Page 1 of 4
QSF-0018295_A
URGENT MEDICAL DEVICE CORRECTION
Mobile App v2.7 Crashing Resulting in Pump Battery Depletion
March 26, 2024
The purpose of this letter is to advise you that Tandem Diabetes Care is voluntarily correcting
the mobile app version 2.7 due to potential crashing resulting in accelerated pump battery
depletion. Your safety is our top priority. You are receiving this letter because our records
indicate you may be operating t:connect
®
mobile app version 2.7 on the Apple iOS
platform with the t:slim X2 insulin pump and you should take actions to update your mobile
app version as soon as possible to help mitigate this risk.
If the t:slim X2 insulin pump mobile app has been updated to version 2.7.1 or later, there is no
update action required.
What is the potential issue?
Pump Battery Depletion:
During normal use, the mobile app may crash and be automatically relaunched by the iOS
operating system. This cycle intermittently repeats, which leads to excessive Bluetooth
communication that may result in pump battery drain and may lead to the pump shutting
down sooner than typically expected.
Risk
As a result of a fully depleted battery, the pump may shut down earlier than typically
expected. Pump shutdown will cause insulin delivery to suspend, which could lead to an
under-delivery of insulin and may result in hyperglycemia, including severe hyperglycemia.
The pump will provide notification prior to shut down by declaring a low power alert and
alarm. In severe cases of hyperglycemia due to a prolonged period of no insulin delivery, the
user may experience diabetic ketoacidosis and may require hospitalization or intervention
from a medical professional.
Users may be at higher risk if the accelerated pump battery depletion occurs during the
night when one is more vulnerable to missing alerts, including severe hyperglycemia due to
a prolonged period of no insulin delivery.
There have been 81 reported adverse events, and one reported injury requiring medical
intervention. There have been no reports of death.
Mitigations and Actions to be taken by the Customer/User
On March 18, 2024, Tandem released a new version of the mobile app, t:connect
®
mobile app
version 2.7.1, to address the above issue.
Page 2 of 4
QSF-0018295_A
1. Please update your mobile app to version 2.7.1 or later, available in the Apple App
Store, to access quality improvements.
2. If your mobile app has been updated to version 2.7.1 or later, there is no update
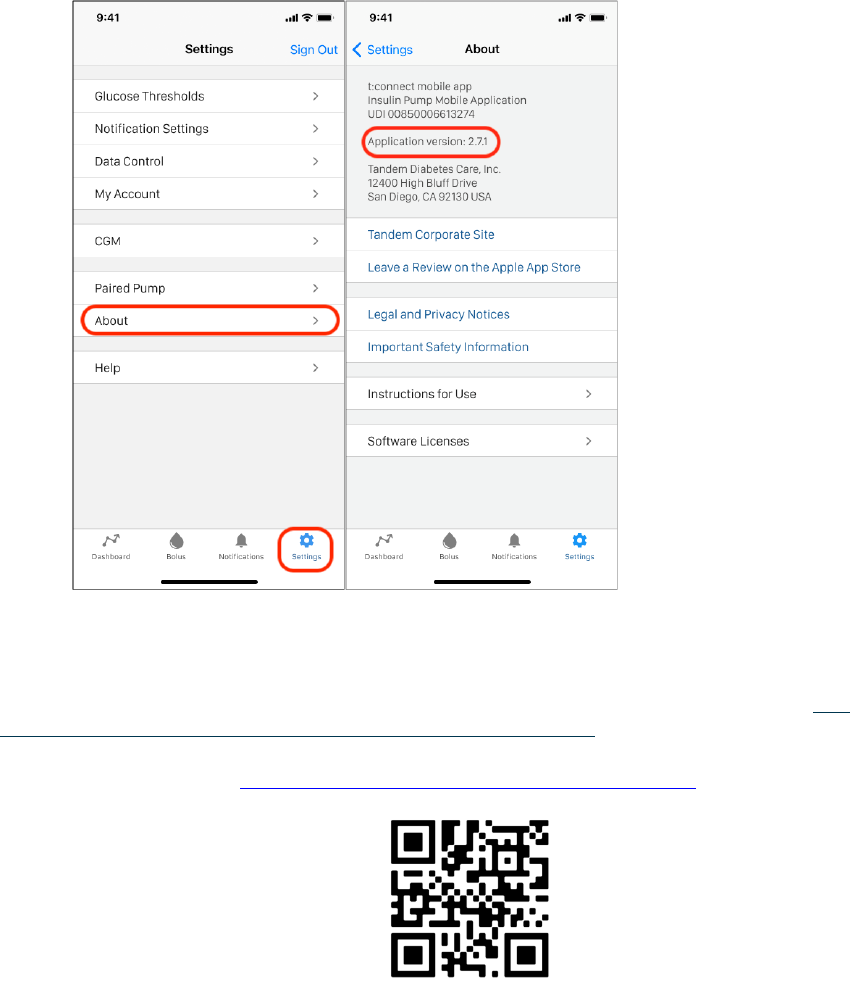
action required. To identify the software version of the t:connect
®
mobile app, open
the app, click the ‘Setting’ icon on your iPhone’s screen, and then click ‘About’. More
details provided below.
It is important to acknowledge receipt of this notice by completing the online form
available at the following link or by using the QR code below. Even if you do not plan on
updating your mobile app to version 2.7.1 or later, Tandem requests that you still
complete the online form or by using the QR code below:
www.tandemdiabetes.com/battery-depletion
3. Continue using your Tandem pump as described in the User Guide.
4. Pay attention to all system alerts and alarms.
5. Please monitor your pump battery level closely to ensure the pump is at or near full
charge before going to sleep to help prevent pump shutdown.
Page 3 of 4
QSF-0018295_A
6. Always carry back-up supplies.
7. To avoid pump shut down, if for any reason battery life is depleted more quickly than
you are used to, Tandem strongly recommends you begin charging you device after
the first low battery alert.
If you have concerns, please email Tandem Diabetes Care Customer Technical Support
Techsupport@tandemdiabetes.com. Our team is available 24/7/365.
Adverse reactions or quality problems experienced with the use of this product may be
reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular
mail, or by fax.
We appreciate your time and attention in reading this important notification.
Thank you for being a part of the Tandem family.
Sincerely,
Tandem Diabetes Care
Frequently Asked Questions (FAQs)
1. How do I know what software version my t:slim X2 t:connect Mobile App is?
To identify the version of t:connect
®
mobile app on your iPhone, open the app, click
the ‘Setting’ icon on your iPhone’s screen, and then click ‘About’.
2. How do I get the latest mobile app version?
To download the new t:connect
®
mobile app 2.7.1 or later version:
• Access the Apple App Store on your iPhone.
• Search for ‘t:connect
®
mobile’ and select it from the search results.
• If an update is available, tap “Update” next to the app.
• Approve with Face ID, Touch ID, or your password if prompted.
To learn more about how to perform a mobile app software update, visit
support.tandemdiabetes.com.
3. Who is affected?
If you are using t:connect
®
mobile app version 2.7, you may be affected.
4. What solution is Tandem offering to 2.7 customers?
Your t:connect
®
mobile app is eligible for an update that includes these changes and
enhancements. To access this update, please visit the Apple App Store and update
your t:connect
®
mobile app to version 2.7.1 or later.

Page 4 of 4
QSF-0018295_A
5. What extra precautions should I take?
Update your t:connect
®
mobile app version as described above. Regularly check your
blood sugar as recommended in your training and user guide to ensure you are not
having unexpectedly high or low readings. Please monitor your pump battery level
closely and always carry back-up supplies.
6. What is a field correction notice?
Correction means repair, modification, adjustment, relabeling, destruction, or
inspection (including patient monitoring) of a product without its physical removal to
some other location according to the FDA. In this instance we are alerting you to a
potential safety risk from using older versions of the t:connect
®
mobile app.
7. Why are you not physically recalling these Tandem Insulin Pumps?
By updating to version 2.7.1 of your t:connect
®
mobile app, this incorporates quality
improvement made to the app software that mitigates the potential issues.
8. What can be the potential risk?
Serious injury might occur if the battery fully depletes resulting in an under-delivery of
insulin, which may result in hyperglycemia. In severe cases of hyperglycemia, the user
may experience diabetic ketoacidosis and may require hospitalization or intervention
from a medical professional.
9. I haven’t had any problems. Do I need to worry about my pump?
Even if you haven’t experienced these issues, ensure your t:connect
®
mobile app has
been updated to the latest mobile app version 2.7.1 as described above.
10. Is there any training required to update to the latest mobile app version?
No training is required to use the new mobile app version.
11. What do I do if I experience an adverse event of quality problem?
If you experience any adverse reactions or quality problems with the use of our
products, please email Techsupport@tandemdiabetes.com or call Tandem Diabetes
Care Customer Technical Support at 1-877-801-6901. Our team is available 24/7/365.
Alternatively, you can also utilize the FDA’s MedWatch Adverse Event Reporting
program either online (www.fda.gov/medwatch/report.htm), by regular mail or by fax
(1-800-332-0178). As always, if you are having a medical emergency, call 911.
