifically valid
standards;
b. subjected to ethical review independent of the
Advertiser, e.g. hospital ethics committee; and
c. initiated and conducted for scientifically and/or
medically legitimate purposes.
10.2 Where such a commissioned article is used
solely or primarily for the purpose of supporting
a claim, including a comparative claim, the article
cannot be the sole or primary evidence relied on
to substantiate the claim – the claim must also
be separately substantiated by other, additional
evidence of sufficient quality and quantity to
meet the standards specified in these Guidelines.
Investigator-initiated or Advertiser-initiated reports
which meet the conditions set out in the Preamble,
Interpretation of these Guidelines may, however,
provide independent substantiation for the safety
or performance of a device or Related Service.
11. TESTIMONIALS AND
ENDORSEMENTS
11.1 For testimonials and/or endorsements (including
but not limited to blogs) to be used in or in support
of any Advertisement, the Advertiser must ensure
that the testimonials or endorsements (whether
from patients, suitably qualified Healthcare
Professionals, celebrities or members of the
public generally) whether written or spoken, are
documented, genuine, not misleading and illustrate
typical examples only, except where these are
obviously fictitious.

60
In this context “typical” means something that is
experienced by the great majority of patients or
users, as applicable. If a testimonial or endorsement
refers to a condition or situation experienced
by only one or very few patients or users, this
must be made clear. Also in this context “suitably
qualified Healthcare Professionals” means persons
who can provide suitable credentials evidencing
relevant professional expertise or qualifications
and accreditation by a professional or regulatory
body that has systems for dealing with complaints
and taking disciplinary action and has registration
based on minimum standards for training and
qualifications.
If a testimonial or endorsement which is used in
Advertising is presented by a person other than
the person originally providing the testimonial /
endorsement – for example when presented by
an actor or model – the effect must not mislead
or otherwise distort the nature of the testimonial
or endorsement. For example, Advertisers could
include a suitable caption or other notice where an
actor speaks the words of the actual provider of the
testimonial to make the actual position clear.
Testimonials or endorsements must relate to the
product being Advertised.
If a testimonial or endorsement is used in or in support
of any Advertisement, the Advertiser must hold
signed and dated documentary evidence, including
contact details, for the provider of the testimonial
or endorsement in question. The Advertiser must
also ensure that he has the consent of the person
providing the testimonial or endorsement both to use
it in connection with Advertising and (if necessary)
to disclose it in connection with the substantiation of
any claim.
Testimonials or endorsements taken from published
articles should be treated in the same way as
quotations (Section 9 above) and in accordance with
any other relevant provisions in these Guidelines.
12. REIMBURSEMENT OF
EXPENSES FOR PROVIDERS
OF TESTIMONIALS OR
ENDORSEMENTS
12.1 Any payment made to a person providing a
testimonial statement or endorsement (or any other
in-kind benefit or advantage provided) must not be
an inducement or reward for giving that statement.
Similarly, no testimonial or endorsement (including
any blog) by any person should be used in or to
support Advertising if the provider of the testimonial
or endorsement has been paid directly or indirectly
(including via in-kind benefits or advantages) on
behalf of the Advertiser to endorse the device or
Related Service or to provide the testimonial.
Reimbursement of reasonable and proportionate
expenses either by or on behalf of an Advertiser,
where the expenses have been incurred by an
individual or a company providing a testimonial or an
endorsement for use in or in support of Advertising,
is permitted always provided such reimbursement:
a. is limited to out-of-pocket expenses reasonably
incurred by the provider of the testimonial or
endorsement in connection with its provision
(e.g. reasonable travel costs to reach the filming
location provided the location chosen and the
mode of travel do not themselves constitute
an inducement or reward for providing the
testimonial / endorsement); and
b. is either paid to a third party travel agent /
vendor to organise the travel / accommodation
or only reimbursed to the testimonial /
endorsement provider against original invoices
or receipts; and
c. is kept entirely separate from any payments or
other arrangements relating to any background
collaboration or sponsorship activities so that
the reimbursement is not, and does not appear
to be, a payment made in connection with, or as
part of a broader scheme of, paid collaboration
or sponsorship between the provider of the
testimonial / endorsement and the Advertiser
(or any person or organisation connected to
him); and
d. specifically in the case of Healthcare
Professionals, such reimbursement is only paid
in circumstances where the reimbursement
payment does not in any way amount to a

61
payment (i.e. as an inducement or reward) for
the testimonial or endorsement to be provided
as a service by the Healthcare Professional (nor
does it have the appearance of being such a
payment).
12.2 There should be a written agreement (e.g. letter
agreement) signed and dated by or on behalf of both
the Advertiser and the provider of the testimonial /
endorsement setting out which expenses will be
reimbursed and the mechanism of reimbursement
(as well as also meeting the other requirements
stated at sections 11 and 12.1 above). Expenses
should only be reimbursed to the testimonial
/ endorsement provider by or on behalf of the
Advertiser against original invoices or receipts.
If the provider of the testimonial / endorsement is
a Healthcare Professional, the written agreement
must also comply with the requirements of Part 1,
Chapter 6 of the ABHI Code (Arrangements with
Consultants).
It is important that any reimbursement of out-
of-pocket expenses should not be, or appear
to be, a contrived payment for a testimonial or
endorsement. No payment is permitted simply
to obtain the testimonial / endorsement of a
Healthcare Professional or any other person, and
the written agreement must make this clear.
13. EFFECT OF BACKGROUND
COLLABORATION OR
SPONSORSHIP ON
TESTIMONIALS AND
ENDORSEMENTS
13.1 Advertisers may use testimonials or endorsements
of a device or a Related Service by patients,
celebrities, suitably qualified Healthcare
Professionals or members of the public in, or in
support of, Advertising where there is background
collaboration or sponsorship by or on behalf of the
Advertiser only if:
a. the background collaboration with or sponsorship
by the Advertiser of the individual or company
providing the testimonial or endorsement is
made clear in the Advertisement; and
b. the testimonial or endorsement meets
the requirements for testimonials and
endorsements contained in these Guidelines
(see Sections 11 and 12).
“Background collaboration or sponsorship” in this
context means any relationship or arrangement(s)
(including for example consultancy arrangements
or any other type of service provision) between the
provider of the testimonial or the endorsement and
the Advertiser (or its representatives or affiliates)
which is not connected with the provision of the
testimonial or the endorsement, irrespective of
whether such relationship or arrangement involves
any payments (or any other in-kind benefit or
advantage) or not.
Examples of background collaboration or
sponsorship could include arrangements for
research, participation on advisory boards or
product development.
Reports of clinical investigations shall not be
regarded as testimonials or endorsements of
a product or Related Service. Rather, they may
provide independent substantiation for the safety
or performance of a device or Related Service.
Statements made of his/her own volition by a
Healthcare Professional (including but not limited
to off-the-cuff statements) shall not be treated as
testimonials or endorsements even if they have
been made at an occasion (such as a conference
or meeting) where the meeting organizer is directly
or indirectly supported by the Advertiser, always
provided such support is incidental to the statement
made. If the Advertiser subsequently wishes to use
such a statement in or in support of Advertising the
Advertiser must ensure all the requirements of these
Guidelines regarding testimonials or endorsements
(see under sections 11 and 12) are met.
13.2 When Advertisers enter into written agreements
covering potential background collaboration or
sponsorship activities, Advertisers should anticipate
the possibility that the party providing the services
under the relevant agreement may at some future
time provide a testimonial or endorsement in relation
to a device or a Related Service. Advertisers should
therefore include provisions in such agreements to
cover this eventuality (specifically points (i) and (ii)
below) in order to ensure that they will be able to
meet the requirements in these Guidelines relating
to transparency of background collaboration
and sponsorship and use of testimonials and
endorsements generally. Such agreements should

62
therefore include written agreement by the potential
future testimonial / endorsement provider to:
a. disclose clearly and conspicuously in any
testimonial / endorsement any background
collaboration with, or sponsorship by or on
behalf of, the Advertiser; and
b. promptly withdraw, or revise and re-publish any
testimonial / endorsement at the Advertiser’s
reasonable request where the request is made
on objective grounds of ensuring safety and
effective use within the Intended Purpose of the
device or Related Service.
Such agreements should be in writing and signed
and dated by both the Advertiser and the potential
provider of the testimonial / endorsement.
14. ANNEX 1 GENERAL
ADVERTISING LAW
AND CODES
The Business Protection from Misleading Marketing
Regulations 2008
The Consumer Protection from Unfair Trading
Regulations 2008
The UK Code of Non-Broadcast Advertising, Sales
Promotion and Direct Marketing (CAP Code)
The UK Code of Broadcast Advertising (BCAP Code)

63
COMPLAINTS PROCEDURE
AND PANEL CONSTITUTION
PART 4

64
INTRODUCTION
A Code of Ethical Business Practice (“Code”) has been
adopted by the Association of British Healthcare Industries
(“ABHI”) to set standards for ethical behaviour and to govern
ethical promotion and sales practices in the medical devices
industry in the UK (“the Industry”). The Code is administered
by the ABHI secretariat in conjunction with the
“Chairman” of the Complaints Adjudication Panel,
“Panel”, which comprises a number of individuals with
relevant industry and/or other experience. Compliance
with the Code and with this Procedure is mandatory
for members of ABHI and companies which (although
not members) (together “Applicable Companies”) have
agreed to comply with the Code and this Procedure
and accept the jurisdiction of the Panel. “Complaints”
made under the Code include direct complaints, as well
as indirect complaints made to MedTech Europe and
referred to ABHI for adjudication, and may also include
issues raised in the media or otherwise that fall within the
remit of the Code.
The Panel is not an investigatory body as such. It asks the
company whose activities are the subject of a complaint
(“respondent”) for a complete response and may ask
the parties to a case for further information in order to
clarify the issues. The company or individual making the
complaint (“complainant”) has the burden of proving their
complaint on the balance of probabilities.
Any company wishing to make a complaint against an
Applicable Company utilising this Complaints Procedure
must initially attempt to reconcile any dispute with that
company through conciliation or mediation procedures
or mutual settlement. Any individual wishing to make a
complaint against an Applicable Company utilising this
Complaints Procedure must initially attempt to resolve
the complaint utilising that company’s internal or external
whistleblowing and/or dispute resolution procedures. If, in
either case, this does not prove possible then complaints
are initially considered by the Chairman who will determine,
if appropriate in consultation with the complainant and/or
respondent, whether there is a case to answer.
Anonymous complaints (where the complainant does not
disclose their identity to the Panel or Chairman) may be
accepted in exceptional circumstances at the discretion
of the Chairman, however the weight to be attached to
any evidence may be adversely affected if the source
is anonymous, and thus in many instances it will not be
possible for such a complaint to proceed.
Confidential complaints (where the complainant does
disclose their identity to the Panel or Chairman but
requests (whether at the outset or during the course of the
complaint) that their identity remains confidential) are not
encouraged but may be accepted at the discretion of the
Chairman, however the ability of the respondent to respond
properly to information or matters put to them and therefore
the Panel’s ability to adjudicate properly on any particular
complaint may be adversely affected if the identity of the
complainant is kept confidential, and therefore in certain
instances it will also not be possible for such a complaint to
proceed. Confidential complaints will not be accepted from
Applicable Companies.
Reports on cases that are subject to a Panel decision
are published and are available on request and on
the ABHI Code of Ethical Business Practice website
www.abhicodeofpractice.org.uk. Information on cases that
are resolved through mediation are published in a summary
report although the names of the companies or individuals
involved will not be disclosed.
Complaints about the conduct of any Applicable Company
under the Code should be submitted to:
Telephone: +44 (0)20 7960 4360,
E-mail: complaints@abhi.org.uk.

65
DISPUTE RESOLUTION PRINCIPLES
General framework
The procedures set out below are intended to provide an
effective and efficient complaint-handling process, the
object of which is to ensure compliance with the Code. It is
based on principles of proportionality, speed, due process,
fairness and transparency.
Applicability of the Code
Member Companies must comply with the Code as a
minimum standard when:
a. Member Companies interact with Healthcare
Professionals and Healthcare Organisations registered
and practising in the UK irrespective of where the
activity takes place; and/or
b. Activities take place in MedTech Europe Geographic
Area, irrespective of where Healthcare Professionals
and Healthcare Organisations are registered and
practicing.
MedTech Europe Geographic Area includes the countries
in the European Economic Area as well as those countries
where MedTech Europe Member Associations are located.
STRUCTURE AND
RESPONSIBILITIES
1. The Panel
1.1 The Panel is responsible for resolving complaints
made under the Code. It may also assist in
arranging for conciliation and/or mediation
between companies when requested to do so.
1.2 The Panel and Chairman report to the ABHI Board
in respect of their activities and the operation and
administration of this Complaints Procedure.
2. The Panel – Constitution and Procedure
2.1 The members of the Panel and the Chairman and
Vice-Chairman shall be appointed yearly by the
ABHI Board. The Panel shall comprise individuals
with a background in the industry and who have
relevant expertise for assessing Complaints under
the Code. The names of the members of the Panel
shall be published on the ABHI website. There is
no limit on the length of time that the Chairman,
Vice-Chairman or other members of the Panel
may serve in those capacities.
2.2 In the event that a Complaint cannot be resolved
through mediation by the Secretariat, Chairman
and such members of the Panel who may be
enlisted for this mediation, the Complaint shall
be referred to the Panel for formal adjudication.
Each individual Panel appointed to consider any
particular complaint shall, so far as possible,
comprise of an appropriate cross-section of Panel
members and shall be appointed by the Chairman
from the wider list of Panel members referred to.
The Chairman shall ensure that a Panel contains
as far as possible individuals with expertise in
relevant areas, e.g. legal, ethical compliance,
advertising and promotion, scientific, business, as
appropriate. Each individual Panel shall comprise
of a minimum of the Chairman and three further
Panel members and decisions shall be made by
majority voting. The Chairman, and in his absence,
the Vice-Chairman, acts as Chairman of the Panel
and has both an original and a casting vote.
2.3 Rulings are made on the basis that a complainant
has the burden of proving their complaint on the
balance of probabilities.
2.4 In advance of their appointment to an individual
Panel to consider any particular complaint, all
Panel members involved shall sign an agreed form
statement of independence confirming that they
have no conflict of interest in adjudicating on the
particular complaint.
2.5 The Panel may obtain expert assistance in any
field. Expert advisers who are consulted may be
invited to attend a meeting of the Panel, but have
no voting rights. Each such expert shall also be
required to confirm that they have no conflict of
interest in providing expert assistance on any
particular case.
2.6 Subject to paragraphs 2.7 and 2.8 below, any
decision of the Panel shall be final, and there
shall be no appeals procedure against any Panel
rulings.
2.7 At any time during a complaint handling process
the Chairman or the Panel shall be entitled to refer
questions of interpretation of the Code in writing
to the MedTech Europe Compliance Panel. The
MedTech Europe Compliance Panel may at its
discretion either decline to entertain the matter if
it is felt that no question of principle is at issue or
accept the interpretation referral, and review and
provide guidance on the interpretation of the Code.
Where such a request has been made, the
Chairman and the Panel shall be obliged to
follow and apply any such guidance provided by

66
MedTech Europe unless so doing would conflict
with UK law. For the avoidance of doubt MedTech
Europe shall not rule on the merits or facts of
any particular complaint but only on questions of
interpretation of the Code.
2.8 This Procedure shall not preclude complainants
from having recourse to courts or other
tribunals to seek resolution of complaints and
any complaints made under the Code and this
Procedure should not be initiated or should be
suspended in case of initiation of formal civil court
proceedings with respect to the same subject
matter. Where a governmental or regulatory
investigation or criminal proceedings are either
initiated or threatened against an Applicable
Company with respect to the same subject
matter, that company shall notify the Chairman of
the same in confidence, who shall then have the
discretion whether or not to suspend any relevant
proceedings under this Procedure.
2.9 For the avoidance of doubt, all companies or
individuals that wish to utilise this Complaints
Procedure to submit a Complaint, and all
Applicable Companies that have agreed to
submit to the jurisdiction of the Panel in respect
of a Complaint, shall not, save in respect of fraud,
fraudulent misrepresentation, manifest error
or gross negligence by the Panel (or a member
thereof) in arriving at a Panel ruling, commence
legal proceedings or any analogous contentious
or complaint proceedings against ABHI, the Panel,
or any Panel member, in respect of any loss or
damage they may suffer as a consequence of any
such Panel ruling.
COMPLAINTS PROCEDURE
3. Action on Complaints
3.1 Prior to lodging a formal complaint against an
Applicable Company under this Procedure, any
company wishing to make a complaint against an
Applicable Company shall first attempt a genuine
mediation with that company in an attempt to
reach an amicable solution. For complaints
between Applicable Companies, such genuine
attempt at mediation shall be a pre-condition
before a complaint can be made utilising this
Procedure and any such complainant shall
adduce sufficient evidence to the Panel to prove
such genuine attempts at mediation have been
made. Any individual wishing to make a complaint
against an Applicable Company utilising this
Complaints Procedure must initially attempt to
resolve the complaint utilising that company’s
internal or external whistleblowing and/or
dispute resolution procedures. If, in either case,
no amicable resolution of the complaint can be
reached through such means within a reasonable
timeframe however, the complainant shall be
entitled to pursue the matter further directly via
this Procedure.
3.2 Any individual or company making a complaint
under this Procedure that is not an Applicable
Company shall be required (in the case of a company
for a minimum of 18 months, and in the case of
an individual for the duration of the Procedure) to
undertake to abide by the provisions of the Code
and of this Procedure as a pre-condition before a
complaint can be made utilising this Procedure.
3.3 If a complaint is received about a company other
than an Applicable Company, such company will
be invited to agree to comply with the Code and
accept the jurisdiction of the Panel. In the absence
of such agreement however, the complaint will not
be accepted for adjudication using this Procedure.
Notwithstanding the foregoing, where a complaint
is brought in respect of activities undertaken or
instigated by an Applicable Company’s parent
or other affiliated company which is not itself an
Applicable Company, the Applicable Company will
be deemed as the respondent company for the
purposes of this Procedure and the complaint will
proceed accordingly.
3.4 When the Chairman receives information from
which it appears that an Applicable Company
may have contravened the Code, the Chairman
shall undertake an initial review of the complaint
and will determine (if appropriate, in consultation
with the complainant and/or respondent) whether
there is a prima facie case to answer.
3.5 If, in the view of the Chairman, a complaint does
not show that there may have been a prima facie
breach of the Code, the complainant shall be so
advised. If the complainant does not accept that
view, the following paragraphs of this Section 3
shall apply.
3.6 In the event that the Chairman determines that
there is either a prima facie case to answer, or
(pursuant to paragraph 3.5) the complainant
insists that the complaint is referred to the Panel
for adjudication, then the Chairman shall write
to the managing director or chief executive or
equivalent of the Applicable Company against
whom the complaint has been made requesting
that it provide a complete response to the matters
of complaint.

67
3.7 The respondent company shall provide such
a response in writing to the Chairman within 10
working days. If no such response is provided by
the respondent company within these timescales
then, save as otherwise provided in paragraph 7,
the Panel shall make it’s adjudication on the basis
of the information provided by the complainant
only. Following receipt by the Chairman of the
respondent company’s response, the case shall
be referred to the Panel to determine whether or
not there has been a breach of the Code.
3.8 To assist companies in ensuring that a complete
response is submitted, the Chairman may suggest
relevant supporting material to be supplied,
although it is the responsibility of the respondent
to ensure that a full response is submitted.
3.9 In addition, the Chairman may request (whether
at the suggestion of the complainant or
respondent or at the behest of the Panel) such
further clarifications or documents from either
the complainant or respondent within such
reasonable timescales as he shall deem prudent
and necessary to assist the Panel in making its
determination.
3.10 As an alternative to proceeding directly to the
Panel, the Chairman may offer to facilitate a
time-limited mediation between the parties. Such
mediation shall normally be limited to six working
weeks but may be extended at the Chairman’s
discretion. Both parties shall make every effort
to ensure that relevant personnel are available to
participate in the process as and when they are
required.
The parties shall agree at the outset to the terms
of mediation and that the costs incurred in running
the process shall be divided equally between
them. Only Applicable Companies shall be liable
for mediation costs.
3.11 If the complainant is not an Applicable Company,
the Chairman may suggest the paragraphs of
the Code to be addressed, however when the
complaint is from an Applicable Company, the
complaint must be signed or authorised in writing
by the company’s managing director or chief
executive or equivalent and must state those
paragraphs of the Code which are alleged to have
been breached.
3.12 U n l e s s the information is disclosed in the
complaint, any complainant other than an
Applicable Company will be asked to confirm in
writing whether or not they have any commercial,
financial or other interest in the matter of
complaint or in the company concerned, such
as whether the complainant is an employee or
ex-employee, a consultant or ex-consultant.
Adjudication of a complaint without this written
confirmation will not be permitted to proceed.
Such interests will be disclosed to the respondent
company and will normally be included in the case
report.
3.13 W h e n an Applicable Company advises the
Chairman or Panel that it may have breached the
Code, the Chairman shall treat the matter as a
complaint if it relates to a potential breach of the
Code or if the company fails to take appropriate
action to address the matter. The company’s
response is invited and the procedure set out in
this Section 3 shall be followed.
4. Complaints Arising from Media Criticism
4.1 If it appears to the Chairman from media reports
that an Applicable Company may have breached
the Code, the Chairman may at his discretion
treat such reports as a complaint if it relates to
a potential breach of the Code or if the company
fails to take appropriate action to address the
matter. The company’s response is invited and the
procedure set out in Section 3 shall be followed.
4.2 The author or editor (as applicable) of the relevant
media report may be asked if they want to be
involved in the case and whether they have any
additional information to submit. If the editor or
author declines involvement, this is stated in the
case report.
4.3 A published letter from which it appears that
an Applicable Company may have breached the
Code may also at the discretion of the Chairman
be treated as a complaint. The procedure set out
in Paragraph 4.1 above shall be followed.
5. Panel Rulings
5.1 Where the Panel rules that there is a breach of the
Code, the Panel shall advise the complainant and
the respondent of such in writing and give their
reasons for the decision.
5.2 The respondent company has ten working days
to provide a written undertaking that the activity
in question (if not already discontinued) will cease
forthwith and that all possible steps will be taken
to avoid a similar breach of the Code in the future.
This undertaking must be signed by the managing
director or chief executive or equivalent of the
company and must be accompanied by details of
the actions taken by the company to implement
the undertaking, including dates and timings and
training undertaken.

68
5.3 In extenuating circumstances, an extension
in the time allowed in which to respond may be
granted at the discretion of the Chairman in
accordance with paragraph 7.
5.4 The respondent company must also pay within
twenty working days an administrative charge
based on the cost of convening the Panel and
dealing with the complaint as determined by
the Chairman.
5.5 Where the Panel rules that there is no breach of
the Code, the Panel shall advise the complainant
and the respondent of such in writing and
give their reasons for the decision. Where the
complaint is from an Applicable Company, the
complainant must pay within twenty working
days an administrative charge based on the
cost of convening the Panel and dealing with the
complaint as determined by the Chairman.
5.6 In addition to the foregoing, the Panel may impose
additional or alternative sanctions on either of the
respondent company (in the event of its breach of
the Code) or complainant company (in the event of
no breach of the Code) as appropriate in respect of
any particular complaint. In particular, the Panel may:
a. Require the relevant company to publish any
communication required by the Panel, including
but not limited to explanatory information or
statements of future intent or policy;
b. Issue a formal reprimand;
c. Recommend to the ABHI Board to suspend
the offender from membership of ABHI for
a specified period and impose conditions on
readmission;
d. Recommend to the ABHI Board to expel the
offender from ABHI;
e. Set time-limits for compliance with any
sanction imposed or order made by the Panel
in addition to those specified in paragraphs
5.1 to 5.5 above;
f. Order that either party pay the costs of the
Panel, in whole or part, having regard to a
standard scale published by ABHI and any
other matters considered appropriate;
g. Provide for further sanctions in the event
of further breaches of or non-compliance
with the Code or any order, sanction or
requirement of the Panel (including time
limits), with or without the right to make
further representations before such further
sanctions are to take effect.
6. Case Reports
6.1 At the conclusion of any case under the Code,
the Panel shall advise the complainant and the
respondent of the outcome and a report shall be
published summarising the details of the case.
6.2 In a case where the complaint was initiated by
an individual, other than in those circumstances
where an anonymous or confidential complaint is
accepted for adjudication, that individual shall be
named in the report.
6.3 In a case where the complaint was initiated by a
company or by an organisation or official body,
that company or organisation or official body shall
be named in the report. The respondent company
and the product(s) concerned will usually be
named in the report unless the Chairman in
his discretion deems this inappropriate. Any
information given must not, however, be such
as to identify any individual person within such
company, organisation or official body.
6.4 Where expert assistance has been obtained by
the Panel, the report will include the name and
qualifications of the expert concerned.
6.5 Where guidance has been sought from MedTech
Europe, the question raised by the Chairman or the
Panel and the guidance received from MedTech
Europe shall be included in the report.
6.6 Where a company has been required to issue a
statement of its corrective actions, the report will
reproduce its text and provide details of how the
corrective actions statement was disseminated.
6.7 A copy of the report on a case is made available
to both the complainant and the respondent
company prior to publication. Any amendments
to the report suggested by these parties are
considered by the Chairman, consulting with the
other party where appropriate. The Chairman’s
decision is final.
6.8 Full case reports will appear on a specified
section of the ABHI Code of Ethical Business
Practice website. Access to the relevant section
of the relevant ABHI website referring to cases or
decisions is unrestricted.

69
GENERAL PROVISIONS
7 Amendments to Time Periods
7.1 The Chairman shall, in extenuating circumstances
and at his discretion, be entitled to grant any party
to this Procedure an extension in time or amend
any timescales specified in this Procedure to the
extent that to do so would be fair and reasonable
in the circumstances.
8. Withdrawal of Complaints
8.1 A complaint may be withdrawn by a complainant
with the consent of the respondent company up
until such time as the respondent company’s
comments on the complaint have been received
by the Chairman, but not thereafter. In either
case, the complainant shall pay an appropriate
administrative charge.
9. Charges
9.1 The administrative charges referred to in
Paragraphs 5.4, 5.5 and 8.1 above are determined
by the Chairman based on the costs of formally
convening the Panel and in dealing with the
complaint.
9.2 Administrative charges are payable only by
Applicable Companies, and these companies are
liable for such charges whether they are members
of the ABHI or not.
9.3 Where two or more companies are ruled in breach
of the Code in relation to a matter involving a joint
activity, each company shall be separately liable to
pay any administrative charge which is payable.
9.4 Failure to pay any of the administrative charges
provided for by this paragraph must be reported
by the Chairman or the Panel to the ABHI Board.
In such circumstances, the Panel shall be entitled
to impose or recommend such further sanctions
as it deems appropriate, including (but not limited
to) those referred to in paragraph 5.6.
10. Anonymity and Confidentiality
10.1 Any complainant or respondent shall be entitled
to request that any document or information
provided to the Panel or Chairman pursuant to this
Procedure is not disclosed further on the grounds
of confidentiality, in particular to either the
complainant or respondent as the case may be
or in any case report. The Chairman shall decide
in his discretion whether to grant such request, in
particular taking in to account the ability of either
the complainant or the respondent to respond
properly to information or matters put to them if
such documents or information is excluded and
therefore the Panel’s ability to properly adjudicate
on any particular complaint. The Chairman’s
decision on this issue shall be final.
10.2 Anonymous complaints (where the complainant
does not disclose their identity to the Panel
or Chairman) may be accepted in exceptional
circumstances at the discretion of the Chairman,
however the weight to be attached to any evidence
may be adversely affected if the source is
anonymous, and thus in many instances it will not
be possible for such a complaint to proceed.
10.3 Confidential complaints (where the complainant
does disclose their identity to the Panel or
Chairman but requests (whether at the outset
or during the course of the complaint) that their
identity remains confidential) are not encouraged
but may be accepted at the discretion of the
Chairman, however the ability of the respondent
to respond properly to information or matters
put to them and therefore the Panel’s ability to
adjudicate properly on any particular complaint
may be adversely affected if the identity of the
complainant is kept confidential, and therefore
in certain instances it will also not be possible
for such a complaint to proceed. Confidential
complaints will not be accepted from Applicable
Companies.
11. Amendments to the Code of Ethical Business Practice
and Complaints Procedure
11.1 The Code and this Procedure may be amended by
a simple majority of those present and voting at
the ABHI Board.
11.2 The Panel may, in the light of their experience,
make recommendations for amendment of the
Code and this Procedure.

GLOSSARY AND
DEFINITIONS
PART 5
71
ABHI: Association of British HealthTech Industries
Advertiser: the ABHI Member by or on behalf of whom
an Advertisement is placed and/or the ABHI Member
supplying the relevant product or Related Service if he has
approved the Advertisement or the Advertisement has been
approved or placed by the Member’s affiliated company
which is not a Member of ABHI.
The ABHI Member shall be treated as the Advertiser
where the ABHI Member, or the Member’s affiliated
company which is not a Member of ABHI, has approved
Advertisements placed by a third party distributor or other
service provider.
Advertisement or Advertising: any marketing
communication or Advertorial issued by or on behalf of
an Advertiser in whatever form (including but not limited
to verbal communications) and through whatever media
(including the world wide web) that is intended wholly or
mainly to influence Healthcare Professionals or Healthcare
Organisations directly or indirectly in (i) their choice of
medical devices (or Related Services) to be purchased,
leased, used or supplied for use by, or in connection with the
treatment of, human patients or in (ii) any recommendation
that they make to others about such purchase, lease, use
or supply.
An example of Advertising intended to influence Healthcare
Professionals or Healthcare Organisations indirectly would
be information provided by or on behalf of an Advertiser
to journalists working for publications which are directed
primarily at Healthcare Professionals or Healthcare
Organisations.
For the avoidance of doubt product labelling, packaging
and instructions for use shall not in the ordinary course be
treated as Advertising for the purpose of these Guidelines.
Advertorial: any communication, feature, announcement
or promotion in a form that resembles independent editorial
comment published by or on behalf of an ABHI Member,
the content of which is controlled by the Advertiser, not the
publisher, irrespective whether it is disseminated in return
for a payment or other reciprocal arrangement, or free of
charge.
Applicable Company: a member of ABHI or an organisation
that has undertaken to comply with the provisions of the
ABHI Code of Ethical Business Practice.
Charitable Donations: means provision of cash,
equipment, company product or relevant Third Party
product, for exclusive use for charitable or philanthropic
purposes and/or to benefit a charitable or philanthropic
cause. Charitable Donations may only be made on an
unrestricted basis and to bona fide charities or other non-
profit entities or bodies whose main objects are genuine
charitable or philanthropic purposes.
CoBP or Code: the code of ethical business practice
published by ABHI as amended from time to time.
Company Events: means activities of any type that are
planned, budgeted, managed and executed in whole or
in part by or on behalf of Member Companies to fulfil a
legitimate, documented business need of the Member
Company, including but not limited to a legitimate business
need to interact with customers including Healthcare
Professionals and/or Healthcare Organisations.
Conference Vetting System (CVS): means the
centralised decision-making process which reviews the
compliance of Third Party Organised Educational Events
with the Code and which is managed independently of ABHI
under the supervision of the MedTech Europe Compliance
Panel. For more information see:
www.ethicalmedtech.eu
Disclosure Guidelines: means the Code provisions
setting out the public disclosure requirements under the
Code.
Demonstration Products (Demos): means either single-
use or multiple-use products provided free of charge by
or on behalf of a Member Company to HCOs or HCPs,
who are equipped and qualified to use them. Demos are
supplied solely for the purpose of demonstrating safe and
effective use and appropriate functionality of a product and
are not intended for clinical use. Demos do not include the
following:
•
Samples;
•
Evaluation Products;
•
Products provided at no charge as part of a Charitable
Donation or as part of a Research or Educational Grant;
or
•
Products provided at no additional charge as part of
the overall purchase price in a commercial supply
arrangement, e.g. as part of an agreed discount
arrangement, or as substitute products provided
pursuant to a warranty agreement.
72
Educational Grants: means provision of funding, Member
Company or third party products or other in-kind support
to a Healthcare Organisation by or on behalf of a Member
Company on a restricted basis for use solely for the support
and the advancement of genuine medical education of
Healthcare Professionals, patients and/or the public on
clinical, scientific and/or healthcare topics relevant to
the therapeutic areas in which the Member Company is
interested and/or involved.
Employer Notification: means the prior written
notification provided to a Healthcare Organisation (e.g.
hospital administration), a Healthcare Professional’s
superior or other locally-designated competent authority
of any interaction, collaboration or other matter concerning
any Member Company and any Healthcare Professional,
the purpose and/or scope of which requires notification
under this Code.
Entertainment: Entertainment includes, but is not limited
to, dancing or arrangements where live music is the main
attraction, sight-seeing trips, theatre excursions, sporting
events (e.g. skiing, golf or football match) and other leisure
arrangements. For the avoidance of doubt, incidental,
background music shall not constitute Entertainment.
Evaluation Products: means either single-use or multiple-
use products and/or equipment provided free of charge to
a Healthcare Organisation by or on behalf of a Member
Company for purposes of obtaining defined, evaluative user
feedback over a defined period of use when used within the
scope of their intended purpose, as per the authorisation in
the country where the supply occurs. Evaluation Products
do not include the following:
• Demos;
• Samples;
• Products provided at no charge as part of a Charitable
Donation or as part of a Research or Educational Grant;
or
• Products provided at no additional charge as part of
the overall purchase price in a commercial supply
arrangement, e.g. as part of an agreed discount
arrangement, or as substitute products provided
pursuant to a warranty agreement.
Event: means either a Company Event or Third Party
Organised Educational Event.
Faculty: means a podium speaker, moderator and/or chair,
who presents during a Third Party Organised Educational
Event. Poster- and abstract-presenters are not considered
to be Faculty.
Financial Hardship: means in relation to a Healthcare
Organisation extreme and unavoidable financial
distress resulting from matters outside the Healthcare
Organisation’s control where the Healthcare Organisation
is unable to operate and where patient care is consequently
jeopardised. Financial distress resulting in whole or in part
from mismanagement of the Healthcare Organisation’s
funds or other matters within its control is not considered
to be financial hardship. Financial Hardship must be
documented and objectively substantiated.
Grants: means either an Educational Grant or a Research
Grant, or both.
Guests: means spouses, partners, family or guests of
Healthcare Professionals, or any other person who does
not have a bona fide professional interest in the information
being shared at an Event.
Healthcare Organisation (HCO): means any legal entity
or body (irrespective of its legal or organisational form)
that is a healthcare, medical or scientific association or
organisation which may have a direct or indirect influence
on the prescription, recommendation, purchase, order,
supply, utilisation, sale or lease of health technologies or
related services such as a hospital or group purchasing
organisation, clinic, laboratory, pharmacy, research
institution, foundation, university or other teaching
institution or learned or professional society (except for
patient organisations); or through which one or more
Healthcare Professionals provide services.
Healthcare Professional (HCP): means any individual
(with a clinical or non-clinical role; whether a government
official, or employee or representative of a government
agency or other public or private sector organisation;
including but not limited to, physicians, nurses, technicians,
laboratory scientists, researchers, research co-ordinators
or procurement professionals) that in the course of their
professional activities may directly or indirectly purchase,
lease, recommend, administer, use, supply, procure or
determine the purchase or lease of, or who may prescribe
health technologies or related services.
Intended Purpose: the use for which the device is intended
according to the data supplied by the manufacturer on the
label, in the instructions or in promotional or sales materials
or statements and as specified by the manufacturer in
the clinical evaluation (Medical Devices Regulation (EU)
2017/ 745).
73
Members: means all full and associate corporate
members (“Member Companies”) of ABHI as well as
association members of ABHI (“Member Associations”), as
defined in the ABHI statutes, as applicable and as amended
from time to time.
Professional Conference Organiser (PCO): a for-
profit company or organisation which specialises in the
management of congresses, conferences, seminars and
similar events.
Product and Procedure Training and Education
Event: means a type of Company Event that is primarily
intended to provide Healthcare Professionals with genuine
education, including information and/or training on:
• The safe and effective use of health technologies,
therapies and/or related services, and/or
• The safe and effective performance of clinical
procedures, and/or
• Related disease areas.
In all cases the information and/or training directly concern
a Member Company’s health technologies, therapies and/
or related services.
Related Service: in relation to a medical device, a related
service means an activity necessary to make the device
available for use. This may include specialist installation
services; repair and maintenance services; or end-of-use
services such as specialist disposal of the device.
Research Grants: means the provision by or on behalf
of a Member Company of funding, products/equipment
and/or in-kind services to any organisation that conducts
research which is made for the sole, restrictive purpose of
supporting the development or furtherance of bona fide,
scientifically valid and legitimate research by the recipient
the purpose of which is to advance medical, scientific and
healthcare knowledge, health technologies and/or clinical
techniques designed to improve patient outcomes.
Sales, Promotional and Other Business Meetings:
means any type of Company Event the objective of
which is to effect the sale and/or promotion of a Member
Company’s health technologies and/or related services,
including meetings to discuss product features, benefits
and use and/or commercial terms of supply.
Samples: means single-use or multiple-use products
provided free of charge by or on behalf of a Member
Company to HCOs or HCPs who are equipped and
qualified to use them in order to enable HCPs to familiarise
themselves with the products in clinical use. Samples do
not include the following:
• Demos;
• Evaluation Products;
• products provided at no charge as part of a Charitable
Donation or as part of a Research or Educational Grant;
or
• products provided at no additional charge as part of
the overall purchase price in a commercial supply
arrangement, e.g. as part of an agreed discount
arrangement, or as substitute products provided
pursuant to a warranty agreement.
Scholarships and Fellowships: means Educational
Grants provided to a Healthcare Organisation by or on
behalf of a Member Company to support fellowships
or scholarships offered by the Healthcare Organisation.
Scholarships in this context means an Educational Grant
provided to support a medical school undergraduate
whereas a fellowship is a period of intensive training for
post-graduate physicians in a chosen clinical sub-specialty
(e.g. medical training after a residency). “Scholars” and
“Fellows” shall be understood accordingly.
Sponsored Posts: Sponsored posts are positions within a
Healthcare Organisation that are funded, in whole or in part,
by a Member Company. Sponsored posts can offer benefits
to the delivery of care, providing expertise, extra capacity
and capability that might not otherwise be provided if
funding was to be found from within the Healthcare
Organisation’s budget.
Third Party Organised Educational Events: means
activities of any type that are planned, budgeted, managed
and executed in whole or in part by or on behalf of a person
or entity other than a Member Company to fulfil Healthcare
Professional medical educational needs.
Third Party Organised Educational Conferences:
means a type of Third Party Organised Educational Event
that is a genuine, independent, educational, scientific, or
policy-making conference organised to promote scientific
knowledge, medical advancement and/or the delivery
of effective healthcare and which is consistent with
relevant guidelines established by professional societies
or organisations for such educational meetings. These
typically include conferences organised by national,
regional, or specialty medical associations/societies,
hospitals, Professional Conference Organisers (PCOs),
patients organisations or accredited continuing medical
education providers.

74
Third Party Organised Procedure Training: means
a type of Third Party Organised Educational Event that is
primarily intended to provide Healthcare Professionals
with information and training on the safe and effective
performance of one or more clinical procedures in
circumstances where the information and training concern:
• Specific therapeutic, diagnostic or
rehabilitative procedures, namely clinical
courses of action, methods or techniques
(rather than the use of health technologies); and
• Practical demonstrations and/or training for HCPs, where
the majority of the training programme is delivered in a
clinical environment.
For the avoidance of doubt, proctorship and preceptorship
are not considered to constitute Third Party Organised
Procedure Training.
Transition Period: means the period from 1 January
2017 up to and including 31 December 2018, following
which Member Companies shall no longer provide financial
or in-kind support direct to Healthcare Professionals to
cover costs of their attendance at Third Party Organised
Educational Events with the exception of Third Party
Organised Procedure Training meetings or pursuant to
a consulting agreement with a Healthcare Professional
speaker engaged by a Member Company to speak at a
satellite symposium.

75
ANNEXES
PART 6
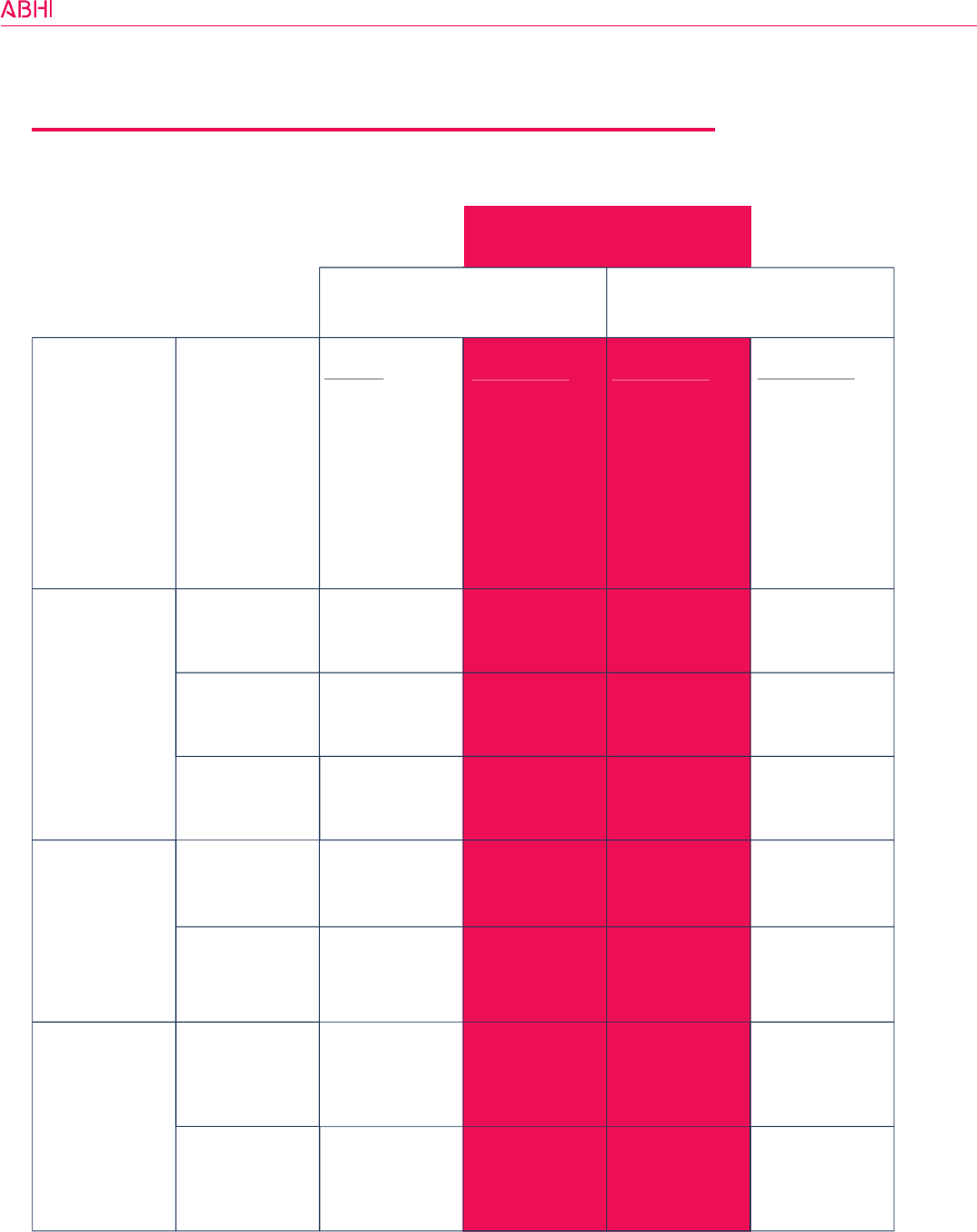
76
WHICH TYPE OF
SUPPORT CAN
MEMBER COMPANIES
PROVIDE TO
WHICH THIRD PARTY
ORGANISED
EDUCATIONAL
EVENTS?
EDUCATIONAL
GRANTS
4
PROVIDED TO
SUPPORT A
ORGANISED
THIRD PARTY
CONFERENCE
Educational Grant to
support the general
running of a conference
Out of scope of the
application of the Code
6
Out of scope of the
application of the Code
N/A
N/A
N/A
N/A
N/A
INTERNATIONAL
(Third Party Organised
Educational Events to
which no Healthcare
Professionals registered
and practicing in the
MedTech Europe
Geographic Area attend,
neither as speakers or
delegates)
NATIONAL
Third Party Organised
Educational Events
attended by delegates
which are local HCPs
only)
IN MEDTECH EUROPE
GEOGRAPHIC AREA
OUTSIDE MEDTECH EUROPE
GEOGRAPHIC AREA
Educational Grants that
includes funds to support
HCP attendance to the
conference
Educational Grants that
includes funds to support
Faculty
Consultancy agreement
for speakers in satellite
symposia
Direct sponsorship of
HCPs as delegates
(passive participation)
Direct sponsorship of
HCPs as Faculty
(active p
articipation)
Booths/advertising
COMMERCIAL
ACTIVITIES
DIRECT SPONSORSHIP
OF HCPs REGISTERED
AND PRACTISING IN
THE MEDTECH EUROPE
GEOGRAPHIC AREA
2018 – Allowed.
Not subject to CVS decision
2019 – Subject to CVS
decision
2018 – Allowed
5
.
2019 – Allowed.
2018 – Allowed.
2019 – Allowed.
2018 – Allowed.
2019 – Allowed.
2018 – Allowed.
2019 – Allowed.
2018 – Allowed.
2019 – Allowed.
2018 – Allowed.
2019 – Not allowed.
2018 – Allowed.
2019 – Not allowed.
2018 – Allowed.
Not subjec
t to CVS decision
2019 – Subject to CVS
decision
2018 – Allowed.
Not subject to CVS decision
2019 – Subject to CVS
decision
2018 – Allowed.
Not subject to CVS decision
2019 – Subject to CVS
decision
2018 – Allowed.
Not subject to CVS decision
2019 – Subject to CVS
decision
2018 – Allowed.
Not subject to CVS decision
2019 – Subject to CVS
decision
2018 – Allowed.
Not subject to CVS
decision
7
2019 – Not allowed
2018 – Subject to CVS
decision
2019 – Not allowed
2018 – Allow
ed.
Not subject to CVS
decision
2019 – Not allowed
2018 – Subject to CVS
decision
2019 – Not allowed
2018 – Allowed.
Not subject to CVS decision
2019 – Allowed.
Not subject to CVS decision
2018 – Allowed.
Not subject to CVS decision
2019 – Allowed.
Not subject to CVS decision
2018 – Allowed.
Not subject to CVS decision
2019 – Allowed.
Not subject to CVS decision
2018 – Allowed.
Not subject to CVS decision
2019 – Allowed.
Not subject to CVS decision
INTERNATIONAL
(Third Party Organised
Educational Events
attended by delegates
coming from at least two
countries of the
MedTech Europe
Geographic Area
1
,
2
)
INTERNATIONAL
(Third Party Organised
Educational Events
attended by delegates
who are Healthcare
Professionals registered
and practising in the
MedTech Europe
Geographic Area
3
)
PRIOR CVS SUBMISSION
1. MedTech Europe Geographic Area includes the countries in the European Economic Area (EEA), as well as those other countries where Member Associations are located.
2.
Formerly referred to as “Cross-border Events”.
3.
For avoidance of doubt, in 2019, this category of “Third Party Organised Educational Events attended by delegates who are Healthcare Professionals registered and practicing in the
MedTech Europe Geographic Area” has to be understood as covering only Healthcare Professionals from the MedTech Europe Geographic Area benefiting from an Educational
Grant.
4. Educational Grants: means provision of funding, Member Company or third party products or other in-kind support to a Healthcare Organisation by or on behalf of a Member
Company on a restricted basis for use solely for the support and the advancement of genuine medical education of Healthcare Professionals, patients and/or the public on clinical,
scientific and/or healthcare topics relevant to the therapeutic areas in which the Member Company is interested and/or involved.
5. Allowed means no CVS decision is required but the provisions of the ABHI Code of Ethical Business Practice and national laws and regulations still apply.
6. Out of scope: Means the Code does not apply given that the situation does neither involve a Member Company interacting with an HCP or HCO registered and practicing in the
MedTech Europe Geographic Area nor does the activity take place in the MedTech Europe Geographic Area.
7. Please note that although international/cross-border Events are eligible to be submitted in CVS, the decisions rendered by CVS in 2018 will only pertain to the direct sponsorship of
HCPs to Third- Party Organised Events.
ANNEX I (ADDED IN MAY 2017)
CVS SCOPE: WHEN ARE CVS ASSESSMENTS REQUIRED?
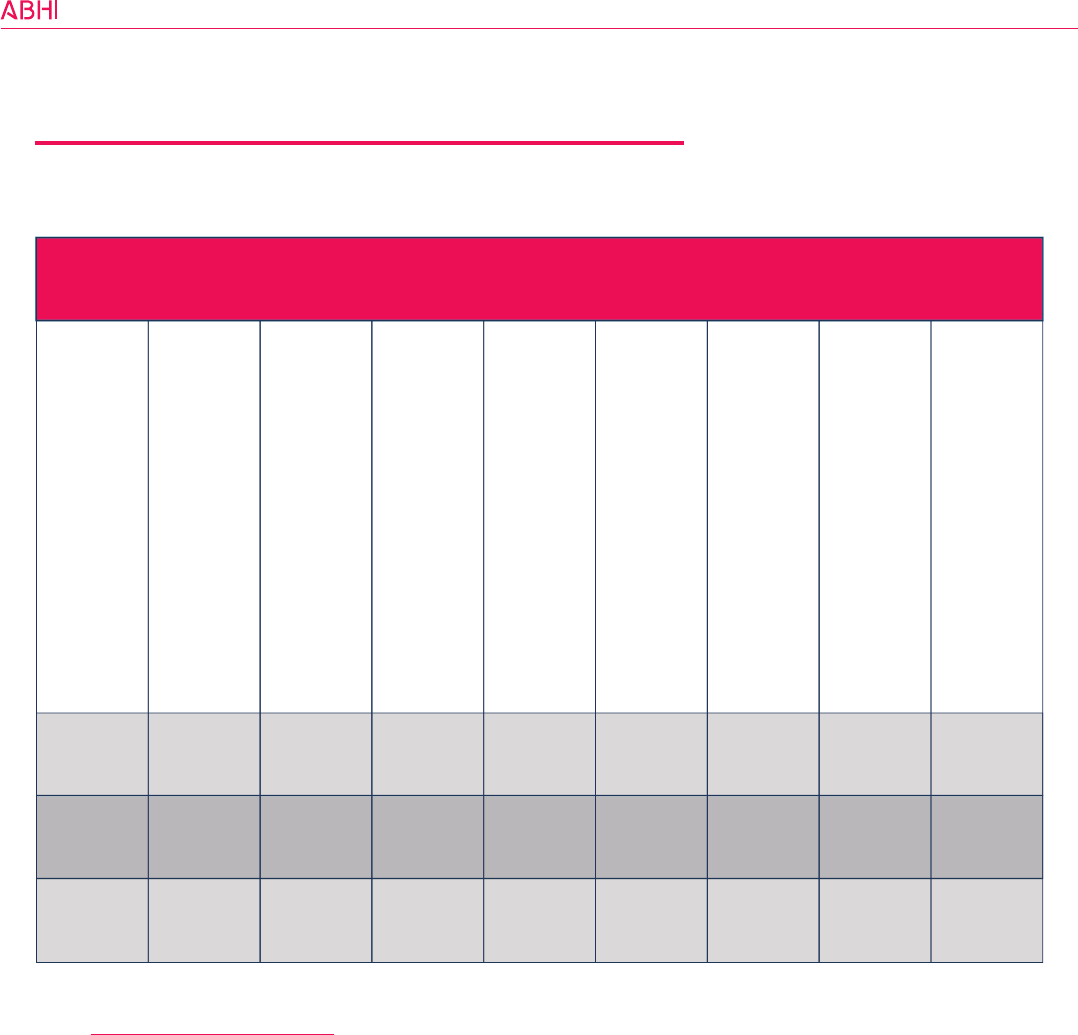
77
A.
Educational
Grants
to Support
Third Party
Organised
Events /or to
Support HCP
Participation
at Third
Party
Organised
Educational
Events)
B.
Other
Educational
Grants to
HCOs
(including
Scholarships,
Fellowships
and Grants
for Public
Awareness
Campaigns).
TEMPLATE
Full HCO
Name
HCO/PCO 1 Yearly amount Optional
Optional
Optional
Yearly amount
Yearly amount
Yearly amount Optional
Optional
Optional
Yearly amount
Yearly amount
HCO/PCO 2
etc.
HCOs:
city where
registered
Country of
Principal
Practice /
Activity
Registered
Address
Unique
country
local
identifier
Object
(Optional)
Object
(Optional)
*Please note that this template is for illustrative purposes only. The template to be used for reporting purposes will be available
in the TransparentMedTech website.
ANNEX II (ADDED IN MAY 2017)
DISCLOSURE GUIDELINES TEMPLATE EXAMPLE*
Q: What is a “unique country local identifier”?
A: It refers to a specific, unique and sustainable reference to identify Healthcare Organisations. Reporting companies will use the Healthcare
Organisation’s VAT as unique country local identifier by default. In those countries where Healthcare Organisations do not necessarily have a VAT
number, another identifier, unique for this Healthcare Organisation and common for all reporting companies will be provided.

78
The MedTech Europe Geographic Area currently includes:
a) countries with National
Associations:
Austria
Belgium
Bulgaria
Czech Republic
Denmark
Finland
France
Germany
Greece
Hungary
Ireland
Italy
The Netherlands
Norway
Poland
Portugal
Romania
Russia
Slovakia
Slovenia
Spain
Sweden
Switzerland
Turkey
The United Kingdom
the countries where Mecomed
is active
b) countries party to the European
Economic Area agreement
without a MedTech Europe
National Association:
Croatia
Cyprus
Estonia
Iceland
Latvia
Liechtenstein
Lithuania
Luxembourg
Malta
c) countries where Medcomed
is active
Algeria
Bahrain
Egypt
Iran
Iraq
Jordan
Kuwait
Lebanon
Libya
Mauritania
Morocco
Oman
Pakistan
Quatar
Saudi Arabia
Sudan
Syria
Tunisia
UAE
Yemen
Please note that countries covered by Mecomed, the Middle East Medical Devices and Diagnostics association, are not currently
under the scope of the Disclosure Guidelines.
ANNEX III (ADDED IN MAY 2017)
MEDTECH EUROPE GEOGRAPHICAL AREA

79
How can a Member Company verify that the Educational Grant is in fact used for the intended purpose as agreed in the
Educational Grant agreement?
A Member Company should set up an internal verification process for the purpose of ensuring that the funds provided through
an Educational Grant are used for the agreed intended purpose. For example, such a process could include verification of every
single Grant provided by a Member Company or periodic verification of a selected sample of Grants, after the Event takes place
or before any subsequent application for an Educational Grant.
Examples of the documents requested by a Member Company for verification purposes could include, but are not limited to,
the following:
Grant to support Healthcare Professionals’ attendance at the Third Party Organised Educational Event:
• Attendance proof (e.g. hotel check-out form, signed attendance list, a digital certificate issued by the Event organiser etc.)
• Travel proof (e.g. flight/train tickets)
• Copy of the receipts of taxi fairs, meals etc.
• Where allowed, pictures of the Event.
Grant to support the costs related to organisation of the Third Party Organised Educational Event:
• Budget breakdown listing the general expenses of the Event
• Accounting records, copies of invoices, receipts
• Verifications performed by company staff on-site during the Event.
• Written confirmation from the Event Organiser that the funds were spent as intended
• Documentation of the speaker’s presentation (e.g. slides)
Grant provided in the form of a Scholarship or Fellowship:
• Activity records of the educational programme
• Certification of enrolment from the institution or professor in charge
• Progress report by or of the beneficiary
If a Grant recipient fails to provide the requesting Member Company with the documents or if a Member Company determines
that the Grant funds were not used as provided in the Grant agreement, the Member Company:
• Should take this into account when assessing any future funding request from the same Healthcare Organisation.
• May consider requesting MedTech Europe to withdraw the right to use the Logo, if the HCO/PCO is an MTE “Chartered
Organisation” under the Ethical Charter (https://www.ethicalmedtech.eu/ethical-charter/general-overview/)
ANNEX IV (ADDED IN JULY 2018)
VERIFICATION OF THE USE OF FUNDS

80
STRUCTURE
1 Introduction
2 Executive summary of the methodologies used for disclosure purposes and countries specificities
3 Definitions
• Recipients
• Types of Educational Grants
4 Disclosure scope and timelines
5 Disclosures in case of partial performance or cancellation
6 Cross-border activities
7 Specific considerations:
• Multi-year agreements
• Consent management (please note that some jurisdictions may require the legal entity’s consent for publication of
data)
– Consent collection
– Management of recipient consent withdrawal
– Management of recipient’s request
– Partial consent
8 Disclosure Form
• Date of submission
• Currency in case of aggregated payments made in different currencies
• VAT included or excluded and any other tax aspects
9 Disclosure financial data and amount of Educational Grants provided
10 Calculation rules
Disclaimer: This Methodology note is provided as a template to support Member Companies in the implementation of
these Disclosure Guidelines. Any other template may be equally valid provided it complies with the general
requirements set out in Section 2.4 Methodology.
ANNEX V (ADDED IN MAY 2017)
EXAMPLE OF DISCLOSURE GUIDELINES METHODOLOGY NOTE
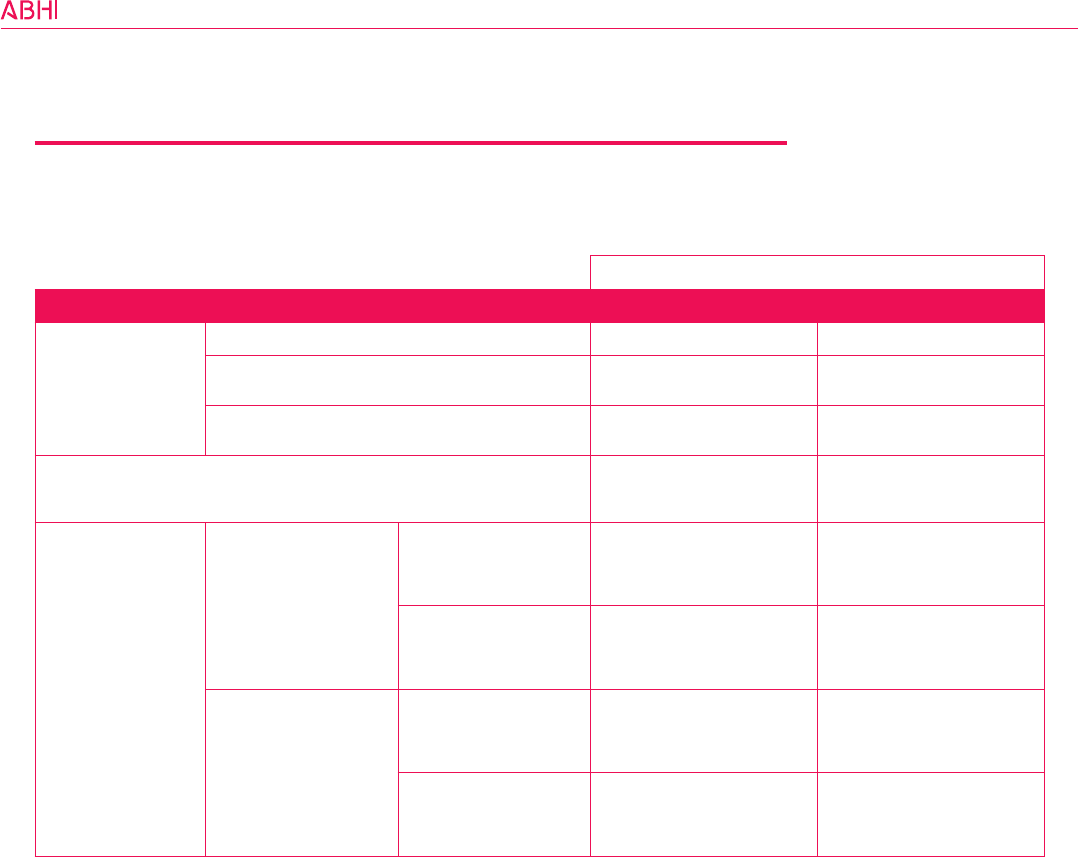
81
ANNEX VI (ADDED IN JULY 2018)
DIRECT SUPPORT TO HCP PARTICIPATION IN EVENTS AS
OF 1ST JANUARY 2019
DESCRIPTION
Delegate: “Delegate” is any Healthcare Professional who is attending passively a Company Event or a Third Party Organised
Event (TPOE) and cannot be considered as “Faculty”.
For avoidance of doubt, poster- and abstract-presenters are considered to be Delegates.
Satellite Symposium: Common elements of Satellite Symposia are:
• It takes place at a TPOE and it is part of the TPOE official programme (i.e. not focused on marketing specific products);
• The Company is responsible for the content subject to review by the Organiser where required;
• It is open to any delegate, not only to selected individuals;
• It has Company branding and the Company can promote the Satellite Symposia to customers.
Speaker/Faculty: “Faculty/speaker” in this chart is someone who is considered as speaker, for example someone who gives a
presentation whether in a Company Event or a TPOE; is moderating/chairing a session and therefore needs to prepare ahead
of the presentation/moderation.
GUIDANCE
Speaker/Faculty: In order to determine whether an event is a TPOE or a Company Event, the following aspects should be taken
into account:
• Open events (not only Company’s customers) are typical of TPOE, and in this case, it is a Third Party that chooses HCPs
attending;
• Who is primary initiator of the Event: How much is the Third-Party vs. the Company involved, who is driving the agenda?
• CME accreditation is an TPOE indication;
• TPOE are generally broader than one or few products;
• Single-sponsored events are often Company Events.
Direct Support for HCP attendance(*)
Event Setting Faculty/speaker(s) Delegates
Third Party Organised
Educational Conference
Main Event / Independent Scientific Program Not allowed Not allowed
Satellite Symposium
Allowed
(through a consulting agreement)
Not allowed
Booth
Allowed
(through a consulting agreement)
Not allowed
Third Party Organised Procedure Training meeting*
*Criteria to distinguish a Third Party Organised Procedure Training meeting
can be found in Q&A 20
Allowed Allowed
Company Events
Product and
Procedure Training and
Education Event
NOT taking place around
or at the same time as a
Third Party Organised
Educational Event
Allowed Allowed
Taking place around or
at the same time as a
Third Party Organised
Educational Event
Allowed Not allowed
Sales, Promotional and
Other Business Meeting
NOT taking place around
or at the same time as a
Third Party Organised
Educational Event
Allowed
(through a consulting agreement)
Not allowed
(except for the demonstration of
non-portable equipment)
Taking place around or
at the same time as a
Third Party Organised
Educational Event
Allowed Not allowed