
667
Primary Ameloblastoma of the Sinonasal Tract
A Clinicopathologic Study of 24 Cases
BACKGROUND.
Ameloblastomas are locally aggressive jaw tumors with a high pro-
Duane R. Schafer,
D.D.S., CDR, DC, USN
1
pensity for recurrence and are believed to arise from the remnants of odontogenic
Lester D. R. Thompson,
M.D., LCDR, MC,
epithelium. Extragnathic ameloblastomas are unusual and primary sinonasal tract
USNR
2
origin is extraordinarily uncommon.
Brion C. Smith,
D.D.S., LTC, DC, USA
1
METHODS.
Twenty-four cases of ameloblastoma confined to the sinonasal tract
Bruce M. Wenig,
M.D.
2
were retrieved from the Otorhinolaryngic-Head & Neck Pathology and Oral-Maxil-
lofacial Pathology Tumor Registries of the Armed Forces Institute of Pathology
1
Department of Oral-Maxillofacial Pathology,
between 1956 and 1996.
Armed Forces Institute of Pathology, Washing-
ton, DC.
RESULTS.
The patients included 5 females and 19 males with an age range of 43–
81 years, with a mean age at presentation of 59.7 years. The patients presented
2
Department of Endocrine and Otorhinolaryn-
with an enlarging mass in the maxillary sinus or nasal cavity (n Å 24), sinusitis (n
gic-Head & Neck Pathology, Armed Forces Insti-
tute of Pathology, Washington, DC. Å 9), or epistaxis (n Å 8). Unilateral opacification of the maxillary sinus (n Å 12)
was the most common radiographic finding. Histologically, the tumors exhibited
the characteristic features of ameloblastoma, including peripherally palisaded co-
lumnar cells with reverse polarity. The majority of the tumors showed a plexiform
growth pattern. Fifteen tumors demonstrated surface epithelial derivation. Surgical
excision is the treatment of choice, ranging from conservative surgery (polypec-
tomy) to more aggressive surgery (radical maxillectomy). Five patients experienced
at least 1 recurrence, usually within 1 year of initial surgery. With follow-up inter-
vals of up to 44 years (mean, 9.5 years), all 24 patients were alive without evidence
of disease or had died of unrelated causes, without evidence of disease.
CONCLUSIONS.
Primary ameloblastoma of the sinonasal tract is rare. In contrast
to their gnathic counterparts, sinonasal tract tumors have a predilection for older
age men. Therapy should be directed toward complete surgical resection to prevent
Presented at the American Academy of Oral and
local tumor recurrence. Cancer 1998;82:667–74. q 1998 American Cancer Society.
Maxillofacial Pathology Annual Meeting, Van-
couver, British Columbia, Canada, May 3–7,
1997.
KEYWORDS: ameloblastoma, nasal cavity, paranasal sinuses, treatment, prognosis.
The authors wish to thank Drs. Dennis K. Hef-
A
fner and Harvey P. Kessler for their critical re-
meloblastomas are locally aggressive jaw tumors with a high pro-
pensity for recurrence that are believed to arise from remnants of
view of the article.
odontogenic epithelium, lining of odontogenic cysts, and the basal
layer of the overlying oral mucosa.
1
Suggested sources for the odonto-
Address for reprints: Duane R. Schafer, D.D.S.,
CDR, DC, USN, National Naval Dental Center,
genic epithelium include cell rests of the dental lamina, a developing
Department of Oral & Maxillofacial Pathology,
enamel organ, the lining of an odontogenic cyst, basal cells of oral
8901 Wisconsin Avenue, Bethesda, MD 20889-
mucosa, or heterotopic embryonic organ epithelium.
1
Ameloblasto-
5602.
mas can occur in either the maxilla or mandible at nearly any age,
but most frequently are discovered as a painless expansion in the
The opinions or assertions contained herein are
the private views of the authors and are not to
mandible of patients in their 20s –40s.
2
The age range is broad and
be construed as official or as reflecting the
the mean age of occurrence has varied from 35–45 years.
3
Gender or
views of the Department of the Navy, Depart-
race predilection in gnathic ameloblastomas has not been demon-
ment of the Army, or the Department of De-
strated.
fense.
Approximately 15–20% of ameloblastomas have been reported
to originate in the maxilla
4–6
with just 2% arising anterior to the pre-
Received May 23, 1997; revision received Au-
gust 18, 1997; accepted August 18, 1997.
molars.
3
There are numerous studies documenting the presence of
q 1998 American Cancer Society
/ 7bb2$$0788 01-24-98 11:03:42 canal W: Cancer
668 CANCER February 15, 1998 / Volume 82 / Number 4
ameloblastomas within the sinonasal cavity.
7–25
In the patient’s race was documented in 21 of the 24 cases.
There were 19 whites and 2 African-Americans.majority of these reports, the tumor was found to have
originated from the maxilla.
7–12,15,21– 23,25
However, rare The patients usually presented with a mass lesion
and nasal obstruction (n Å 15). Additional signs andcase reports document true primary sinonasal amel-
oblastomas without connection to gnathic sites.
13,14,16–20
symptoms included sinusitis (n Å 9), epistaxis (n Å 8),
facial swelling, dizziness, and headaches. The durationIt is due to the rarity of primary sinonasal tract amel-
oblastoma that this study was undertaken. Our goal was of symptoms ranged from 1 month to several years.
Nine of the 24 ameloblastomas involved only the nasalto better characterize the clinicopathologic features of
sinonasal tract ameloblastomas and to determine the cavity (including the nasal septum, lateral wall, middle
or superior turbinate), 6 tumors were confined to theprobable histogenesis of this tumor. To the best of our
knowledge, this represents the single largest report of paranasal sinuses (maxillary, frontal, ethmoid, or
sphenoid) and 9 involved both the nasal cavity andprimary sinonasal tract ameloblastomas.
the paranasal sinuses at presentation.
In contrast to the characteristic multilocular and
MATERIALS AND METHODS
radiolucent presentation of ameloblastomas within
Twenty-four cases of ameloblastoma with primary
the jaws, the sinonasal lesions most often were de-
involvement of the sinonasal tract were retrieved from
scribed radiographically as solid masses or opacifica-
the files of the Otorhinolaryngic-Head & Neck Pathol-
tions filling the nasal cavity, maxillary sinus, or both
ogy and Oral-Maxillofacial Pathology Departments of
(Fig. 1A) Bone destruction, erosion, and remodeling
the Armed Forces Institute of Pathology (AFIP). These
(remnant of bony shell delimiting the lesion as it grew)
cases represent consultative material submitted be-
was noted in a minority of cases (Fig. 1B). Primary
tween 1956 and 1996 from military, Veterans Adminis-
origin in, or continuity with, the maxillary alveolar pro-
tration, and civilian hospitals. Of 19,658 sinonasal tract
cess could not be demonstrated in any of the cases
tumors seen in consultation between 1970 and 1996,
examined.
21 (0.11%) were diagnosed as ameloblastomas. In con-
trast, ameloblastomas comprised 43.1% of all oral cav-
Pathologic Features
ity, mandibular, and maxillary odontogenic cysts or
On gross examination, the tissue specimens ranged
tumors accessioned to the AFIP over the same time
in size from several millimeters to 9.0 cm in greatest
period. Hematoxylin and eosin stained histologic
aggregate dimension. Although several of the lesions
slides were reviewed with characteristic histologic fea-
confined to the nasal cavity had retained a polypoid
tures,
26
precluding the necessity for histochemical, im-
appearance (Fig. 2), most of the tissue had been frag-
munohistochemical, or electron microscopic studies.
mented during surgical harvest. The tissue most fre-
Clinical and demographic data were summarized from
quently was described as predominantly solid in ap-
the admitting history, physical examination, radio-
pearance with glistening gray-white, pink, or yellow-
graphic study reports, and pathology requisitions pro-
tan color and a consistency varying from rubbery to
vided by the contributing facilities. Follow-up data re-
granular. The lesions were not described as cystic. Re-
garding patient outcome was obtained wherever pos-
sidual bone of the sinonasal cavity was present in a
sible. This included 1) a description of the treatment
number of the specimens.
rendered (both initial and subsequent, whether sur-
By light microscopic examination, the plexiform
gery, radiation, or chemotherapy); 2) radiographic or
pattern, comprised of a network of long anastomosing
clinical evidence to verify the site of origin; 3) recur-
cords of odontogenic epithelium, was the sole or pre-
rence data; and 4) morbidity for all cases. Attention
dominant histologic pattern in 22 of 24 cases (92%)
was centered on the review of available imaging stud-
(Fig. 3A). The epithelial strands are bounded at the
ies and/or radiology reports to determine the exact
periphery by a layer of columnar cells exhibiting hyp-
anatomic location and extension of the tumor.
erchromatic, palisaded, and reverse polarized nuclei,
along with subnuclear cytoplasmic vacuolization (Fig.
3B). The stellate reticulum-like component associated
RESULTS
Clinical
with the other patterns of ameloblastoma often is less
conspicuous in association with the plexiform histo-The clinical features are detailed in Table 1. In brief,
the patients included 19 males and 5 females, demon- logic type. The acanthomatous pattern, characterized
by squamous metaplasia and keratin formation of thestrating a male predilection of 3.8:1. The age at presen-
tation ranged from 43–81 years, with an overall mean central portions of the epithelial islands, was the next
most common histologic pattern observed, but wasage at presentation of 59.7 years. This average age was
approximately about 15–25 years later than in patients limited to a secondary or focal component (Fig. 3C).
In two patients, the tumor presented with a predomi-with ameloblastoma occurring within the jaws.
3
The
/ 7bb2$$0788 01-24-98 11:03:42 canal W: Cancer

Sinonasal Tract Ameloblastomas/Schafer et al. 669
TABLE 1
Clinical Information and Follow-up
Age Predominant
Case (yrs) Gender Clinical presentation Location histologic type Recurrence Follow-up interval
1 43 F Nasal obstruction and Left nasal cavity and Plexiform 3 (at 1, 2 and 3 yrs) Alive, NED, 44 yrs
epistaxis with maxillary sinus
paresthesias
2 47 M Nasal obstruction Right maxillary sinus Plexiform 3 (at 1, 3 and 7 yrs) Dead, NED, 23 yrs
3 50 F Epistaxis Left posterior nasal cavity Plexiform N/A LTF
4 65 M Nasal obstruction and Left nasal cavity Follicular 1 (at 1 yr) Alive, NED, 16 yrs
sinusitis
5 66 M Nasal obstruction and Right nasal cavity Plexiform None Alive, NED, 7 yrs
sinusitis
6 62 F Nasal obstruction with Left nasal cavity Plexiform None Alive, NED, 16 yrs
polypoid mass
7 57 M Nasal obstruction with Left nasal cavity Plexiform None Dead, NED, 10 yrs
sinusitis
8 63 M Epistaxis Right nasal cavity Plexiform None Alive, NED, 10 yrs
9 61 M Epistaxis and polypoid Left nasal cavity and Plexiform 1 (13 yrs) Alive, NED, 14 yrs
mass maxillary sinus
10 70 M Nasal obstruction with Left nasal cavity, ethmoid Plexiform None Alive, NED, 11 yrs
sinusitis and maxillary sinus
11 55 M Nasal obstruction and Right nasal cavity, ethmoid Plexiform None Alive, NED 11 yrs
epistaxis and maxillary sinus
12 62 M Nasal obstruction and Left nasal septum, ethmoid Plexiform None Alive, NED, 9 yrs
epistaxis and maxillary sinus
13 72 M Sinusitis Right maxillary sinus Plexiform None Alive, NED, 6 yrs
14 61 M Sinusitis Right nasal cavity and Plexiform 2 (at 10 and 20 yrs) Alive, NED, 24 yrs
maxillary sinus
15 81 M Nasal obstruction Right nasal cavity Plexiform None Alive, NED, 3 yrs
16 50 M Sinusitis and headaches Left maxillary and sphenoid Plexiform None Dead, NED, 2 yrs
sinuses
17 58 M Nasal obstruction and Left nasal cavity and Plexiform None Alive, NED, 2 yrs
epistaxis maxillary sinus
18 62 M Sinusitis Left nasal cavity Plexiform None Alive, NED 2 yrs
19 53 M Facial swelling Right ethmoid and Plexiform None Alive, NED, 2 yrs
maxillary sinus
20 45 F Nasal obstruction Left maxillary sinus Plexiform None Alive, NED, 2 yrs
21 45 F Eye tearing Left nasal cavity, ethmoid Plexiform None Alive, NED, 2 yrs
and frontal sinuses
22 59 M Nasal obstruction with Left nasal cavity and Follicular None Alive, NED, 1 yr
mass maxillary sinus
23 71 M Nasal obstruction, Left maxillary and frontal Plexiform None Alive, NED, 1 yr
epistaxis, and sinusitis sinus
24 74 M Polypoid mass Right nasal cavity Plexiform None Alive, NED, 1 yr
M: male; F: female; NED: no evidence of disease; N/A: nonapplicable; LTF: lost to follow-up.
nantly follicular histologic pattern characterized by is- ratory epithelium in areas adjacent to the ameloblas-
tomatous proliferation, and a mixed chronic inflam-lands of epithelium resembling enamel organ epithe-
lium. In 15 cases, the ameloblastomatous proliferation matory cell infiltrate with edematous changes within
the lamina propria.could be observed arising in direct continuity with the
intact sinonasal surface mucosal epithelium (Fig. 4).
In the remaining nine cases, the surface epithelium
Treatment and Prognosis
Surgical excision was the treatment of choice in allwas not identifiable due to ulceration or sampling,
precluding the identification of an association be- cases. The type and extent of surgery varied per indi-
vidual case and encompassed conservation surgerytween the ameloblastomatous proliferation and the
sinonasal surface epithelium. (i.e., polypectomy) and more aggressive surgical pro-
cedures, including Caldwell-Luc resection, lateral rhi-Other histologic changes identified included the
presence of squamous metaplasia of the surface respi- notomy, and partial or radical maxillectomy. Radiation
/ 7bb2$$0788 01-24-98 11:03:42 canal W: Cancer
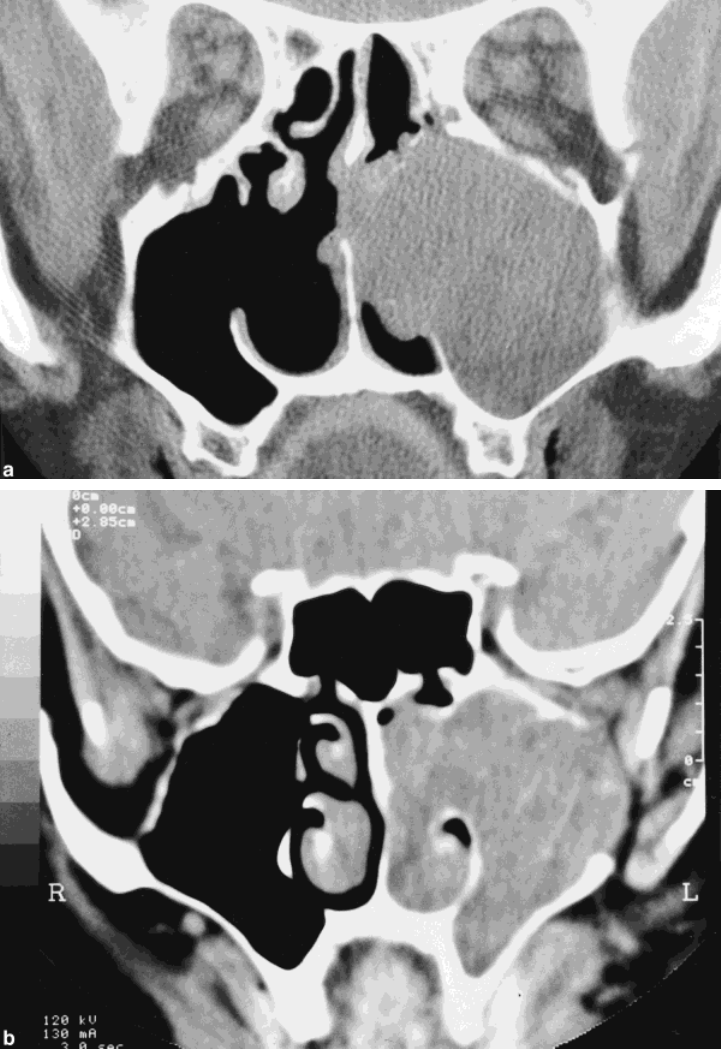
670 CANCER February 15, 1998 / Volume 82 / Number 4
FIGURE 1.
(A) (Case 23). Com-
puted tomography (CT) scan show-
ing an ameloblastoma confined to
the maxillary sinus and nasal cav-
ity. Radiographic survey and surgi-
cal exploration confirmed an intact
sinus floor. (B) (Case 18): Coronal
CT scan of a large ameloblastoma
filling the entire left maxillary sinus
and nasal cavity with bony erosion
of the lateral sinus wall and orbital
floor.
was used in conjunction with maxillectomy in the most positively with complete surgical eradication
when performed in conjunction with thoroughly de-treatment of one patient (Case 2) after the third recur-
rence. Five patients (21%) experienced at least 1 recur- tailed radiographic imaging.
Follow-up was available for 23 of 24 patientsrence. Recurrence of the tumor generally occurred
within 1 to 2 years of the initial procedure but 1 patient (96%), all of whom were either alive without evidence
of disease or had died of unrelated causes without(Case 9) did not experience recurrence until 13 years
after his initial surgery. The histologic appearance of evidence of recurrence. Follow-up periods ranged
from 1–44 years (average, 9.5 years). One patient (Caseall recurrent tumors was identical to that of the pri-
mary neoplasm. Overall treatment success correlated 3) was lost to follow-up.
/ 7bb2$$0788 01-24-98 11:03:42 canal W: Cancer
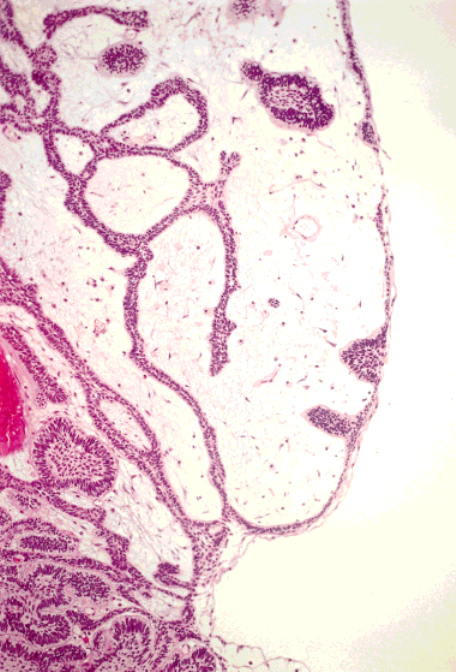
Sinonasal Tract Ameloblastomas/Schafer et al. 671
staxis.
13,14,16–20
Our findings parallel those of the litera-
ture with the average age at presentation in the late 6th
decade of life (59.7 years) and a clinical presentation
of sinonasal-related symptoms, including obstruction
and epistaxis. None of our patients presented prior to
the fifth decade of life and almost all the patients were
men. In contrast to sinonasal tumors, ameloblastomas
of the oral cavity typically occur in younger age pa-
tients (15 –25 years younger) without gender predilec-
tion. We are unsure why the sinonasal cavity amel-
oblastomas show a preference for older age men. The
gender predilection most likely is a chance occurrence.
An explanation for the older age at onset could be that
the sinonasal ameloblastomas require a longer period
of time before attaining sufficient size to present with
symptoms. These tumors may have been present at
an earlier age but remain clinically silent or produce
nonspecific symptoms (e.g., sinusitis) until they are
large enough to become clinically evident. This is en-
tirely speculative but we do not have another explana-
tion for the unusual demographics associated with si-
nonasal ameloblastomas.
The embryologic derivation of the sinonasal tract
and odontogenic apparatus is closely related. The si-
nonasal tract arises from the ectodermally derived na-
sal pits that invaginate to cover the oronasal mem-
brane, conchae, primary and secondary palatine pro-
cesses, and diverticula of the lateral nasal wall. The
odontogenic apparatus is a combination of an endo-
FIGURE 2.
Proliferating cords of ameloblastic epithelium imperceptibly
phytic proliferation of the basal layer of the ectoder-
blend with the surface epithelium. The polypoid architecture and loose
mally derived oral cavity epithelium and the mesoder-
myxoid stroma resemble an inflammatory polyp.
mally derived mesenchyme.
30,31
The sinonasal tract
and oral cavity communicate freely until closure of
the palatine shelves. Although hypothetical, it appears
quite likely that this embryologic approximation
DISCUSSION
Ameloblastomas are epithelial-derived odontogenic allows the sinonasal tract mucosa either to incorporate
odontogenic epithelium or to acquire cells capable oftumors that typically originate in jaw bones, primarily
involving the mandible and less often the maxilla.
2
odontogenesis during development.
The epithelial source of origin for gnathic amel-Ameloblastomas originating separate from normally
situated odontogenic epithelium represent a rare oc- oblastomas still is being debated.
1,27,32–34
Although
there are extragnathic ameloblastomas that have beencurrence.
27–29
Included among the extragnathic sites
of origin of ameloblastomas is the sinonasal tract. Pre- shown to have arisen from misplaced odontogenic
rests,
10
there was no evidence in any of our cases ofvious documentation of sinonasal tract ameloblasto-
mas include those case reports in which the tumors the ameloblastomas arising from a preexisting odonto-
genic lesion. When considering the possible histogen-were identified as a primary sinonasal tract lesion
without extension from a gnathic ameloblas- esis of these sinonasal tract tumors, parallels can be
made with peripheral ameloblastomas, which also aretoma.
13,14,16–20
In contrast, Tsaknis and Nelson
7
re-
ported 24 cases of maxillary ameloblastomas that in- believed to originate outside the boundaries of the
odontogenic apparatus. In the early 1900s, Bakay
32
cluded patients whose tumors were identified clini-
cally extending into the sinonasal region from a proposed the basal cell layer of the surface epithelium
as the origin of these peripheral ameloblastomas. Ap-primary gnathic origin.
Previous case reports of primary sinonasal tract proximately 50 years later, Stanley and Krogh
27
sug-
gested odontogenic epithelial rests outside the jaw-ameloblastomas indicate that these tumors primarily
affect individuals in the sixth to eighth decades of life bone proper as the progenitor cell for peripheral amel-
oblastomas. In addition, Gardner
33
grouped peripheralpresenting with nasal obstruction, sinusitis, and epi-
/ 7bb2$$0788 01-24-98 11:03:42 canal W: Cancer
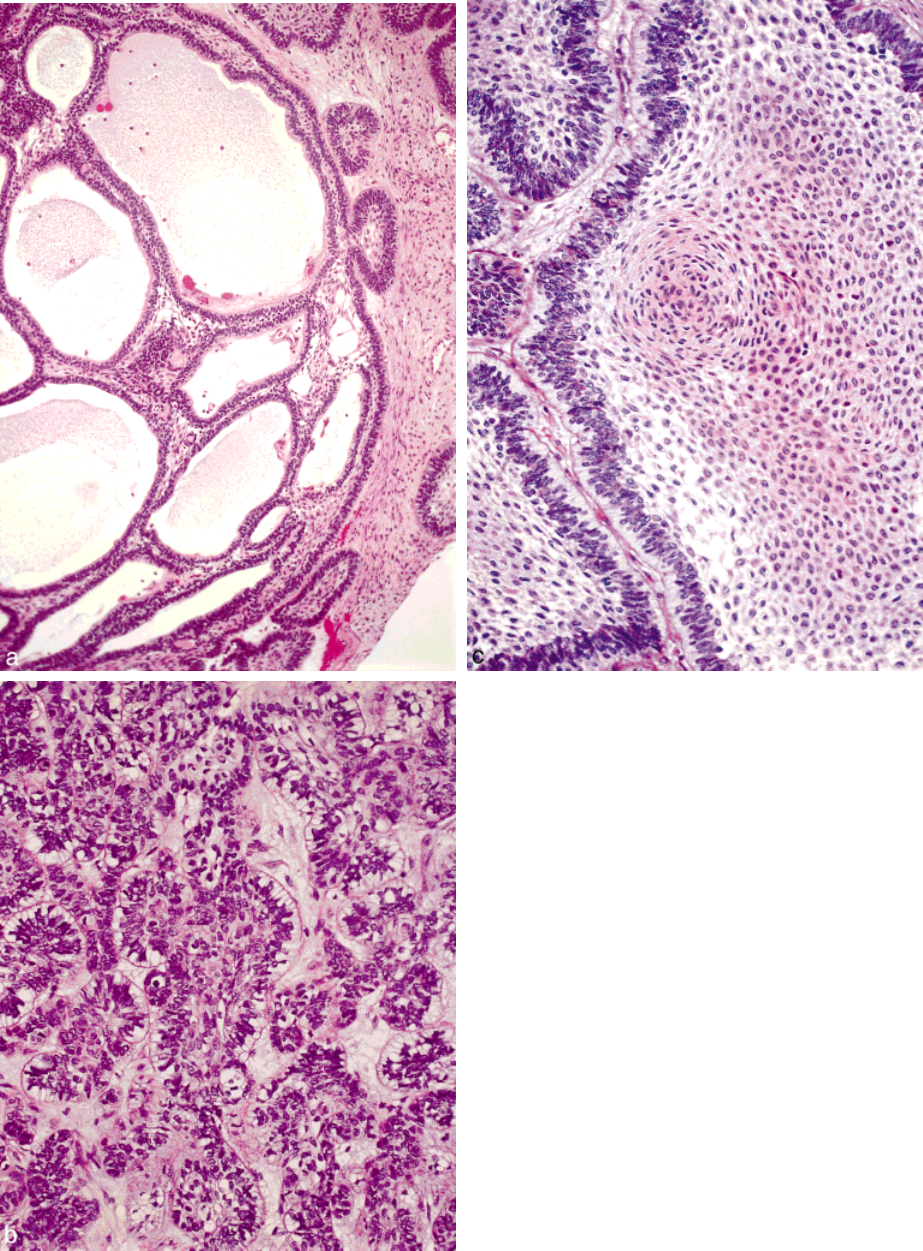
672 CANCER February 15, 1998 / Volume 82 / Number 4
FIGURE 3.
(A) Plexiform histologic pattern of ameloblastoma with back-
to-back anastamosing cords of epithelium. (B) The columnar peripheral cells
with reverse polarity of their nuclei is the most common and recognizable
histologic feature of ameloblastoma. Basal cytoplasmic vacuolization and
hyperchromasia of the nuclei are additional features. (C) Squamous metapla-
sia within the center of the ameloblastoma islands is characteristic of the
acanthomatous growth pattern.
/ 7bb2$$0788 01-24-98 11:03:42 canal W: Cancer
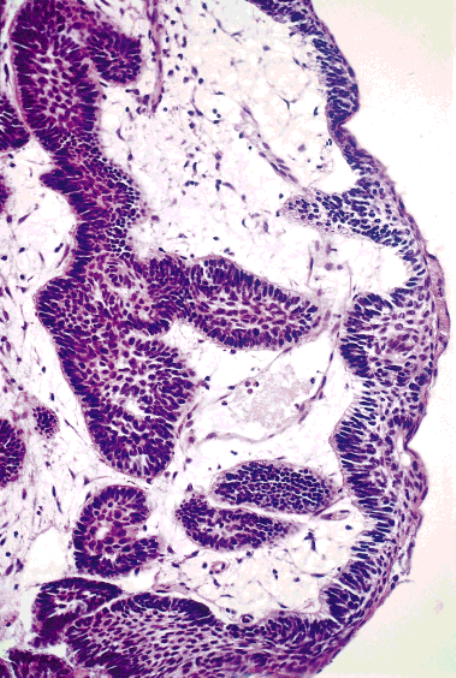
Sinonasal Tract Ameloblastomas/Schafer et al. 673
ameloblastomas occurs after some inductive process
on the sinonasal epithelium that results in the neo-
plastic transformation of retained or acquired odonto-
genic cells toward ameloblastomatous differentiation.
The presence of chronic sinusitis and squamous meta-
plasia in the areas adjacent to the ameloblastoma
could be that initiating event. An argument against
this proposal would be that, if true, other types of
odontogenic tumors also should be found as primary
sinonasal tract neoplasms, but the reality is that this
occurs rarely. Alternatively, the ameloblastomatous
epithelium could have originated in the submucosa
and secondarily extended to involve the surface epi-
thelium rather than originating from this epithelium.
However, we could not identify any cell source, such
as odontogenic dental lamina, within the submucosa
that might represent the source for the development
of these tumors. Furthermore, in our departments’
collective experience, odontogenic remnants rarely, if
ever, are found in sinonasal mucosal tissues. Although
the surface epithelium appears to represent the likely
site of origin, the histogenesis for the primary sinona-
sal ameloblastomas remains unknown.
Given the classic histologic features of these tu-
mors, the differential diagnosis is limited. Of primary
importance is to exclude extension into the sinonasal
tract from a primary gnathic ameloblastoma. The only
other consideration might be a craniopharyngioma.
Craniopharyngiomas arise from Rathke’s pouch in the
area of the pituitary gland or along the developmental
FIGURE 4.
Transition from ciliated respiratory epithelium (right) to an
tract leading to Rathke’s pouch and the pituitary gland.
epithelium with squamous metaplasia from which an ameloblastomatous
Histologically, craniopharyngiomas are epithelial neo-
proliferation at the basal cell layer is observed giving rise to infiltrative
plasms comprised of centrally situated stellate cells
ameloblastoma into the submucosa.
with small nuclei and clear cytoplasm surrounded by
a row of basaloid-appearing columnar cells with polar-
ized nuclei in a palisaded arrangement. Degenerativeameloblastomas with the so-called basal cell carci-
noma of the gingiva, proposing that remnants of den- necrobiotic changes, such as ghost cells and calcifica-
tion, can be identified in the tumor. These featurestal lamina or surface epithelium were the source for
extragnathic ameloblastomas. closely resemble the appearance of gnathic ameloblas-
tomas. However, the clinical features of craniopharyn-In their case report, Woo et al.
34
stated that the
possible origins for peripheral ameloblastoma of the giomas markedly contrast with those of sinonasal tract
ameloblastomas so that the lesions should be readilybuccal mucosa included either pluripotential cells in
the overlying epithelial basal layer or from native or separable.
35
Primary sinonasal tract ameloblastomas are un-ectopic epithelial rests. Woo et al.
34
rejected the origin
was dental lamina rests due to the infrequent, if at all, common tumors. Our findings indicate that amel-
oblastomas can originate in the sinonasal tract withoutoccurrence of these rests in the buccal mucosa. These
authors believed that the peripheral ameloblastomas an association with the gnathic area. These tumors
have a distinct predilection for men in the sixth tomost likely originate from pluripotential cells in the
basal layer of the buccal mucosa, thus reaffirming Ba- eighth decades of life. The signs and symptoms are
those of unilateral nasal obstruction accompanied bykay’s proposition.
32
We concur with Bakay
32
and Woo
et al.
34
As we previously noted, direct continuity with opacification of the adjacent maxillary sinus. The
treatment of choice is complete surgical resection. Ifthe sinonasal surface epithelium was found all of our
cases in which the surface epithelium was identifiable. possible, conservative surgery can be used if an as-
sured complete removal can be performed. Incom-This finding supports a mucosal surface epithelial der-
ivation. Perhaps the development of sinonasal tract plete resection may result in local recurrence. In our
/ 7bb2$$0788 01-24-98 11:03:42 canal W: Cancer
674 CANCER February 15, 1998 / Volume 82 / Number 4
17. Reaume C, Wesley RK, Jung B, Grammer FC. Ameloblastoma
cases, neither distant metastasis nor tumor-related
of the maxillary sinus. Case 31, Part 1. J Oral Surg 1980;38:
deaths occurred. Based on our histologic findings,
435–7.
there is strong evidence to propose origin of these
18. Reaume C, Wesley RK, Jung B, Grammer FC. Ameloblastoma
tumors directly from the sinonasal tract epithelium.
of the maxillary sinus. Case 31, Part 2. J Oral Surg 1980;38:
520–1.
However, further studies are indicated to substantiate
19. Wenig BL, Sciubba JJ, Cohen A, Goldstein A, Abramson AL.
the histogenesis of these tumors.
An unusual case of unilateral nasal obstruction: ameloblas-
toma. Otolaryngol Head Neck Surg 1985; 93:426–32.
REFERENCES
20. Pantoja E, Kopp EA, Beecher TS. Maxillary ameloblastoma:
1. Shafer WG, Hine MK, Levy BM. Odontogenic tumors. In:
report of a tumor originating in the antrum. Ear Nose Throat
A Textbook of oral pathology. 4th edition. Philadelphia:
J 1976; 55:358–61.
W. B. Saunders Co., 1983:258–317.
21. Komisar A. Plexiform ameloblastoma of the maxilla with
2. Waldron CA. Odontogenic cysts and tumors. In: Neville DW,
extension to the skull base. Head Neck 1984; 7:172–5.
Damm DD, Allen CM, Bouquot JE, editors. Oral and maxillo-
22. Bredenkamp JK, Zimmerman MC, Mickel RA. Maxillary
facial pathology. Philadelphia: W. B. Saunders Co., 1995:
ameloblastoma. A potentially lethal neoplasm. Arch Otola-
493–540.
ryngol Head Neck Surg 1989;115:99–104.
3. Regezi JA, Sciubba JJ. Odontogenic tumors. In: Oral pathol-
23. Porter J, Miller R, Stratigos GT. Ameloblastoma of the max-
ogy: clinical-pathologic correlations. 2nd ed. Philadelphia:
illa. Report of a case. Oral Surg Oral Med Oral Pathol
W. B. Saunders Co., 1993:362–97.
1977;44:34–8.
4. Small IA, Waldron CA. Ameloblastoma of jaws. J Oral Maxil-
24. Weissman JL, Snyderman CH, Yousem SA, Curtis HD. Amel-
lofac Surg 1955;8:281–97.
oblastoma of the maxilla: CT and MR appearance. AJNR Am
5. Robinson HBG. Ameloblastoma. A survey of 379 cases from
J Neuroradiol 1993;14:223–6.
the literature. Arch Pathol Lab Med 1937;23:831–43.
25. Kieserman SP, Baker P, Eberle R. Ameloblastoma of the max-
6. Sehdev MK, Huvos AG, Strong EW, Gerold FP, Willis GW.
illa: a series of three cases. Otolaryngol Head Neck Surg 1997;
Ameloblastoma of the maxilla and mandible. Cancer 1974;
116:395–8.
33:324–33.
26. Vickers RA, Gorlin RJ. Ameloblastoma: delineation of early histo-
7. Tsaknis PJ, Nelson JF. The maxillary ameloblastoma: an
pathologic features of neoplasia. Cancer 1970;26:699–710.
analysis of 24 cases. J Oral Maxillofac Surg 1980;38:336–42.
27. Stanley HR Jr., Krogh HW. Peripheral ameloblastoma. Report
8. Weiss JS, Bressler SB, Jacobs EF Jr., Shapiro J, Weber A,
of a case. Oral Surg Oral Med Oral Pathol 1959;12:760–5.
Albert DM. Maxillary ameloblastoma with orbital inva-
28. Klinar KL, McManis JC. Soft-tissue ameloblastoma. Oral
sion. A clinicopathologic study. Ophthalmology 1985;92:
Surg Oral Med Oral Pathol 1969; 28:266–72.
710–3.
29. Wallen NG. Extraosseous ameloblastoma. Oral Surg Oral
9. Kwartler JA, Labagnara J Jr., Mirani N. Ameloblastoma pre-
Med Oral Pathol 1972; 34:95–7.
senting as a unilateral nasal obstruction. J Oral Maxillofac
30. Langman J. Head and neck. In: Medical embryology. 4th
Surg 1988; 46:334–6.
edition. Baltimore: Williams and Wilkins, 1981:268 –96.
10. Scaccia FJ, Strauss M, Arnold J, Maniglia AJ. Maxillary amel-
31. Ten Cate AR. Embryology of the head, face, and oral cavity
oblastoma: case report. Am J Otolaryngol 1991;12:20–5.
and structure of the oral tissues. In: Ten Cate AR, editor.
11. Cheney ML, Wolf C, Cox RH, Kreutziger KL. Ameloblastoma
Oral histology: development, structure, and function. St.
presenting as chronic sinusitis. J La State Med Soc 1986;138:
Louis: C. V. Mosby Company, 1985:13 –77.
11–4.
32. Bakay L. Az a
´
llkapocs ko¨zponti ha
´
mdaganata
´
nak sza
´
rma-
12. Pantazopoulos PE, Nomikos N. Adamantinoma of the maxil-
za
´
sa. Orv Hetil 1908; 52:473–8.
lary sinus. Ann Otol Rhinol Laryngol 1966;75:1160–8.
33. Gardner DG. Peripheral ameloblastoma. A study of 21 cases,
13. De Gandt J-B, Gerard M. A propos d’un ame
´
loblastome du
including 5 reported as basal cell carcinoma of the gingiva.
sinus maxillaire. Acta Otorhinolaryngol Belg 1974;28:365– 8.
Cancer 1977; 39:1625–33.
14. Gaillard J, Haguenauer JP, Pignal JL, Dubreuil C. Ame
´
loblas-
34. Woo S-B, Smith-Williams JE, Sciubba JJ, Lipper S. Peripheral
tome du sinus maxillaire. J Fr Otorhinolaryngol 1981;30:107–
ameloblastoma of the buccal mucosa: case report and re-
10.
view of the English literature. Oral Surg Oral Med Oral Pa-
15. Marsot-Dupuch K. Sinusite maxillaire re
´
va
´
latrice d’une lo-
thol 1987; 63:78–84.
calisation nasosinusienne d’un ame
´
loblastome. Ann Radiol
35. Burger PC, Scheithauer BW. Tumors of the central nervous
(Paris) 1991; 34:131–2.
system. In: Rosai J, Sobin L, editors. Atlas of tumor pathol-
16. Seabaugh JL, Templer JW, Havey A, Goodman D. Ameloblas-
ogy. Fascicle 10. Third series. Washington, DC: Armed Forces
toma presenting as a nasopharyngeal tumor. Otolaryngol
Institute of Pathology, 1994:349 –54.
Head Neck Surg 1986; 94:265–7.
/ 7bb2$$0788 01-24-98 11:03:42 canal W: Cancer
