
AN ABSTRACT OF THE THESIS OF
Christopher A. Letchworth for the degree of Master of Science in Food Science and Technology presented
on April, 24 2020.
Title: Reduction of Salmonella spp. on In-shell Hazelnuts Using Continuous Steam Blanching and Prevalence
of Salmonella spp. on In-shell Oregon Hazelnuts.
Abstract approved:
Robert J. McGorrin
Tree nuts have been implicated in a number of foodborne outbreaks and recalls in recent years linked to
enteric pathogens, particularly Salmonella. Therefore, prior to distribution and marketing, it is necessary to
understand the biological risks associated with the consumption of tree nuts and to find effective methods to
inactivate foodborne pathogens. Steam treatment processes have been validated for use on California
almonds, but little research has been conducted for in-shell hazelnuts. Hazelnuts grown in Oregon were
recalled nearly annually from 2009 to 2017 and were implicated in an outbreak of E. coli O157:H7 that
sickened 10 people in the Midwest and Canada in 2011 (Miller et al. 2014). In 2017, an outbreak of Salmonella
Typhirium sickened 5 people and was traced to an 80-acre Oregon farm and nursery that sold between 32,000
to 48,000 pounds of raw in-shell hazelnuts directly to consumers from a road-side stand (Yada et al. 2019).
To help characterize the biological risk of in-shell hazelnuts, we conducted a prevalence and amounts survey
of Salmonella on in-shell hazelnuts grown in Oregon over two harvest years (2013-2014). In a separate study,
we developed a steam treatment process that inactivates a 5-log reduction of Salmonella spp. on the surface of
in-shell hazelnuts with minimal impact on final product quality.
For the Salmonella prevalence study, raw, green-dried in-shell hazelnut samples (n = 472) were collected by six
of the largest hazelnut handlers in Oregon’s Willamette Valley and tested for the presence of Salmonella spp.
using a modified method from the Food and Drug Administration’s (FDA) Bacteriological Analytical Manual
(BAM). In-shell hazelnut samples (375 g) were enriched in 1:10 (w/v) lactose broth followed by selective
enrichment in Rappaport-Vassiliadis Broth (RV) and Tetrathionate Broth (TT). Selective enrichments were
streaked for isolation onto Hektoen Enteric (HE) and Xylose Lysine Desoxycholate (XLD) Agars. Suspected
colonies displaying Salmonella spp. morphology were confirmed on CHROMagar Salmonella Plus. A most-
probable-number (MPN) method (3 x 333, 33.3, 3.3 g) using the same cultural steps as the initial testing was
used to determine Salmonella population levels on naturally-contaminated in-shell hazelnuts. The prevalence
of Salmonella spp. by year was 21.7% (55/254) and 46.8% (102/218) for 2013 and 2014, respectively.
Salmonella population levels ranged from 0.092 to 30.7 MPN/100 g, with an average of 2.6 MPN/100 g.
These data will help support risk assessment strategies for the Oregon hazelnut industry.
In our second study, we evaluated the efficacy of steam blanching on the reduction of Salmonella spp. on the
surface of in-shell hazelnuts as a potential thermal postharvest treatment for hazelnuts. A pilot-scale steam
blancher was used to deliver a continuous steam treatment at atmospheric pressure. In-shell hazelnuts were
inoculated (~8.5 log CFU/g) with a five-strain Salmonella spp. cocktail and exposed to steam (88
C) for 15 s,
1, 3, 5, and 10 min. Following steam treatment, hazelnut samples were transferred to 0.1% peptone water
(24
C), hand agitated 1 min, serially diluted, plated on Hektoen Enteric agar and incubated at 37
C for 24 h.
D-values (0.82 to 1.53 min) were calculated based on plate counts. Salmonella spp. could not be recovered by
enrichment after hazelnuts inoculated at 5 log CFU/g were treated with steam at 88
C for 10 min. These
data will be useful when developing validated postharvest steam treatments for the hazelnut industry.
©Copyright by Christopher A. Letchworth
April 24, 2020
All Rights Reserved
Reduction of Salmonella spp. on In-shell Hazelnuts Using Continuous Steam Blanching and Prevalence of
Salmonella spp. on In-shell Oregon Hazelnuts
By
Christopher A. Letchworth
A THESIS
submitted to
Oregon State University
in partial fulfillment of
the requirements for the
degree of
Master of Science
Presented April 24, 2020
Commencement June 2020

Master of Science thesis of Christopher A. Letchworth, presented on April 24, 2020
APPROVED:
Major Professor, representing Food Science and Technology
Head of the Department of Food Science and Technology
Dean of the Graduate School
I understand that my thesis will become part of the permanent collection of Oregon State University libraries.
My signature below authorizes release of my thesis to any reader upon request.
Christopher A. Letchworth, Author
ACKNOWLEDGEMENTS
I would like to express my deepest gratitude and appreciation to my advisor, Dr. Robert McGorrin, for giving
me the opportunity to learn and grow as a scientist as well an individual. His continual support of my
professional and personal goals, along with his fundamental understanding of food science and extensive
knowledge of food industry needs, has helped make me a better scientist and person. It has been an honor
and privilege conducting research under his guidance.
I would also like to acknowledge the support of my other thesis committee members: Drs. Lisbeth Goddik, Si
Hong Park, and Bill Braunworth. I wish to thank them for generously offering their time and expertise. I
appreciate their assistance, valuable advice and guidance.
I would like to also express my sincerest appreciation to the Oregon Hazelnut Marketing Board, including
Polly Owen and Michael Klein for providing funding and resources for this research. The generous support
provided by the Hazelnut Marketing Board helped make this research possible.
I am also extremely grateful for the continual support I received from family and friends during my research.
I would like to thank my parents George and Frances Letchworth for their continued support of my
educational and personal goals, as well as my amazing friends in the lab and the FST department for their
assistance and encouragement over the last few years.

TABLE OF CONTENTS
Page
1. INTRODUCTION……….……………………………………………………………………………. 1
2. LITERATURE REVIEW……………………………………………………………………………..... 2
2.1 HAZELNUTS………………...……………………………………………………………… 2
2.1.1 Hazelnuts ……………………………………………………………………………... 2
2.1.2 Geographic Distribution………..……………………………………………………....2
2.1.3 Growth and Production. .………...…………………………………………………… 3
2.1.4 Oregon Hazelnut Industry ……………………………………………………………. 4
2.1.5 Oregon Hazelnut Varieties..…………………………………………………………… 4
2.2 OUTBREAKS AND RECALLS ASSOCIATED WITH TREE NUTS….…………………... 5
2.2.1 Mandatory Pasteurization of Almonds……………………..……...………………….... 7
2.2.2 Salmonella Risk Assessment for Tree Nuts………………….………………………….. 7
2.2.3 Prevalence of Foodborne Pathogens on Tree Nuts……….………..………………….. 8
2.3 VALIDATED POSTHARVEST TREATMENTS……………...…………………………... 11
2.3.1 Propylene Oxide ……………………………………………………………………...11
2.3.2 Oil Roasting .……………….………………………………………………………. 13
2.3.3 Dry Roasting…...…………………………………………………………………….. 14
2.3.4 Steam Treatment……………………………...……………………………………… 14
2.3.4.1 Proprietary Steam Technologies……………………….…………………... 15
2.3.5 Hot Water Blanching………………………………..………………………………... 16
2.4 POTENTIAL SOURCES OF CONTAMINATION ………………………….……………17
2.5 PATHOGENS ASSOCIATED WITH TREE NUTS…..…………………………………... 18
2.5.1 Salmonella spp . ……………………………………………………………………… 18
2.5.2 Escherichia coli O157:H7 …………………………………………………………......... 18
2.6 PERSISTENCE AND SURVIVAL OF PATHOGENS IN TREE NUTS AND OTHER
LOW-MOISTURE FOODS………………………………………………………………...19
2.7 REFERENCES……………………………………………………………………………... 20
3. PREVALENCE AND LEVELS OF SALMONELLA SPP. ON IN-SHELL OREGON
HAZELNUTS OVER THE 2013 AND 2014 HARVESTS………………………………………….. 24
3.1 ABSTRACT ……………………………………………………………………………….... 25
3.2 INTRODUCTION ………………………………………………………………………… 26
3.3 MATERIALS AND METHODS.…………………………………………………………... 28
3.3.1 Hazelnut Sample Collection …………………………………………………………. 28
3.3.2 Salmonella spp. Analysis………………………………………………………………..28
3.3.2.1 Presence/Absence………………………………………………………….28
3.3.2.2 Most Probable Number (MPN)…………………………………………… 29
3.4 RESULTS AND DISCUSSION …………………………………………………………….30
3.4.1 Prevalence of Salmonella spp. in Hazelnuts ……………………………………………30
3.4.2 Concentration of Salmonella spp. in Hazelnuts ……………………………………….. 31
3.5 ACKNOWLEDGEMENTS………………………………………………………………... 35
3.6 REFERENCES……………………………………………………………………………... 36

TABLE OF CONTENTS (Continued)
Page
4. REDUCTION OF SALMONELLA SPP. ON IN-SHELL HAZELNUTS USING CONTINUOUS
STEAM BLANCHING AND THE IMPACT ON PRODUCT QUALITY AND SENSORY
CHARACTERISTICS………………………………………………..………… ……………………... 38
4.1 ABSTRACT………………………………………………………………………………… 39
4.2 INTRODUCTION ………………………………………………………………………… 39
4.3 MATERIALS AND METHODS……………………………………………………………42
4.3.1 Hazelnuts…………………………………………………………………………….. 42
4.3.2 Preparation of Inoculum…………………………………………………………........ 42
4.3.3 Immersion Inoculation Method.….……………………………………………...……43
4.3.4 Spot Inoculation Method…………..………………………………………………… 43
4.3.5 Description of Pilot-Scale Steam Blancher …………………………………………... 44
4.3.6 Sample Preparation and Arrangement………………………………………………... 46
4.3.7 Steam Treatments……………………………………………………………………. 47
4.3.8 Microbial Analysis……………………………………………………………………. 47
4.3.9 Sensory Analysis…………………………………………………………………........ 48
4.3.10 Statistical Analysis…………………………………………………………………... 49
4.4 RESULTS……………………………………………………………………………………49
4.4.1 Reduction of Salmonella spp. After Steam Treatment…………………………………. 49
4.4.2 Temperature Profile of Steam Blancher Chamber During Steam Treatments….……... 52
4.4.3 Endpoint Determination – Verification of 5-log Reduction…….……………………. 53
4.4.3.1 Immersion Inoculated Salmonella spp. Cells………………………………... 53
4.4.3.2 Spot Inoculated Salmonella spp. Cells…………………..………..…………. 54
4.4.5 Sensory Analysis – Attributes Acceptability Test and Triangle Difference Test………. 56
4.5 DISCUSSION………………...……………………………………………………………...58
4.5.1 Steam Treatment Efficacy – This Study Compared to Previous Studies….….………... 58
4.5.2 Variability of Pilot-Scale Steam Treatment Efficacy at Shorter Treatment Times.……..59
4.5.3 Predicted Model versus Verification Results…………………………………………. 60
4.5.4 Physical Characteristics of the Nut and Comparison of Inoculation Methods….……...60
4.5.5 Sublethally Injured Foodborne Pathogens………………….…………….…………... 61
4.6 CONCLUSION……………………………….……………………………………………. 62
4.7 ACKNOWLEDGEMENTS….…………….….………………………...…………………. 62
4.8 REFERENCES….……………….…………….……………………………………..…….. 63
5. OVERALL CONCLUSION…….……….…….……………………………………………………... 65

LIST OF FIGURES
Figure Page
4.1 Pilot-scale steam blancher and sample configuration for in-shell hazelnut treatments.……………… 45
4.2 Sample layout blancher: location of catch compartments used for treatment validation…………….. 47
4.3 Survival of Salmonella spp. on immersion inoculated hazelnuts after exposure to steam at 88
°
C……...52
4.4 Ratio of Salmonella positive samples after enrichment……….……………………………………….53

LIST OF TABLES
TABLE Page
2.1 Outbreaks of foodborne illness associated with the consumption of tree nuts…....………………….. 6
2.2 Prevalence and levels of Salmonella on naturally-contaminated tree nuts in North America....................10
3.1 Prevalence and amounts of Salmonella spp. on in-shell hazelnuts……………...…….…........................ 34
3.2 Recovery frequency of Salmonella during MPN analysis…………………………………………….35
4.1 Salmonella enterica strains included in the inoculation cocktail for this study…….……...…………….. 43
4.2 Estimated times required to achieve 1 and 5-log reductions of Salmonella …….………….................. 51
4.3 Qualitative reduction of immersion-inoculated 5 log CFU/ g of Salmonella on hazelnut........................ 54
4.4 Qualitative reduction of spot-inoculated 5 log CFU/ g of Salmonella on hazelnuts………………….. 55
4.5 Ability of consumers to distinguish steam treated hazelnuts from control samples…………………. 57
4.6 Sensory properties of hazelnuts after steam treatment…………………………………….................. 57
1
1. Introduction
Low moisture foods such as tree nuts have traditionally been considered biologically safe due to their low
water activity (a
w
≤ 0.70) (Danyluk et al. 2007). However, outbreaks of enteric pathogens, particularly
Salmonella spp., have been associated with tree nuts, including in-shell hazelnuts in recent years, prompting the
need for new industry risk assessment strategies. The Oregon hazelnut industry is actively searching to better
understand the biological risks associated with hazelnuts and improve the food safety of hazelnuts.
Currently, there are several validated postharvest treatments for the pasteurization of almonds including,
propylene oxide (PPO) fumigation, steam, oil roasting, dry roasting, and hot water blanching that show
promise of application to other tree nut industries. Steam treatment is an effective alternative for in-shell
hazelnuts and other minimally processed tree nuts that cannot be roasted, hot water blanched, or have
chemical residues left behind from PPO fumigation. Evaluation of the efficacy of steam treatment using a
pilot-scale steam blancher will help determine the potential for steam technologies as thermal postharvest
treatments.
In addition, a two-year Salmonella spp. prevalence and quantities survey of in-shell hazelnut samples collected
from Oregon processors will help quantify the risk of Salmonella spp. associated with in-shell hazelnuts. The
results of the prevalence study will help guide future industry risk assessment strategies.
2
2. Literature Review
2.1 Hazelnuts
2.1.1 Hazelnuts
The hazelnut, commonly referred to as filbert, is the fruit of the hazel (Corylus) tree, a genus of deciduous
trees belonging to the birch family Betulaceaeae that consists of approximately 17 species (Holstein et al. 2018).
While one hazelnut species, Corylus cornuta var. californica, is native to the Pacific Northwest, the European
Hazelnut, Corylus avellana, has been cultivated for commercial use and is primarily used for commercial
production world-wide, including the Pacific Northwest (Olsen 2013a). Hazelnuts are a good source of
protein, unsaturated fats (oleic acid), magnesium and vitamins B and E (Richardson 1997). According to
nutritional research, hazelnuts may potentially be beneficial for the heart, help reduce cancer risks, decrease
inflammation, and aid in digestive health (Richardson 1997). The health benefits of hazelnuts have been
purported for centuries. A Chinese manuscript dated 2838 BC lists hazelnuts as one of the five sacred foods
given to man from heaven (Dreher et al. 1996; Olsen 2013a). The Greek philosopher Theophrastus
described the benefits of hazelnuts in his writings, and the Greek physician Dioscorides wrote about using
hazelnuts to treat common ailments such as colds and even baldness (Dreher et al. 1996). Hazelnuts have
been cultivated for over five centuries in China, and evidence of Mesolithic nut processing on Colonsay, an
island in the Inner Herbides of Scotland, where hundreds of thousands of charred hazelnut shells were found
and carbon dated to over 9000 years ago, suggests this island community was trading processed hazelnuts
with other surrounding communities (Mithen et al. 2001). Cultivation of hazelnuts in the Pacific Northwest
and Oregon began in more recent times, and is thought to have first begun in 1858 when Sam Strickland, an
English sailor, planted the first hazelnut tree outside Scottsburg, Oregon using the European cultivar, Corylus
avellana (Olsen 2013a).
2.1.2 Geographic Distribution
Hazelnut trees grow naturally in a variety of conditions and locations. However, hazelnuts thrive at growing
in temperate oceanic climates along the 45
th
parallel, and this latitudinal line intersects both the Willamette
3
Valley in Oregon and Turkey, the world’s largest producer of hazelnuts (Lupo 2019; Olsen 2013a). The
temperate oceanic climates of the Willamette Valley and the Black Sea region of northern Turkey are ideal for
the commercial production of hazelnuts (Olsen 2013a). It is no coincidence that the top three hazelnut
producing countries – Turkey, Italy and the United States – all have moderate oceanic regions in proximity to
the 45
th
parallel that are ideal for the commercial production of hazelnuts. Turkey produces the majority of
hazelnuts world-wide, accounting for approximately 70% of global production, with Italy and the United
States being responsible for approximately 20% and 4% of global production, respectively (Kilic et al. 2006;
Olsen 2013a). Commercial hazelnut production in the United States is unique to the Pacific Northwest.
Specifically, Oregon’s Willamette Valley is responsible for approximately 99% of the United States annual
crop, with Washington producing the remaining 1% (NASS 2019; Olsen 2013a). While the Pacific Northwest
is responsible for about 4% of world hazelnut production, Oregon-grown hazelnuts have gained a global
reputation for their large size and robust flavor.
2.1.3 Growth and Production
Hazelnut trees are monoecious and self-incompatible, meaning they contain both male and female flowers
but cannot self-pollinate (Germain 1994). Hazelnuts pollinate in the winter before ripening in the fall.
Harvest typically begins in late September or October after nuts have ripened, turned hazel colored, and have
fallen to the ground separated from their husks (HMB 2012), and lasts approximately one month.
Mechanical sweepers then align the hazelnuts into uniform rows before a harvesting machine picks up the
hazelnuts, sorts the nuts from other soil and plant debris, and deposits the nuts in trailers or large totes where
they are then transported to processing facilities throughout Oregon (HMB 2012).
Postharvest processing of hazelnuts includes general washing and drying steps to ensure hazelnuts are clean
and of high quality before being distributed to consumers. Washing steps vary commercially, with processors
rinsing or spraying water or diluted food-safe sanitizers on in-shell hazelnuts to remove excess dirt and debris
from the hazelnut shell. After processing, hazelnuts must contain no more than 0.02 percent (w/w) of
4
foreign material (CFR 2008b). After washing, clean hazelnuts are then dried over several days to reduce the
moisture to less than 6 percent (CFR 2008b). Following drying, hazelnuts can be packaged and distributed as
in-shell nuts or undergo additional processing steps such as shelling and roasting. While hazelnuts are
commonly shelled, roasted and incorporated into confectionaries, the majority of Oregon hazelnuts are sold
in-shell and undergo minimal processing.
2.1.4 Oregon Hazelnut Industry
Oregon’s Willamette Valley is the core of the United States hazelnut industry, responsible for producing over
99 percent of the US annual hazelnut crop (Mehlenbacher et al. 1997). Between 2016-2018, Oregon
produced 42,333 tons of hazelnuts on average from 40,333 bearing acres for a total utilized annual
production value of $94.7 million, according to the National Agricultural Statistics Service (NASS 2019).
Historically, the majority of Oregon Hazelnuts (77 percent in 2014) are sold in-shell and undergo minimal
processing.
2.1.5 Oregon Hazelnut Varieties
Major varieties of Oregon hazelnuts have traditionally included Barcelona, Lewis, and Ennis, with Barcelona
historically being the most popular variety (HGO 2019; Olsen 2013b). However, newly developed cultivars
cross-bred for their resistance to the fungal disease, Anisogramma anoamala, compose the majority of new tree
plantings. The popularity of Barcelona trees has been declining since the 1990s, with only about 1 percent of
new plantings using this varietal. A newer hazelnut cultivar – Jefferson – has become the most commonly
planted varietal in Oregon due to its resistance to eastern filbert blight (EFB) caused by the fungus
Anisogramma anoamala, with over 50 percent of new plantings utilizing this EFB-resistant varietal (Olsen
2013b). Eastern filbert blight is a fungal disease that has decimated hazelnut crops over years by causing
severe branch die-back and loss of susceptible trees. The Oregon State University hazelnut breeding program
has been working to develop new hazelnut varieties that in addition to having EFB-resistance, increase annual
production yield, and have desirable kernel characteristics (Olsen 2013b). In addition to Jefferson, other new
5
hazelnut varieties are becoming popular for planting including, McDonald, Wepster, and most recently Polly
O., all of which display resistance to EFB (HGO 2019; Mehlenbacher et al. 2019).
2.2 Outbreaks and Recalls Associated with Tree Nuts
Historically, tree nuts have been considered microbiologically safe due to their low water activity. However,
recent outbreaks of foodborne illness associated with the consumption of tree nuts has led to a reevaluation
of the risks associated with them, with increasing importance placed on preventative control processes. In
addition, detection of foodborne pathogens in prevalence surveys and in recall-associated tree nuts has led to
several class-1 recalls in the United States and Canada in recent years. In 2009, detection of Salmonella in a
single hazelnut processing facility led to the recall of 114,350 lb of shelled hazelnuts (FDA 2009). Additional
recalls of hazelnuts in 2012, 2013, and 2015 were attributed to Salmonella detection in hazelnut samples.
Outbreaks of salmonellosis in the United States and Canada in 2000-2001 and 2003-2004 were
epidemiologically linked to raw almonds (Chan et al. 2002; CDC 2004). An outbreak of E. coli O157:H7
associated with the consumption of in-shell hazelnuts sickened 8 people in the Midwest in 2011 and was the
first case of E. coli O157:H7 being associated with tree nuts (Miller et al. 2012). In 2017, an outbreak of
Salmonella Typhirium which sickened 5 people was traced to an 80-acre Oregon farm and nursery that sold
between 32,000 to 48,000 pounds of raw in-shell hazelnuts directly to consumers from a road-side stand
(Yada et al. 2019). Walnut kernels were additionally implicated in an outbreak of E.coli O157:H7 in 2011.
Due to the increase of illness associated with the consumption of tree nuts, along with increased detection of
foodborne pathogens on tree nuts, a reevaluation of the health risks and efficacy of preventative control
measures is necessary. A summary of recent outbreaks of foodborne illness associated with tree nuts is
shown in Table 1.

6
a
Data obtained from Yada et al. 2019
Table 1
.Outbreaks of foodborne illness associated with the consumption of tree nuts
a
Type
Product
(source)
Pathogen Year
Number
of Cases
Outbreak location(s)
Almond
raw whole
(California)
S. Enteritidis PT
30
2000-01 168 Canada, USA
raw whole
(California)
S. Enteritidis PT 9c 2004 47 Canada, USA
raw whole
(California)
S. Enteritidis NST
3+ (aka PT 30)
2005-6 15 Sweden
raw whole
(Australia)
S. Typhimurium 2012 27 Australia
Coconut
desiccated
(Pap New
Guinea)
S. Typhi, S.
Senftenberg and
possibly others
1953 >50 Australia
desiccated
(Sri Lanka)
S. Paratyphi B 1960-61 3 England
desiccated
(not
stated)
S. Java PT Dundee 1999 18 United Kingdom
dried, raw
S. Newport, S.
Typhimurium
2017-2018 15
USA (CA, CO, CT, MA, NJ,
NY, OK, PA, WA), Canada
Hazelnut
in-shell
(Oregon)
E. coli O157:H7 2010-11 10 Canada, USA (WI, MN, MI)
raw in-
shell
(Oregon)
S. Typhimurium 2017 5 USA (OR)
Pine nut
whole,
bulk
(Turkey)
S. Enteritidis 2011 43 USA (MD, NY, NJ, PA, VA)
Pistachio
roasted
(Calironia)
S. Senftenberg 2013 8
USA (CA, KS, MA, MD, PA,
WI)
roasted
(California)
S. Montevideo, S.
Senftenberg
2015-16 11
USA (AL, AZ, CT, GA, MI,
MN, ND, VA, WA)
Walnut
raw shelled
pieces
(California)
E. coli O157:H7 2011 14 Canada
7
2.2.1 Mandatory Pasteurization of Almonds
In response to the salmonellosis outbreaks linked to almonds, and with pressure from the FDA, the Almond
Board of California (ABC) – a grower-enacted federal marketing association – proposed mandatory
pasteurization of almonds (7 CFR Part 981). Beginning in September 2007, almonds grown in California and
sold in the U.S., Canada, and Mexico must be subjected to a treatment process or processes that achieve a
minimum 4-log reduction of Salmonella. Almond handlers are required to use treatment processes that have
been recognized by a scientific review panel identified by the Almond Board known as the Technical Expert
Review Panel (TERP), or reviewed by the FDA and issued a letter of determination when a process has
sufficiently demonstrated its effectiveness to achieve a 5-log reduction of Salmonella in almonds. While a
minimum 4-log reduction of Salmonella on almonds is considered the mandatory treatment criterion, a 5-log
reduction is required for labeling almonds as “pasteurized”. The TERP currently recognizes several validated
postharvest treatments for almonds, including propylene oxide (PPO) fumigation, steam, oil roasting, dry
roasting, and hot water blanching (ABC 2007d). The steam treatments recognized by the TERP consist of
two proprietary processes – a continuous conveyor system and a batch system – that have been reviewed and
accepted by TERP. Each process has established critical control points for one or two sets of operating
parameters that have been accepted by TERP, and has undergone extensive validation testing using almonds
inoculated with Salmonella Enteritidis PT 30 (SE PT 30). The almond industry is currently the only nut
industry requiring mandatory use of validated postharvest treatments that inactivate Salmonella. Research
conducted on almonds has helped define sanitation standards for tree nuts, and technologies used for
reduction of Salmonella populations on almonds show promise of application to other tree nut industries.
2.2.2 Salmonella Risk Assessment for Tree Nuts
Tree nut industries, along with the FDA, now recognize the increased risk of nut-associated foodborne
illnesses and began taking steps to improve the microbiological safety of tree nuts, with the almond industry
serving as a primary model. Prompted by increasing outbreaks, recalls, and detection of Salmonella in tree
nuts, the U.S. Food and Drug Administration (FDA) began conducting a risk assessment of Salmonella
8
contamination associated with tree nuts in 2013. The purpose of the risk assessment – a planned, multiyear
study – is twofold: to quantify the public health risk associated with eating tree nuts potentially contaminated
with Salmonella, and to evaluate the impact of interventions currently being used or that could be applied in
the future to prevent or reduce Salmonella contamination levels (FDA 2013). The results of the risk
assessment will help inform public policy on nut safety and help guide nut producers on best practices,
according to the FDA.
The Oregon Hazelnut Industry, represented by the Hazelnut Marketing Board, has actively been working to
better understand the risk of Salmonella contamination associated with hazelnuts and to develop validated
processes that inactivate or sufficiently reduce Salmonella and other pathogens on in-shell hazelnuts. Working
with the Hazelnut Marketing Board, the Oregon State University Food Safety Systems laboratory recently
concluded a multi-year (2013-2015) prevalence survey of Salmonella on in-shell hazelnuts. Data collected from
the Salmonella prevalence survey on in-shell hazelnuts will be provided to the FDA for their risk assessment of
Salmonella contamination in tree nuts. In addition to the prevalence study, our lab has been working with the
Hazelnut Marketing Board to investigate the efficacy of postharvest treatments to inactivate or sufficiently
reduce Salmonella on in-shell hazelnuts, including steam treatment, propylene oxide (PPO) fumigation, and
peroxyacetic acid (PAA) washing.
2.2.3 Prevalence of Foodborne Pathogens on Tree Nuts
Much of the available data for the prevalence of foodborne pathogens on tree nuts is derived from retail
surveys using small sample sizes (25g; Davidson et al. 2015; Harris et al. 2019). Prior to 2017, available data
for the prevalence of Salmonella and E.coli on hazelnuts was restricted to several retail surveys form the UK
and Australia that used a limited amount of samples and small sample sizes for determination of prevalence
levels (Harris et al. 2019). Several larger retail surveys of hazelnuts sold in the United States and Canada have
since found the prevalence of Salmonella ranging from 0.0% to 0.43% on in-shell and shelled hazelnuts sold at
the retail level in the United States and Canada (Table 2). Despite these more recent retail surveys, a
9
comprehensive survey of the prevalence of Salmonella occurring at the hazelnut processing level in Oregon
has not been conducted. However, large prevalence surveys have been conducted on California almonds,
pistachios and walnuts, with hundreds to thousands of samples (100-500 g) collected at the processor level
over multiple harvest years (Table 2). In order to make a direct comparison to data derived from the
California tree nut Salmonella prevalence surveys, we devised an in-shell hazelnut Salmonella prevalence study
with a similar design, collecting 472 samples (375 g) from processors over three harvest years (2013-2015).
Available data prior to 2016 from foodborne pathogen surveys of California tree nuts indicate approximately
a one percent contamination level of Salmonella on almonds, pecans, and pistachios, with the exception of in-
shell walnuts, which had a significantly lower incidence of Salmonella contamination (0.14%) (Table 2). A
survey of almonds grown in California found a prevalence of 0.98% (137/13,972) Salmonella on raw almond
kernel samples (100g) over eight harvest years (2001-2007 and 2010) (Bansal et al. 2010; Danyluk et al. 2007;
Lambertini et al. 2012). The average prevalence of Salmonella on raw in-shell almonds grown in California was
1.5% (100 g; 7/455) in 2006 and 2007 (Bansal et al. 2010). The incidence of Salmonella on in-shell walnuts
was found to be 0.14% (100 to 375 g; 4/3,838) over three California harvest years (2011-2014) (Davidson et
al. 2015). The average prevalence of Salmonella contamination on pistachios grown in California was 0.81%
(100g; 32/968) between 2011-2013 (Harris et al. 2016). A recent survey of in-shell pecans collected over four
harvest years (2010-2014) from seven pecan shelling facilities located across five U.S. states, found 44 of
4,641 (0.95%) samples (100 g) positive for Salmonella (Brar et al. 2015). Salmonella population levels in
naturally-contaminated tree nuts are relatively low, ranging from 0.000095 to 39 MPN/100 g upon retesting
(Table 2). The detection of Salmonella in California tree nut surveys, along with increased foodborne
outbreaks being linked to tree nuts, underscores the need for effective postharvest treatments that are
validated to sufficiently reduce (4 to 5 log) Salmonella in tree nuts.

10
Table 2. Prevalence and levels of Salmonella on naturally-contaminated tree nuts in North America
a
A risk assessment model designed by Danyluk et al. (2006) was used to characterize the risk associated with
consumption of raw almonds. The model was based on Monte Carlo simulations and took into account
many of the factors after almonds reached the processors, such as handler and consumer storage times, and
pre-process, post-process, retail, and consumer reduction levels on Salmonella during storage (Pan et al. 2012).
Type of nut Where Collected Sample size (g)
No. o
f
samples
tested (n)
No. positive
for
Salmonell
a
Percent positive
Concentration (Av
g
MPN/100 g)
References
A
lmond, raw kernel
Processor receiving,
California
100 14,949 146 0.98
96 samples: 0.0044 to
0.15 for 2002-06;
4 samples: 0.00080,
0.00080, 0.00095,
0.0034 for 2010
Bansal et al. 2010;
Danyluk et al. 2007;
Lambertini et al. 2012
Almond, raw in-shell
Processor receiving,
California
100 455 7 1.5 18 samples: 1.4 to 18.3 Bansal et al. 2010
A
lmond, in-shell Retail, Canada 25 86 0 0 0 CFIA, 2017
A
lmond, shelled Retail, Canada 25 319 0 0 0 CFIA, 2017
Hazelnut, in-shell Retail, US 375 80 0 0 0 Zhang et al. 2017
Hazelnut, raw shelled Retail, US
375 577 2 0.35
NA
b
Zhang et al. 2017
Hazelnut, in-shell
Retail, Canada 25 696 3 0.43 NA CFIA, 2017
Hazelnut, shelled Retail, Canada 25 870 0 0 0 CFIA, 2017
Macadamia, raw
shelled
Retail, US 375 355 15 4.2 NA Zhang et al. 2017
Pecan, raw in-shell
Processor receiving,
5 U.S. states
100
4,641 44 0.95
44 samples: 0.47 to 39;
mean of 2.4
Brar et al. 2015
Pecan, in-shell Retail, Canada 25 40 0 0 0 CFIA, 2017
Pecan, shelled Retail, Canada 25 86 0 0 0 CFIA, 2017
Pistachio, raw in-shel
l
Processor receiving,
California
100
3,968 32 0.81
11 samples (sinkers):
0.0046
21 samples (floaters):
0.012 to 0.43
Harris et al. 2016
Pistachio, in-shell Retail, Canada 25 481 0 0 0 CFIA, 2017
Pistachio, shelled Retail, Canada 25 22 0 0 0 CFIA, 2017
W
alnut, raw in-shell
Processor,
California
100
935 0 0 0 Davidson et al. 2015
W
alnut, raw in-shell
Processor,
California
375
2,903 4 0.14
3 samples: 0.0032,
0.0038, 0.0042
Davidson et al. 2015
W
alnut, in-shell Retail, Canada 25 792 2 0.25 NA CFIA, 2017
W
alnut, shelled Retail, Canada 25 874 0 0 0 CFIA, 2017
b
Data not determined
a
Data obtained from Harris et al. 2019
11
The model was able to demonstrate that lack of a pasteurization step led to a greater than 78% probability of
more than one case of salmonellosis occurring per year; however, introduction of a pasteurization step
achieving a minimum 4-log reduction reduced the probability of illness to 0.01% according to the model.
Based on this model, the Almond Board’s TERP concluded that a 4-log reduction was a suitable standard for
almond pasteurization, and recommended that a mandatory treatment program be implemented (Pan et al.
2012). A similar risk assessment model developed by Lambertini et al. (2012) ran a simulation with updated
variables such as total amount ingested by consumer, concentration of Salmonella in almonds, assumed storage
times, and temperature distributions throughout all processing steps. The model showed that, under the
current rule mandating a 4-log minimum reduction of Salmonella on almonds, the estimated risk of
salmonellosis was 0.72 cases annually. The model further showed that if a 3-log reduction was mandated
rather than a 4-log reduction, the risk of salmonellosis would be 7.2 cases annually (Pan et al. 2012).
2.3 Validated Postharvest Treatments
Several chemical and thermal treatment technologies have been investigated on almonds for their efficacy at
inactivating Salmonella and other pathogens (i.e. E. coli O157:H7). Chemical treatments have widely been used
on food commodities for their efficacy at controlling or inactivating microbial pathogens while preserving the
original quality of the product. As an alternative to chemical treatments, which may leave residues on the
final product, thermal treatments have long been used on food commodities. However, thermal treatments
exceeding 60°C may negatively affect final product quality if not well controlled (Pan et al. 2012).
Chemical Treatments
2.3.1 Propylene Oxide
Propylene oxide (PPO), a registered fumigant in the US for reduction of bacteria, yeasts, and mold on raw
nut meats, is among the technologies approved by the US Food and Drug Administration (FDA) for the
pasteurization of raw almond kernels (ABC 2008). Research projects funded by the Almond Board of
California (ABC) and carried out by Dr. Linda Harris of the University of California, Davis (UCD), and ABC
12
staff in collaboration with Blue Diamond Growers, Inc. (Sacramento, CA) and Industrial Sterilization (Sparks,
NV) demonstrated that PPO fumigation was effective at achieving a 5-log reduction of Salmonella Enteritidis
PT30 (SE PT30) on inoculated raw almond kernels (Danyluk et al. 2005). The FDA issued a Letter of
Determination confirming the validity of PPO as a pasteurization treatment for raw almond kernels in
September 2004 after reviewing the research findings from the ABC-sponsored studies on the efficacy of
PPO in reducing Salmonella in almonds. Almonds which are fumigated in accordance with the PPO treatment
parameters described by Danyluk et al. (2005) can be labeled as “pasteurized” (ABC 2008). Similar PPO
treatment parameters for in-shell almonds have been conditionally accepted by TERP that achieve a
minimum 4-log reduction of Salmonella. The PPO treatment conditions for hazelnuts must be the same as
those approved for the use in the pasteurization of almond kernels, except that in-shell hazelnuts must be
held for a minimum of 5 days at 15-18
C for post-treatment for ventilation, whereas almond kernels have the
option of be held for 2 days at 38-43
C for post-treatment ventilation.
The US Environmental Protection Agency (EPA) requires that the exposure time to PPO does not exceed 4
h and that the residue on the product is less than 300 ppm (Danyluk et al. 2005). Consequently, the ABC
published a standard operating procedure (SOP) for treatment of almonds kernels and in-shell almonds using
PPO, establishing parameters that are effective at inactivating Salmonella on almonds while following the EPA
requirements of no more than 4 h PPO exposure time and a final product residue less than 300 ppm. A brief
summary of the SOP is as follows: Almonds are pre-warmed to (30
C) before being loaded into a sealed and
pre-heated chamber (47-51
C). The pressure of the chamber is lowered to approximately 9.9 kPa before
PPO is injected into the chamber at a minimum concentration of 0.5 kg/m
3
. Following injection of PPO, an
inert gas (i.e. nitrogen) is pumped into the chamber to maintain a pressure of 84.3 kPa during the 4-h
treatment. A series of aeration cycles (4 to 14 cycles) follow the 4 h process, where a cycle is the decrease of
the chamber pressure to 9.9 kPa followed by an increase to atmospheric pressure with an inert gas or air.
Following the aeration cycle, almonds are transferred to a post-ventilation treatment room for a minimum of
2 days at 38-43
C (almond kernels) or for 5 days above 15
C (almond kernels and in-shell almonds) to
13
achieve a PPO residue of 300 ppm or less on the final product (ABC 2008). Treatment with PPO provides
biologically safe final products while maintaining the integrity and sensory parameters of almonds (Danyluk et
al. 2005). However, public acceptance – particularly in export markets – and the high cost of operation make
PPO treatment impractical for some nut processors. Foreign markets such as the European Union have
strict guidelines on the importation of food commodities treated with PPO and other chemicals, and the
Federal Register estimates the cost of a PPO chamber is between $500,000 and $1,250,000, with alternative
off-site contract processing costing between $0.04 and $0.05 per pound (CFR 2008a).
Thermal Treatments
2.3.2 Oil Roasting
Roasting causes almonds to have a more crunchy texture and alters the flavor profile of nut products (Du et
al. 2010). Oil roasting is used by the almond industry to obtain crunchy and roasted flavors in almond
products (ABC 2007d). Du et al. (2010) demonstrated that immersion of almonds in hot oil (127
C) achieved
a 5-log reduction of SE PT30 in 1.5 min. The authors partly attributed the rapid and large reduction of
Salmonella to washing-off of loosely attached, less protected, and more heat-sensitive pathogen cells (Du et al.
2010). In addition, the efficacy of oil roasting may also result from the high temperature of the oil (127
C)
and the high rate of heat transfer from the oil to the kernel.
Oil roasting parameters (oil temperature and time) are dictated by the desired degree of roast, throughput
rate, initial temperature and initial moisture levels of the almonds, volume of the heated oil, etc. At oil
temperatures of 138
C to 177
C (280
F to 350
F) roasting times of 3 to 15 minutes are typically needed to
achieve crunchy and crispy oil-roasted almond products (ABC 2007d). While oil roasting is able to meet
pasteurization requirements (5-log reduction of SE PT30), it is only applicable to roasted almonds and not to
raw almonds.
14
2.3.3 Dry Roasting
In general, oil roasting is a much faster process than dry roasting using hot air (129
to 154
C). Several
studies initiated by the ABC found that certain existing dry-roasting parameters used by the almond industry
did not deliver a minimum 4-log reduction of SE PT30 on almonds. Yang et al. (2010) demonstrated that dry
roasting almond kernels to a medium level at 130
C was insufficient to achieve a 4-log reduction on SE PT30.
The heat resistance of SE PT30 during dry roasting (hot air) is well documented, with SE PT30 having a D-
value of 25 min at 121.1
C and a Z-value of 26.1
C (ABC 2007c). While dry roasting typically takes much
longer than oil roasting, it has been recognized by TERP as a validated technology to achieve a minimum 4-
log reduction of Salmonella on almonds. Common temperatures for dry roasting range from 129 to 154
C for
10 to 45 minutes. Dry roasting may not achieve the required 4-log reduction of Salmonella on almonds
without significantly impacting the sensory properties of almonds, and is therefore not applicable as a
treatment for raw almonds or other minimally processed tree nuts such as in-shell hazelnuts.
2.3.4 Steam Treatment
Steam at 100
C has a higher heat capacity than the same amount of water at that temperature. One of the
advantages of steam pasteurization is the large transfer of heat when steam condenses on the surface of
foods, which rapidly raises the surface temperature (James et al. 2000). Another attractive feature of
condensed steam team is its ability to penetrate small cavities and condense on cold surfaces that water is
unable to reach. Water vapor molecules are much smaller in diameter (mean free path of steam molecule at
140
C is 0.4 µm) than Salmonella cells (approximately 0.7 µm), making steam capable of reaching bacteria that
occur in cavities (Morgan et al. 1996). Unlike steam, the surface tension of water makes it unable to penetrate
pores of this size. Consequently, water cannot reach all the contaminated surfaces that are large enough to
contain bacterial cells of this size (Morgan et al. 1996).
Several studies have investigated steam pasteurization of Salmonella Enteritidis inoculated on the surface of
almond kernels with mixed results. Using the same almond variety (Nonpareil) and same SE strains (S.
15
Enteritidis 43353, ME-13, ME-14), Chang et al. (2010) were able to demonstrate a 5-log reduction of SE after
exposure to pressurized steam (143 kPa) at 25 s using a batch style, custom made, almond pasteurizer. In
contrast, Lee et al. (2006) were unable to achieve a 4-log reduction of SE even after 35-s exposure to
atmospheric steam using conventional steaming methods. The efficacy of the pressurized steam treatment
observed by Chang et al. (2010) was partly attributed to the rapid increase of temperature within the
pressurized treatment chamber in contrast to the heat dissipation that occurs in open air using conventional
steaming, with the author concluding that the efficacy of steam at inactivating SE is dependent on the
condition of steam applied (Chang et al. 2010). Both authors noted negative impacts on final product quality,
with increasing moisture content and loss in visual quality of almond kernels exposed to steam for prolonged
periods (35 s). A separate study conducted by Bari et al. (2010), demonstrated that a combination of
superheated steam (115
C) for 70 s followed by infrared heating for 70 s was able to achieve a 5-log reduction
of Salmonella on almond kernels without significantly affecting final product quality.
2.3.4.1 Proprietary Steam Technologies
There are currently two proprietary steam technologies that have been reviewed and accepted by TERP.
Both proprietary processes include specific sets of parameters for the treatment of almond kernels and have
undergone extensive validation testing using SE PT30 and established critical control points for one or two
sets of operating parameter that have been accepted by TERP (ABC 2007b).
The two accepted proprietary processes are: the FMC JSP-1 pasteurization system installed at Going Nuts
(Madera, CA) and the H
2
O Express pasteurization system Chamber 1, installed at Stewart & Jasper Company
(Newman, CA) (ABC 2007b). The FMC JSP-1 pasteurization unit is an inline, continuous conveyor system
that treats almonds prior to packaging. Two sets of processing parameters have been accepted by TERP for
the FMC process: one to achieve a 5-log reduction of Salmonella and one to achieve a 4-log reduction. The
FDA, after reviewing the 5-log reduction validation results, issued a Letter of Determination to acknowledge
16
that the FMC JSP-1 pasteurization unit achieves a minimum 5-log reduction of SE PT30 on natural almonds
when operated at defined parameters including belt speed and loading capacity (ABC 2007b).
The H
2
O Express pasteurization system is a batch type system that treats almonds in their final packaging.
For this system, TERP accepted operating parameters for a 4-log reduction of Salmonella on almonds for
three chambers in Newman, CA. The current acceptance only applies to almonds packed in 50-lb cartons for
Chamber 1 and 2,200-lb-tri-wall fiber totes for chambers 1,2, and 3 (ABC 2007b).
Steam treatment is an effective alternative to chemical treatments such as PPO fumigation which may leave
chemical residues.
2.3.5 Hot Water Blanching
Blanching with hot water or steam-injected water is a thermal process used by almond handlers to remove the
pellicle (skin) from almond kernels. Typical hot water blanching processes include scalding and drying steps
where almonds are exposed to heat. Scalding is the step of interest for validation and involves soaking
almond kernels in hot water or steam-injected water (ABC 2007a). Harris et al. (2012) studied the efficacy of
heated water on the reduction of Salmonella on almonds in a hot water bath. In their study, Salmonella could
not be recovered by enrichment after almonds inoculated with SE PT30 and Salmonella Senfentburg 775W at
5-log CFU/g were heated at 88
C for 2 min (Harris et al. 2012). Based on information from this study, the
TERP determined that a minimum process of 2.0 min or more of exposure to hot water at 88
C (199
F) or
above will provide a 5-log or greater reduction of Salmonella on almonds (ABC 2007a). Subsequently, the
FDA reviewed the information and issued a Letter of Determination acknowledging the process was suitable
for pasteurization. Almond products processed utilizing those conditions may be labeled as pasteurized
(ABC 2007a). While approved for pasteurization of almond kernels, hot water blanching may not be a
suitable treatment technology for in-shell hazelnuts and other minimally processed tree nuts due to its effect
on quality and sensory characteristics of the final product – particularly moisture content. In a preliminary
17
trial conducted at the OSU pilot plant, hot water blanching was determined to have negative impacts on the
sensory and quality characteristics of in-shell hazelnuts, with a large increase in moisture content compared to
steam blanching.
2.4 Potential Sources of Microbial Contamination During Tree Nut Production and Processing
Microbial contamination of tree nuts can occur during several stages of nut production and processing.
Salmonella contamination on almonds has mainly been traced to the orchard and huller/sheller facilities.
Contamination in the orchard during harvest was considered the most likely source of Salmonella for
outbreak-associated almonds and Salmonella has been shown to survive and persist in almond huller/sheller
processing facilities (Davidson et al. 2015).
Similarly to almonds, there are several points during the harvest and post-harvest handling of hazelnuts when
Salmonella could easily be introduced. At harvest, hazelnuts fall to the ground before they are mechanically
swept up. Any pathogens introduced to orchard soils could potentially contaminate hazelnuts while they are
exposed to the orchard floor. Studies on almond orchard soils show that Salmonella may persist long-term
and even multiply in contaminated soils (Danyluk et al. 2008). Uesugi et al. (2007) observed that a Salmonella
strain associated with a foodborne outbreak in 2001 was able to persist in an almond orchard for over 5 years.
Microbial contamination of orchard soils is a likely source of contamination for hazelnuts and other tree nuts
that fall to the orchard floor before harvest.
Following harvest, hazelnuts are washed to remove any dirt and plant debris and then dried. Ineffective
washing processes may lead to contamination or cross contamination of hazelnuts because Salmonella is able
to easily cross-contaminate products in a liquid medium. Effective washing procedures are critical for the
biological safety of in-shell hazelnuts and other minimally processed tree nuts.
18
2.5 Pathogens Associated with Tree Nuts
2.5.1 Salmonella spp.
Salmonella is a genus of gram negative, non-spore forming, rod-shaped (bacillus) bacteria of the
Enterobacteriaceae family. Salmonella is divided into two species – Salmonella enterica and Salmonella bongori –
with S. enterica further divided into six subspecies and over 2,500 serovars. Salmonella enterica serovar
Enteritidis and Salmonella enterica serovar Typhimurium are the most common serotypes isolated from
salmonellosis patients in the United States (CDC 2011). Nontyphoidal salmonellosis refers to illnesses caused
by all serotypes of Salmonella except Typhi, Paratyphi A, Paratyphi B, and Paratyphi C. Nontyphoidal
salmonellae are a leading cause of bacterial diarrhea worldwide, causing an estimated 94 million cases of
gastroenteritis and 115,000 deaths globally each year (CDC 2011).
Investigations of Salmonella Enteritidis outbreaks frequently implicate contaminated poultry and egg products
as the source of infection (Patrick et al. 2004). While eggs and poultry products continue to be major vehicles
for the transmission of salmonellae, raw fruits and vegetables have been increasingly implicated in outbreaks
due to modern agricultural practices, such as irrigation with polluted water or fertilization with manure,
sewage sludge, and animal excrement (Beuchat et al. 2013). Outbreaks of Salmonella Enteritidis were linked to
raw almonds in 2000 to 2001 and 2003 to 2004.
2.5.2 E. coli O157:H7
Escherichia coli is a gram-negative, facultatively anaerobic, rod-shaped (bacillus) bacterium of the genus
Esherichia and family Enterobacteriaceae. E. coli are a diverse group of bacteria that naturally inhabit the
gastrointestinal tracts of people and animals. Similarly to Salmonella, over 200 serotypes of E.coli have been
classified serologically. While most E. coli are harmless and an important component of a healthy human
intestinal tract, pathogenic types that can cause diarrhea can be transmitted through contaminated water or
food, or through contact with animal or persons. Pathogenic E. coli strains are categorized into six
pathotypes based upon virulence and host clinical symptom and include: (i) enteropathogenic E. coli (EPEC),
19
which causes diarrhea in children and animals; (ii) enterohemorrhagic E. coli (EHEC), which is responsible for
hemorrhagic colitis and hemolytic-uremic syndrome; (iii) enterotoxigenic E. coli (ETEC), which causes
traveler’s diarrhea and porcine and bovine diarrhea; (iv) enteroaggregative E. coli (EAEC), which causes
persistent diarrhea in humans, and diffusely adherent E. coli (DAEC), a subclass of EAEC which causes
diarrhea in children; (vi) uropathogenic E. coli (UPEC), which causes urinary tract infections in humans and
animals; and (vii) neonatal meningitis E. coli (NMEC), which is responsible for meningitis and sepsis
(Palaniappan et al. 2006).
EHEC, also referred to as Shiga-toxin producing E. coli (STEC), are the most common cause of E. coli
foodborne illnesses, and are estimated to cause more than 265,000 illnesses each year in the United States,
with more than 3,600 hospitalizations and 30 deaths (CDC 2014). Most outbreaks of EHEC infection in the
United States have been caused by EHEC O157:H7 (E. coli O157:H7), which is responsible for more than
75 percent of EHEC infections (Jay et al. 2005).
2.6 Persistence and Survival of Pathogens in Tree Nuts and Other Low-Moisture Foods
Foodborne pathogens are unable to multiply on low moisture foods (water activity <0.70), but are capable of
persisting for long periods on dry surfaces. Studies have shown that Salmonella and E. coli O157:H7 are able
to survive long-term on tree nuts and other low moisture foods, and that pathogenicity may be associated
with survival advantages such at desiccation or thermal resistance (Hiramatsu et al. 2005). In addition, low
water activity has been shown to increase the resistance of Salmonella to thermal (Izurieta et al. 2012) and
chemical treatments (Kieboom et al. 2006). Any treatment process designed for mitigation of pathogens such
as Salmonella on tree nuts will need to address these issues.
20
2.7 References
[ABC] Almond Board of California. 2007a. Guidelines for Validation of Blanching Processes. Almond Board
of California [Online]. Available from: https://www.almonds.com/sites/default/files/blanching-validation-
guidelines.pdf. Accessed: Mar 27, 2020.
[ABC] Almond Board of California. 2007b. Considerations for Proprietary Processes for Almond
Pasteurization and Treatment. Almond Board of California [Online]. Available from:
http://www.almonds.com/sites/default/files/content/attachments/proprietary-processes.pdf Accessed: Mar
27, 2020.
[ABC] Almond Board of California. 2007c. Guidelines for Validation of Dry Roasting Processes. Almond
Board of California [Online]. Available from: https://www.almonds.com/sites/default/files/dry-roast-
validation-guidelines.pdf . Accessed: Mar 27, 2020.
[ABC] Almond Board of California. 2007d. Guidelines for Validation of Oil Roasting Processes. Almond
Board of California [Online]. Available from: https://www.almonds.com/sites/default/files/oil-roast-
validation-guidelines.pdf. Accessed: Mar 28, 2020.
[ABC] Almond Board of California. 2008. Guidelines for Validation of Propylene Oxide Pasteurization.
Almond Board of California [Online]. Available from:https://www.almonds.com/sites/default/files/ppo-
validation-guidelines.pdf. Accessed: Mar 28, 2020.
Bansal, A., Jones, T.M., Abd, S.J., Danyluk, M.D., and Harris, L.J. 2010. Most-probable number
determination of Salmonella levels in naturally contaminated raw almonds using two sample preparation
methods. J. Food Prot. 73:1986–1992.
Bari, M.L., Nei, D., Sotome, I., Nishina, I.Y., Hayakawa, F., Isobe, S., and Kawamoto, S. 2010. Effectiveness
of superheated steam and gas catalytic infrared heat treatments to inactivate Salmonella on raw almonds.
Foodborne Path. Dis. 7:845–850.
Beuchat, L.R., Komitopoulou, E., Beckers, H., Betts, R.P., Bourdichon, F., Fanning, S., Joosten, H.M., and
Ter Kuile, B.H. 2013. Low-water activity foods: increased concern as vehicles of foodborne pathogens. J.
Food Prot. 1:150–172.
Brar, P.K., Strawn, L.K., and Danyluk, M.D. 2015. Prevalence, Level, and Types of Salmonella Isolated from
North American In-shell Pecans over Four Harvest Years. J. Food Prot. 79:352-360.
Canadian Food Inspection Agency (CFIA). 2017. Bacterial pathogens on in-shell nuts and in shelled nuts and
nut butters (2012–2015). Available at: http://www.inspection.gc.ca/food/chemical-
residuesmicrobiology/microbiology/eng/1324284849823/1324285064868.
[CDC] Centers for Disease Control and Prevention. 2004. Outbreak of Salmonella serotype Enteritidis
infections associated with raw almonds-United States and Canada, 2003–2004. MMWR Weekly 53(22):484-
487.
[CDC] Centers for Disease Control and Prevention. 2011. CDC Estimates of Foodborne Illness in the
United States CDC 2011 Estimates: Findings. Centers for Disease Control and Prevention [Online]. Available
from: http://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html. Accessed: Apr 9, 2020.
[CDC] Centers for Disease Control and Prevention. 2014. E. coli general information homepage. [Online].
Available from: http://www.cdc.gov/ecoli/general/index.html. Accessed: Mar 11, 2020.

21
Chan, E. S., Aramini, J.,Ciebin, B., Middleton, D., Ahmed, R., Howes, M., Brophy, I., Mentis, I.,
Jamieson, F., Rodgers, F., Nazarowec-White, M., Pichette, S. C., Farrar J., Gutierrez, M., Weis, W. J.,
Lior, L., Ellis, A .and Isaacs, S. 2002. Natural or raw almonds and an outbreak of a rare phage type of
Salmonella Enteritidis infection. Can. Commun. Dis. Rept. 28(12):97–99. Available at:
http://publications.gc.ca/collections/Collection/H12-21-28-12.pdf. Accessed Apr 10, 2020.
Chang, S.S., Han, A.R., Reves-De-Corcuera, J.I., Powers, J.R., and Kang, D.H. 2010. Evaluation of steam
pasteurization in controlling Salmonella serotype Enteritidis on raw almond surfaces. Lett. Appl. Microbiol.
50:393–398.
[CFR] Code of Federal Regulations. 2008a. Almonds Grown in California. 7 CFR 981. Washington, D.C.:
U.S. Department of Agriculture, Office of the Federal Register.
[CFR] Code of Federal Regulations. 2008b. Hazelnuts Grown in Oregon and Washington. 7 CFR 982.
Washington, D.C.: U.S. Department of Agriculture, Office of the Federal Register.
Danyluk, M.D., Uesugi, A.R., and Harris, L.J. 2005. Survival of Salmonella Enteritidis PT 30 on inoculated
almonds after commercial fumigation with propylene oxide. J. Food Prot. 68:1613–1622.
Danyluk, M.D., Jones, T.M., Abd, S.J., Schlitt-Dittrich, F., Jacobs, M., and Harris, L.J. 2007. Prevalence and
amounts of Salmonella found on raw California almonds. J. Food Prot. 70:820–827.
Danyluk, M.D., Nozawa-Inoue, M., Hristoya, K.R., Scow, K.M., Lampinen, B., and Harris, L.J. 2008. Survival
and growth of Salmonella Enteritidis PT 30 in almond orchard soils. J. Appl. Microbiol. 104:1391–1399.
Davidson, G. R., Frelka, J. C., Yang, M., Jones, T. M., and Harris, L. J. 2015. Prevalence of Escherichia coli
O157:H7 and Salmonella on Inshell California Walnuts. J. Food Prot. 78(8):1547–53.
Dreher, M., Maher, C., and Kearney, P. 1996. The traditional and emerging role of nuts in healthful diets.
Nutrition Reviews. 54(8):241-245.
Du, W.X., Abd, S.J., McCarthy, K.L., and Harris, L.J. 2010. Reduction of Salmonella on inoculated almonds
exposed to hot oil. J. Food Prot. 73:1238–1246.
[FDA] U.S. Food and Drug Administration. 2009. Willamette Shelling Recalls Shelled Hazelnuts Because of
Possible Health Risk. U.S. Food and Drug Administration [Online]. Available from: https://wayback.archive-
it.org/7993/20170404195211/https://www.fda.gov/Safety/Recalls/ArchiveRecalls/2009/ucm194806.htm.
Accessed: Apr, 15, 2020.
[FDA] U.S. Food and Drug Administration. 2013. FDA to assess risk of salmonellosis associated with eating
tee nuts. U.S. Food and Drug Administration [Online]. Available from:
http://www.fda.gov/Food/NewsEvents/ConstituentUpdates/ucm361206.htm. Accessed: Apr 12, 2020.
Germain, E. 1994. The reproduction of hazelnut (Corylus Avellana): A review. Acta Hortic. 351: 195-210.
Available from: https://doi.org/10.17660/ActaHortic.1994.351.19. Accessed: Apr 17, 2020.
Harris, L.J., Lieberman, V., Mashiana, R.P., Atwill, E., Yang, M., Chandler, J.C., Bisha, B., Jones, T. 2016.
Prevalence and amounts of Salmonella found on raw California inshell pistachios. J. Food Prot. 79: 1304-1315
Harris, L.J., Uesugi, A.R., Abd, S.J., and McCarthy, K.L. 2012. Survival of Salmonella Enteritidis PT 30 on
inoculated almond kernels in hot water treatments. Food Res. Int. 45:1093–1098.
22
Harris, L.J., Yada, S., Beuchat, L.R., and Danyluk, M.D. 2019. Prevalence and levels of foodborne pathogens
on naturally contaminated nuts and edible seeds (version 2) [Tables 1–4 and references]. In Surveys for
foodborne pathogens on nuts. Available at: https://ucfoodsafety.ucdavis.edu/low-moisture-foods/nuts-and-
nut-pastes. Accessed: Apr 9, 2020.
[HGO] Hazelnut Growers of Oregon. 2019. Hazelnut Varieties [Online]. Available from:
https://www.hazelnut.com/about-hazelnuts/varieties/. Accessed Apr 11, 2020.
Hiramatsu R, Matsumoto, M., Sakae, K., Miyazaki, Y. 2005. Ability of Shiga Toxin-Producing Escherichia coli
and Salmonella spp. to Survive in a Desiccation Model System and in Dry Foods. Appl. Environ. Microbiol.
71(11):6657-6663.
[HMB] Hazelnut Marketing Board. 2012. Oregon Hazelnuts. Production. Hazelnut Marketing Board.
Available from: http://www.oregonhazelnuts.org/about-us/production/. Accessed: Mar 19, 2020.
Holstein, N., Tamer, S., and Weigend, M. 2018 . The nutty world of hazel names – a critical taxonomic
checklist of the genus Corylus (Betulaceae). European J. of Taxonomy. 409:1-45. Available from:
https://doi.org/10.5852/ejt.2018.409. Accessed Apr 17, 2020.
Izurieta W.P., Komitopoulou, E. 2012. Effect of moisture on Salmonella spp. heat resistance in cocoa and
hazelnut shells. Food Res Int 45:1087-1092.
James, C., Goksoy, E.O., Corry, J.E.L., and James, S.J. 2000. Surface pasteurization of poultry meat using
steam at atmospheric pressure. J Food Process Eng. 45:111-117.
Jay J.M., Loessner, M.J., Golden, D.A. 2005. Foodborne Gasteroenteritis Caused by Escherichia coli. Modern
Food Microbiology. Springer Science. New York, NY. p. 637-655.
Kieboom, J., Kusumaningrum, H.D., Tempelaars, M.H., Hazeleger, W.C., Abee, T., Beumer, R.R. 2006.
Survival, Elongation, and Elevated Tolerance of Salmanella enterica Serovar Enteritidis at Reduced Water
Activity. J Food Prot 69(11):2681-2686.
Kiliç, O. and Alkan, I. 2006. The developments in the world hazelnut production and export, the role of
Turkey. J. of Applied Sciences. 6(7): 1612-1616.
Lambertini, E., Danyluk, M.D., Schaffner, D.W., Winter, C.K., and Harris, L.J. 2011. Risk of
salmonellosis from consumption of almonds in the North American market. Food Res. Int.
45:1166–1174.
Lee, S.Y., Oh, S.W., Chung, H.J., Reyes-de-Corcuera, J.I., Powers, J.R., and Kang, D.H. 2006.
Reduction of Salmonella enterica serovar Enteritidis on the surface of raw shelled almonds
by exposure to steam. J. Food Prot. 69:591–595.Lupo, L. 2019. Hazelnuts: the next almond? Seeding new ways
from the old, HGO seeks to grow hazelnut consumption to new heights. Quality Assurance and Food Safety
Magazine. Available at: https://www.qualityassurancemag.com/article/hazelnuts-the-next-almond/.
Accessed Apr 9, 2020.
Mehlenbacher, S.A. and Olsen, J. 1997. The Hazelnut Industry in Oregon, USA. Acta Hortic 445:337–345.
Mehlenbacher, S.A., Smith, D.C. , and McCluskey, R.L. 2019. ‘Polly O’ Hazelnut. HortScience 54(8):1429-1432.
23
Miller, B.D., Rigdon, C.E., Ball, J., Rounds, J.M., Klos, R.F., Brennan, B.M., Arends, K.D., Kennelly, P.,
Hedberg, C., and Smith, K.E. 2012. Use of traceback methods to confirm the source of a multistate
Escherichia coli O157:H7 outbreak due to in-shell hazelnuts. J. Food Prot. 75:320–327.
Mithen, S., Finaly, N., Carruthers, W., Carter, S., and Ashmore, P. 2001. Plant use in the Mesolithic: evidence
from Staosnaig, Isle of Colonsay, Scotland. J Archaeol Sci 28(3): 223–234.
Morgan, A. I., Goldber, N., Radewonuk, E.R., and Scullen, O.J. 1996. Surface pasteurization of raw poultry
meat by steam. LWT-Food Science and Technology. 29: 447-451.
[NASS] National Agricultural Statistics Service. 2019. NW Noncitrus Fruits and Nuts 2018 Summary. USDA
National Agricultural Statistics Service. [Online]. Available from:
https://www.nass.usda.gov/Publications/Todays_Reports/reports/ncit0619.pdf . Accessed Apr 9, 2020.
Olsen, J. 2013a. Growing Hazelnuts in the Pacific Northwest. Oregon State University Extension
publication. [Online]. Available from:
http://ir.library.oregonstate.edu/xmlui/bitstream/handle/1957/43804/em9072.pdf. Accessed: Mar 3, 2020.
Olsen, J. 2013b. Growing Hazelnuts in the Pacific Northwest. Oregon State University Extension
publication. [Online]. Available from:
http://ir.library.oregonstate.edu/xmlui/bitstream/handle/1957/43808/em9073.pdf. Accessed: Mar 3, 2020.
Palaniappan, R., Zhang, Y., Chiu, D., Torres, A., DebRoy, C., Whittam, T.S., and Chang, Y. 2006.
Differentiation of Eschericia coli Pathotypes by Oligonucleotide Spotted Array. J. Clinical Microbiology.
44(4):1495-1501.
Pan, Z., Bingol, G., Brandl, M.T., and McHugh, T.H. 2012. Review of current technologies for
reduction of Salmonella populations on almonds. Food Bioprocess Technol. 5:2046–2057.
Patrick, M.E., Adcock, P.M., Gomez, T.M., Altekruse, S.F., Holland, B.H., Tauxe, R.V., and Swerdlow, D.L.
2004. Salmonella Enteritidis infections, United States, 1985-999. Emerg Infect Dis. 10: 1-7.
Richardson, D.G. 1997. The health benefits of eating hazelnuts: implications for blood lipid profiles,
coronary heart disease, and cancer risks. Acta Hortic. 445:295-300. Available from:
https://doi.org/10.17660/ActaHortic.1997.445.39. Accessed: Apr 16, 2020.
Uesugi, A.R., Danyluk, M.D., Mandrell, R.E., and Harris, L.J. 2007. Isolation of Salmonella Enteritidis phage
type 30 from a single almond orchard over a 5-year period. J. Food Prot. 70:1784–1789.
Yada, S., and Harris, L.J. 2019. Recalls of tree nuts and peanuts in the U.S., 2001 to present (version 2) [Table
and references]. In U.S. recalls of nuts. Available at: http://ucfoodsafety.ucdavis.edu/Nuts_and_Nut_Pastes.
Accessed: Apr 9, 2020.Yang, J., Bingol, G., Pan, Z., Brandl, M.T., McHugh, T.H., and Wang, H. 2010.
Infrared heating for dry-roasting and pasteurization of almonds. J. Food Eng. 101:273–280.
Zhang, G., Hu, L., Melka, D., Wang, H., Laasri, A., Brown, E. W., Strain, E., Allard, M., Bunning, V. K.,
Musser, S. M., Johnson, R., Santillana Farakos, S. M., Scott, V. N., Pouillot, R.,Van Doren, J. M., and
Hammack, T. S. 2017. Prevalence of Salmonella in cashews, hazelnuts, macadamia nuts, pecans, pine nuts,
and walnuts in the United States. J. Food Prot. 80:459–466. Available at:
http://www.jfoodprotection.org/doi/pdf/10.4315/0362-028X.JFP-16-396.
24
3. PREVALENCE AND LEVELS OF
SALMONELLA
SPP. ON IN-SHELL OREGON
HAZELNUTS OVER THE 2013 AND 2014 HARVESTS
Authors
Christopher A. Letchworth, Joy Waite-Cusic, Robert J. McGorrin*
Department of Food Science and Technology, Oregon State University, Corvallis, Oregon
97331, U.S.A.
*Corresponding author: Robert J. McGorrin, robert.mcgorrin@oregonstate.edu
Research Note
25
3.1 Abstract
A multi-year Salmonella spp. prevalence study was conducted to support risk assessment strategies for
Oregon’s hazelnut industry. During the 2013 and 2014 harvest seasons, raw, green-dried in-shell hazelnut
samples (n = 472) were collected by six of the largest industry handlers and tested for the presence of
Salmonella spp. using a modified method from the Food and Drug Administration’s (FDA) Bacteriological
Analytical Manual (BAM). Samples of in-shell hazelnuts (375 g) were enriched in 1:10 lactose broth followed
by selective enrichment in Rappaport-Vassiliadis Broth (RV) and Tetrathionate Broth (TT). Selective
enrichments were isolated onto Hektoen Enteric (HE) and Xylose Lysine Desoxycholate (XLD) Agars.
Colonies displaying typical morphology for Salmonella spp. were confirmed on CHROMagar Salmonella Plus.
When a sample was positive for Salmonella, the pathogen level was determined by a most-probable-number
(MPN) method (3 x 333, 33.3, 3.3 g) following the same cultural steps as the initial testing. Salmonella spp.
prevalence on in-shell hazelnuts by year was 21.7% (55/254) and 46.8% (102/218) for 2013 and 2014,
respectively. Contamination levels averaged 2.6 MPN/100 g with a range of 0.092 to 30.7 MPN/100 g.
Salmonella prevalence on in-shell hazelnuts is drastically higher compared to prevalence studies for other tree
nuts. Further investigation is needed to understand the contributing factors leading to these high rates of
contamination as well as mitigation factors to improve the food safety of hazelnuts.
26
3.2 Introduction
Outbreaks of foodborne illness associated with the consumption of tree nuts have increasingly been
documented in recent years, including in-shell hazelnuts. Raw almonds were implicated in outbreaks of
salmonellosis in the United States and Canada in 2000-2001 (Isaacs et al. 2005) and 2003-2004 (CDC 2004),
prompting the California almond industry to voluntarily adopt mandatory pasteurization of raw almonds
beginning in 2007. In 2011, outbreaks of E. coli O157:H7 were epidemiologically linked to in-shell hazelnuts
and walnut kernels (CDC 2011; Davidson et al., 2015). A 2017 outbreak of Salmonella Typhirium sickened 5
people and was traced to an 80-acre Oregon farm and nursery that sold between 32,000 to 48,000 pounds of
raw in-shell hazelnuts directly to consumers from a road-side stand (Yada et al. 2019). In 2013, prompted by
outbreaks and recalls of Salmonella contamination in tree nuts, the U.S. Food and Drug Administration (FDA)
began conducting a multiyear, planned risk assessment of Salmonella contamination associated with tree nuts.
The risk assessment is intended to quantify the public health risk associated with eating tree nuts potentially
contaminated with Salmonella and to evaluate the impact of interventions to prevent Salmonella contamination
or reduce its contamination levels (FDA 2013). Foodborne pathogen surveys from tree nuts that quantify the
prevalence and amounts of pathogens, such as Salmonella, provide valuable data when making quantitative risk
assessments.
Oregon’s Willamette Valley produces approximately 99 percent of the United States annual hazelnut crop.
According to the National Agricultural Statistics Service (NASS), between 2016-2018, Oregon produced
42,333 tons of hazelnuts on average from 40,333 bearing acres for a total utilized production value of
$94.7million (NASS 2019). Historically, the majority of Oregon hazelnuts (77 percent of total yield in 2014)
have been sold in-shell and undergone minimal processing (NASS 2015). However, recent trade tariffs with
China have disrupted the supply chain of in-shell hazelnuts that are typically exported to China, and more
hazelnuts are being sold shelled. For example, between 2016-2018, almost half (47 percent) of the total yield
of Oregon Hazelnuts were sold shelled, compared to only 23 percent in 2014 (NASS 2019).
27
Salmonella is unable to multiply on the surface of tree nuts due to the low water activity (generally less than
0.70), but is able to persist on tree nuts for prolonged periods and has been shown to survive in production
and processing environments. There are several points during the harvest and post-harvest handling of
hazelnuts when Salmonella could feasibly be introduced. Before harvest, hazelnuts ripen and fall to the
orchard floor before they are mechanically swept up. Hazelnuts may be exposed to pathogens in soil, water,
and manure on the orchard floor. After harvest, hazelnuts are washed to move any dirt and debris and then
dried. Inadequate washing procedures may lead to contamination or cross-contamination as Salmonella is able
to easily contaminate products in a liquid medium. Cross-contamination of hazelnuts may occur during
processing, handling and storage (GMA 2016). Salmonella populations were found to be stable on inoculated
almonds after over a year in cold storage (4°C) (Kimber et al. 2012; Uesugi et al. 2006). In addition, Salmonella
strains associated with foodborne outbreaks have been shown to survive in contaminated almond orchard
soils for over 5 years (Uesugi et al. 2007). Persistence of Salmonella has led to recalls of tree nuts, including
hazelnuts grown in Oregon. Between 2009 to 2015, there were 4 class-1 recalls of hazelnuts in the United
States and Canada due to Salmonella contamination (Yada et al. 2019).
Large-scale prevalence surveys of California almonds, walnuts, pecans, and pistachios have been conducted
over multiple harvest years with hundreds to thousands of samples (100-500 g) collected at the processor
level (Harris et al. 2019). Available data for the prevalence of Salmonella on hazelnuts is limited to several
retail surveys in the UK and Australia that used a limited amount of samples and small sample size (25g)
(Davidson et al. 2015; Harris et al. 2019). This study was undertaken to characterize the likelihood of
Salmonella contamination among in-shell hazelnuts produced in Oregon over several years. The primary study
objective was to determine the prevalence and levels of Salmonella in minimally processed in-shell hazelnuts
collected from processors throughout Oregon’s Willamette Valley.
28
3.3 Materials and Methods
3.3.1 Hazelnut Sample Collection
In-shell hazelnuts from the 2013 and 2014 harvests were collected from six large hazelnut handlers located in
the Willamette Valley in Oregon. Hazelnut samples were collected three times a week during active season
(middle September – late October) following the first drying stage (green dry). Personnel from the Hazelnut
Marketing Board collected samples from the handlers and delivered samples to Oregon State University each
afternoon to ensure the identity of the processors and origin of samples remained anonymous to lab
personnel. In 2013, no information was collected about any of the samples. In 2014, random numerical
codes were used to unmask sample results to handlers after all nuts were out of commerce.
Each handler was asked to provide samples from four separate lots of hazelnuts per collection day. Each
sample consisted of four subsamples (>100g/subsample) collected from various locations throughout the lot.
Subsamples were collected in sterile Whirl-Pak bags (Nasco, Salida, CA) and placed inside a larger zipper-style
plastic bag to maintain sample organization. Sample analysis began upon receipt by the laboratory.
3.3.2
Salmonella
spp. Analysis
3.3.2.1 Presence/Absence
Enrichment and isolation of Salmonella spp. was performed using a modified version of the Food and Drug
Administration’s Bacteriological Analytical Manual (FDA-BAM) method (Andrews et al. 2020). A total of
375 g of each hazelnut sample (~93.75 g/subsample) were aseptically transferred to a sterile 5.4 L Whirl-Pak
bag (Nasco). Lactose broth (3.375L; Neogen, Lansing, MI) was added to each sample bag and vigorously
shaken for 20 seconds and incubated at 37C for 24 ± 2 h. Following pre-enrichment, 0.1 ml and 1.0 ml of
samples were transferred to 10 ml of Rappaport-Vassiliadis (RV) broth and Tetrathionate (TT) broth,
respectively, and incubated at 37C for 24 ± 2 h. Enrichments were streaked for isolation onto Hektoen
Enteric agar (HE; Neogen) and Xylose Lysine desoxycholate agar (XLD; Neogen) and incubated at 37C for
24-48 h. Presumptive Salmonella colonies (4 per selective enrichment) were transferred to CHROMagar

29
Salmonella Plus (DRG International Inc., Springfield, NJ) and incubated at 37C for 24 ± 2 h. Colonies
displaying pink/mauve coloration were considered to be confirmed as Salmonella spp. and were transferred to
Tryptic Soy Broth (TSB; Neogen) and incubated 24 hrs. Following incubation, TSB cultures were mixed in a
1:1 ratio of 80% (v/v) glycerol and stored at -80
C for future analyses. Remaining hazelnut samples were
stored at ambient temperature (2013) or at 4
C (2014) pending testing results.
3.3.2.2 Most Probable Number (MPN)
Hazelnut samples determined to be positive for Salmonella spp. were further evaluated for enumeration. To
determine the level of Salmonella contamination, a three-tube MPN analysis was performed. Hazelnuts were
divided into nine samples (3 x 3 g; 3 x 33 g; 3 x 333 g) into sterile Whirlpak bags and combined with a 1:10
ratio (w/v) of Lactose broth and incubated at 37C for 24 ± 2 h. Each MPN bag was analyzed to detect the
presence of Salmonella as described above.
Concentrations of Salmonella were calculated using the Thomas approximation (Blodget et al. 2006; Swanson
et al. 2001) of MPN per gram (Bansal et al. 2010) for the three-tube Salmonella MPN analysis:
MPN/g = P/
√
𝑁𝑇
where P is the number of positive tubes, N is total grams of sample in all negative tubes, and T is total grams
of samples in all tubes. The 95% confidence interval was also estimated using the equation:
log(MPN/g) ±(1.96)(0.55)
where a is the dilution ratio and n is the number of tubes per dilution. The limit of detection (0.090
MPN/100 g) was determined by calculating the value for an MPN of 0/3, 0/3, and 1/3 for the 333 g, 33 g,
and 3 g samples, respectively.
30
3.4 Results and Discussion
3.4.1 Prevalence of Salmonella spp. in Hazelnuts
A total of 472 in-shell hazelnut samples were analyzed over the 2013 and 2014 harvest, and 157 (33.3%)
samples were positive for Salmonella upon initial screening (Table 1). The highest annual prevalence occurred
in 2014, where 102 (46.8%) of 218 samples were positive for Salmonella. In contrast, Salmonella prevalence in
2013 was less than half that of 2014, with 55 (21.7%) of 254 samples positive for Salmonella upon initial
screening.
The average prevalence of Salmonella on in-shell hazelnuts of 33.3% (375g; 157 of 472) was determined over
the 2013 and 2014 harvests. The prevalence is significantly higher than previously reported for other tree
nuts in similarly conducted prevalence surveys (hundreds to thousands of 100 to 375 g samples collected
from processors over multiple years). Average Salmonella prevalence levels for California tree nuts are
comparable, with approximately a 1% prevalence of Salmonella, with the exception of in-shell walnuts, which
is significantly lower. The average prevalence of Salmonella in raw almond kernels collected over eight
California harvests (2001 to 2007 and 2010) was 0.98% (100g; 137 of 13,972) (Bansal et al.2010; Danyluk et
al. 2007; Lambertini et al. 2012). The prevalence of Salmonella for in-shell almonds was determined to be
1.3% (100g; 6 of 455) in a 2006 and 2007 survey (Bansal et al. 2010). A three-year survey of in-shell walnuts
found the incidence of Salmonella was 0.14% (100 to 375g; 4 of 3,838) between 2011 to 2014 (Davidson et al.
2015). A survey of in-shell pecans collected over four harvest seasons (2010 to 2014) from seven pecan
shelling facilities located across five U.S. states, found the average prevalence of Salmonella to be 0.95% (100 g;
44 of 4,641). A survey of California pistachios determined the average prevalence of Salmonella to be 0.81%
(100 g; 32 of 3,968) between 2010 to 2012.
Available data for the prevalence of Salmonella on hazelnuts is limited to several retail surveys from Australia
and the UK which used limited amounts of samples and small sample sizes (25 g). Two surveys of edible
hazelnut kernels (25g; 0 of 233) for sale in England in 2008 and 2010 did not identify any Salmonella in retail
31
samples (Little et al. 2009; Little et al. 2010). A survey of hazelnut kernels (25 g; 0 of 48) collected from
Australian processors at the point of receipt, prior to any processing, also did not find any Salmonella
contaminated product (Eglezos et al. 2008; Eglezeos et al. 2010). A survey of RTE packages collected from
an Australian hazelnut processor (25 g; 0 of 51) in 2010 failed to find any Salmonella contamination (Eglezos
2010). In another study, hazelnut kernel samples (25 g; 0 of 34) were collected from retailers, processors, and
growers and did not document any Salmonella contamination on hazelnut kernels (NSW Food Authority,
2012).
3.4.2 Concentration of
Salmonella
spp. in Hazelnuts
For MPN analysis, Salmonella was recovered in 116 of the 157 samples initially testing positive for Salmonella
over the 2013 and 2014 harvests. The detection limit of the MPN method was 0.092 MPN/100 g (Table 2).
Salmonella was recovered from 43.4% and 90.2% of samples originally testing positive in 2013 and 2014,
respectively. Average concentration levels of Salmonella were 0.879 MPN/100 g in 2013 and 3.103 MPN/100
g in 2014. Hazelnut samples collected in 2013 were stored under ambient conditions for 3 to 5 months
before MPN testing was performed. In contrast, 2014 hazelnut samples were stored at 4
C for approximately
one month before MPN analysis was conducted.
Discrepancies in storage time and temperature of Salmonella-positive hazelnut samples prior to MPN analysis
between 2013 and 2014 likely contributed to differences in Salmonella concentration (MPN/100g) and
recovery during MPN analysis. In 2013, hazelnuts were stored under ambient conditions for 3 to 5 months
prior to MPN testing; in contrast, in 2014 hazelnut samples were stored at 4
C for approximately one month
prior to MPN analysis. Studies have shown that Salmonella population levels are stable on nuts stored at 4°C
for months with no reduction in population levels, while Salmonella population levels decline over time on
nuts stored at ambient temperature (Kimber et al. 2012). Samples collected in 2014 were held in cold storage
at 4
C, stabilizing and preventing Salmonella populations from declining, likely contributing to higher recovery
and concentration rates for 2014 MPN samples versus 2013 MPN samples.
32
When Salmonella is detected in tree nuts, its levels are often near or less than 1 cell per gram, even in outbreak
associated product (Danyluk et al. 2007, Lambertini et al. 2012). Lambertini et al (2012) estimated that the
levels of Salmonella could have been 120 MPN/g during the 2001 almond outbreak. Of the survey hazelnut
samples initially determined to be positive for Salmonella, 66.92% remained positive in a subsequent MPN
analysis. The levels of Salmonella estimated for in-shell hazelnuts averaged 0.879 MPN/100g and 3.103
MPN/100g for the 2013 and 2014 harvests, respectively. The levels of Salmonella found in hazelnuts are
similar to those determined for Salmonella in walnuts (0.32 to 0.42 MPN/100g) (Davidson et al.2015) and raw
almond kernels (0.79 to 16.0 MPN/100g) (Bansal et al. 2010, Danyluk et al. 2007, Lambertini et al. 2012).
Hazelnuts can be exposed to Salmonella at a number of points in the orchard and during harvesting and post-
harvest handling. After ripening, hazelnuts fall to the orchard floor where they are mechanically swept into
long, narrow piles between rows of trees in the orchard (Waterbury 2016). Harvesting machines pick up the
hazelnuts, separate them from other plant debris, and deposit the nuts in tote boxes or trailers (Waterbury
2016). Nuts are transported to processing facilities throughout the region. General processing steps involve
washing and drying, with washing methods varying by process company. Washing involves spraying or
rinsing the nuts with water or a diluted food-safe sanitizer to remove excess dirt and debris from the hazelnut
shells. Washed hazelnuts are immediately dried to reduce the moisture to less than 6 percent (CFR 2008).
Hazelnuts can be dried in warm, dry locations over several weeks or over several days in food dryers. Once
dried, hazelnuts can be distributed as in-shell nuts or be shelled, roasted and incorporated into
confectionaries.
Hazelnut samples used in this survey were collected from processors after washing and during the initial
drying stage of processing. In similar large-scale surveys of tree nuts, samples were collected at receipt, prior
to any processing (Davidson et al. 2015). Thus, contaminants in those surveys could only have been
introduced before any processing steps, namely in the orchard or during harvest or immediate postharvest
handling (Davidson et al. 2015). While the orchard remains a likely environmental source for the
33
introduction of Salmonella to in-shell hazelnuts, the process of washing hazelnuts provides several feasible
opportunities for the introduction of Salmonella to the shell surface as well. Cross-contamination of Salmonella
during the initial washing of in-shell hazelnuts may have attributed to the significantly higher levels of
Salmonella prevalence of in-shell hazelnuts compared to other tree nuts.
The comingling of in-shell hazelnuts during washing provides an opportunity for cross-contamination of
Salmonella between in-shell hazelnuts. If hazelnut lots are not carefully kept separated during washing, a
potentially contaminated lot from one orchard could cross-contaminate other lots. In addition, if the water
used for washing hazelnuts is continually reused or recycled, it could potentially become infected with
Salmonella and cross contaminate product. Also, inadequate washing methods that fail to spray or rinse the
complete surface of shell surfaces could contribute to Salmonella contamination during processing. Lack of lot
separation, contaminated washing water, and inadequate washing techniques are some of the potential ways
Salmonella can contaminate in-shell hazelnuts during the initial washing process.
While hazelnuts can be roasted or blanched, the majority of hazelnuts (76%) are sold in-shell and undergo
minimal processing (NASS 2015). There is considerable interest in processes that are able to reduce
Salmonella in hazelnuts without affecting the quality or sensory characteristics of the nut.
34
Table 1. Frequency and quantity of Salmonella spp. detected on in-shell hazelnut samples from 2013 and 2014 harvests.
Harvest
Year
Samples
Analyzed
Samples
Positive for
Salmonella
spp.
Prevalence
Number of Samples Containing Salmonella spp. in MPN Range
Average
MPN/100 g
e
Frequency
of MPN
Recovery
<0.092
MPN/100 g
c
0.092
MPN/100 g
0.092-1.0
MPN/100 g
1.0-10.0
MPN/100 g
10.0-30.0
MPN/100 g
>30.0
MPN/100 g
ND
d
2013
a
254 55 21.7% 29 0 17 6 0 0 3 0.879 44.2%
2014
b
218 102 46.8% 10 2 46 37 6 1 0 3.103 90.2%
Total 472 157 33.3% 39 2 63 43 6 1 3 2.643 74.7%
a
2013: Hazelnut samples stored under ambient temperature conditions between initial Salmonella analysis and MPN enumeration.
b
2014: Hazelnut samples stored at 4C between initial Salmonella analysis and MPN enumeration.
c
Detection limit for MPN analysis was 0.092 MPN/100 g.
d
Not determined: MPN analysis not completed due to excessive mold growth during storage.
e
Average MPN/100 g calculated using only samples with at least one
p
ositive
sample in MPN analysis (0.092 MPN/100 g).
35
Table 2. Recovery frequency of Salmonella during MPN analysis
No. of Samples in Salmonella MPN
range (MPN/100g)
Harvest
year
No. of
positive
samples
<0.092 0.092
c
>0.092 ND
d
Frequency of
recovery%
Avg
(MPN/100g)
2013 55
a
29 24 2 43.64 0.879
2014 102
b
10 2 90 90.20 3.103
Total 157 39 2 114 2 66.92 2.643
a
Samples stored under ambient conditions prior to MPN analysis
b
Samples stored at 4
°
C prior to MPN analysis
c
Detection limit (MPN/100g)
d
Not determined - mold contamination
3.5 Acknowledgments
Funding for this research was provided by the Hazelnut Marketing Board. The authors would like to thank
Alex Emch, Eva Pearson, Daniel Wright, Joey Minarsich, Selena Callahan, Sam Mertz, and Whitney Nielson
for assistance with media preparation and sample analysis. The authors would also like to thank Dr. Linda
Harris of University of California, Davis and Dr. Michelle Danyluk at the University of Florida for assistance
with sample size calculations and methodological considerations.
36
3.6 References
Andrews, W. H., Wang, H., Jacobson, A., Ge, B., Zhang, G., and Hammack, T. 2020. FDA Bacteriological
Analytical Manual (BAM). Chapter 5 Salmonella [Online]. Available from
https://www.fda.gov/food/laboratory-methods-food/bam-chapter-5-salmonella. Accessed July 12, 2020.
Bansal, A., Jones, T.M., Abd, S.J., Danyluk, M.D., and Harris, L.J. 2010. Most-probable number
determination of Salmonella levels in naturally contaminated raw almonds using two sample preparation
methods. J. Food Prot. 73:1986–1992.
Blodgett, R. 2006. Most probable number from serial dilutions, Appendix 2. In U.S. Food and Drug
Administration Bacteriological Analytical Manual. Available at:https://www.fda.gov/food/laboratory-
methods-food/bam-appendix-2-most-probable-number-serial-dilutions. Accessed July 12, 2020.
Centers for Disease Control and Prevention. 2004. Outbreak of Salmonella serotype Enteritidis infections
associated with raw almonds—United States and Canada, 2003–2004. MMWR Weekly 53(22):484–487.
Centers for Disease Control and Prevention. 2011. Investigation update: multistate outbreak of
E. coli O157:H7 infections associated with in-shell hazelnuts. Available at:
http://www.cdc.gov/ecoli/2011/hazelnuts0157/index.html.
Danyluk, M.D., Jones, T.M., Abd, S.J., Schlitt-Dittrich, F., Jacobs, M., and Harris, L.J. 2007. Prevalence and
amounts of Salmonella found on raw California almonds. J. Food Prot. 70:820–827.
Davidson, G. R., Frelka, J. C., Yang, M., Jones, T. M., and Harris, L. J. 2015. Prevalence of Escherichia coli
O157:H7 and Salmonella on Inshell California Walnuts. J. Food Prot. 78(8):1547–53.
Eglezos, S., Huang, B., and Stuttard, E. 2008. A survey of the bacteriological quality of preroasted peanut,
almond, cashew, hazelnut and Brazil nut kernels received into three Australian nut-processing facilities over a
period of 3 years. J. Food Prot. 71:402–404.
Eglezos, S. 2010. The bacteriological quality of retail-level peanut, almond, cashew, hazelnut, Brazil, and
mixed nut kernels produced in two Australian nut-processing facilities over a period of 3 years. Foodborne
Pathog. Dis. 7:863–866.
[FDA] U.S. Food and Drug Administration. 2013. Risk assessments: Salmonella on tree nuts. U.S. Food and
Drug Administration [Online]. Available from: https://www.fda.gov/food/cfsan-risk-safety-
assessments/risk-assessments-salmonella-tree-nuts . Accessed: Apr 12, 2020.
Federal Register. 2008. Almonds grown in California; outgoing quality control requirements. 7 CFR part 981.
Fed. Reg. 72:15021–15036.
[GMA]Grocery Manufacturers Association. 2016. Industry handbook for safe processing of nuts. GMA
Nut Safety Task Force, Grocery Manufacturers Association, Washington, DC. Available at:
https://ucfoodsafety.ucdavis.edu/sites/g/files/dgvnsk7366/files/inline-files/261404.pdf . Accessed: Apr
20, 2020.
Harris, L.J., Yada, S., Beuchat, L.R., and Danyluk, M.D. 2019. Prevalence and levels of foodborne pathogens
on naturally contaminated nuts and edible seeds (version 2) [Tables 1–4 and references]. In Surveys for
foodborne pathogens on nuts. Available at: https://ucfoodsafety.ucdavis.edu/low-moisture-foods/nuts-and-
nut-pastes. Accessed: Apr 9, 2020.
37
Isaacs, S., J. Aramini, B. Ciebin, J. A., Farrar, R., Ahmed, D., Middleton, D., Chandran, A.U., Harris, L.J.,
Howes, M., Chan, E., Pichette, A.S., Campbell, K., Gupta, A., Lior, L.Y., Pearce, M., Clark, C., Rodgers, F.,
Jamieson, F., Brophy, I., and Ellis, A. 2005. . An international outbreak of salmonellosis associated with raw
almonds contaminated with a rare phage type of Salmonella Enteritidis. J. Food Prot. 68(1):191–198.
Kimber, M.A., Kaur, H., Wang, L., Danyluk, M.D., and Harris, L.J. 2012. Survival of Salmonella, Escherichia coli
O157:H7, and Listeria monocytogenes on inoculated almonds and pistachios stored at -19, 4, and 24°C. J. Food
Prot. 75:1394–1403.
Lambertini, E., Danyluk, M.D., Schaffner, D.W., Winter, C.K., and Harris, L.J. 2012. Risk of salmonellosis
from consumption of almonds in the North American market. Food Res. Int. 45:1166–1174.
Little, C.L., Jemmott, W., Surman-Lee, S., Hucklesby, L., and de Pinna, E. 2009. Assessment of
microbiological safety of edible roasted nut kernels on retail sale in England, with a focus on Salmonella. J.
Food Prot. 72:853–855.
Little, C.L., Rawal, N., de Pinna, E., and McLauchlin, J. 2010. Survey of Salmonella contamination of edible
nut kernels on retail sale in the UK. Food Microbiol. 27:171–174.
[NASS] National Agricultural Statistics Service. 2015. NW Noncitrus Fruits and Nuts 2014 Summary. USDA
National Agricultural Statistics Service. [Online]. Available from:
http://www.nass.usda.gov/Statistics_by_State/Oregon/Publications/Fruits_Nuts_and_Berries/FR07_12015
.pdf. Accessed Apr 16, 2020.
[NASS] National Agricultural Statistics Service. 2019. NW Noncitrus Fruits and Nuts 2018 Summary. USDA
National Agricultural Statistics Service. [Online]. Available from:
https://www.nass.usda.gov/Publications/Todays_Reports/reports/ncit0619.pdf . Accessed Apr 9, 2020.
NSW Food Authority. 2012. Report on the prevalence of Salmonella and E. coli in ready to eat nuts and nut
products sold in Australia. Available at:
https://www.foodauthority.nsw.gov.au/sites/default/files/_Documents/scienceandtechnical/national_nut_s
urvey.pdf . Accessed: Apr 21, 2020.
Swanson, K. M. J., Peteran, R. I., and Hanlin, J. H. 2001. Culture methods for enumeration of
microorganisms. Compendium of methods for the microbiological examination of foods, 4
th
ed. American
Public Health Association, Washington, D.C.: p. 52-57.
Uesugi, A.R., Danyluk, M.D., and Harris, L.J. 2006. Survival of Salmonella Enteritidis phage type 30 on
inoculated almonds at -20, 4, 23 and 35°C. J. Food Prot. 69:1851–1857.
Uesugi, A.R., Danyluk, M.D., Mandrell, R.E., and Harris, L.J. 2007. Isolation of Salmonella Enteritidis phage
type 30 from a single almond orchard over a 5-year period. J. Food Prot. 70:1784–1789.
Waterbury, M. 2016. Fall harvest: Oregon hazelnuts. Portrait Magazine [Online]. Available from:
https://www.portraitmagazine.com/fall-harvest-oregon-hazelnuts. Accessed Apr 5, 2020.
Yada, S., and Harris, L. J. 2019. Recalls of tree nuts and peanuts in the U.S., 2001 to present (version 2)
[Table and references]. In U.S. recalls of nuts. Available at:
http://ucfoodsafety.ucdavis.edu/Nuts_and_Nut_Pastes. Accessed: Apr 9, 2020.
38
4. REDUCTION OF
SALMONELLA
SPP. ON IN-SHELL HAZELNUTS USING
CONTINUOUS STEAM BLANCHING AND THE IMPACT ON PRODUCT QUALITY AND
SENSORY CHARACTERISTICS
Authors and Affiliations
Christopher A. Letchworth
a
, Ann Colonna
b
, Michael Morrissey
b
, Joy G. Waite-Cusic
a
, Robert J. McGorrin
a
*
a
Department of Food Science and Technology, Oregon State University, Corvallis, Oregon
97331, U.S.A.
b
Food Innovation Center, Oregon State University, Portland, Oregon
97209, U.S.A.
*Corresponding author: Robert J. McGorrin, robert.mcgorrin@oregonstate.edu
Prepared for submission to:
Food Research International
39
4.1 Abstract
This study was conducted to evaluate the efficacy of steam blanching on the reduction of Salmonella spp. on
the surface of in-shell hazelnuts for Oregon’s hazelnut industry. A pilot-scale steam blancher was used to
deliver a continuous steam treatment at atmospheric pressure. In-shell Barcelona hazelnuts (size large; 3
g/nut) (50 g /sample) were inoculated (8 to 9 log CFU/g) with Salmonella spp. and exposed to steam (88
C)
for 15 s, 1, 3, 5, and 10 min. Following treatment, hazelnuts were transferred to 0.1% peptone water (24
C),
hand agitated 1 min, serially diluted, and plated on Hektoen Enteric agar and incubated at 37
C for 24 h. D-
values of 0.82 to 1.53 min were calculated for exposure of Salmonella spp. to steam treatment at 88
C for
different sample positions in the steam blancher. Salmonella spp. could not be recovered by enrichment after
hazelnuts inoculated at 5 log CFU/g were treated with steam at 88
C for 10 min. These data will be useful to
validated hazelnut industry blanching processes.
4.2 Introduction
Tree nuts have been identified as a potential source for enteric pathogens, particularly Salmonella spp.
Globally, there have been 15 reported outbreaks of salmonellosis and 2 outbreaks of E. coli O157:H7
associated with the consumption of tree nuts since 1953 (Harris et al. 2019). Outbreaks of salmonellosis were
first linked to tree nuts when outbreak-associated Salmonella strains were isolated from coconuts in three
separate outbreaks involving coconuts in 1953, 1960, and 1999 that sickened over 75 people. Since 2000,
there has been an increase in reported outbreaks of foodborne illness associated with tree nuts, with 12
reported outbreaks of salmonellosis responsible for 383 cases of illness associated with tree nuts including,
almonds, cashew, pine nuts, and pistachios. In 2011, in-shell hazelnuts and raw shelled walnuts were
implicated in outbreaks of E. coli O157:H7 that sickened 24 people in Canada and the Midwest United States
(Harris et al. 2019). In addition, since 2001 there have been 101 recalls of tree nuts involving foodborne
pathogen contamination or possible contamination (Yada et al. 2019). The majority of product recalls has
been for Salmonella contamination or possible contamination and has involved numerous types of tree nuts
including hazelnuts, almonds, pecans, walnuts, pine nuts, macadamia nuts, and cashews. Detection of
40
Salmonella in hazelnut samples has led to several class I recalls in the United States and Canada since 2009. In
2009, detection of Salmonella in shelled hazelnuts was traced to a single hazelnut processing facility and
resulted in the recall of 114,350 lb of product (Beuchat et al. 2013; Yada et al. 2019). Detection of Salmonella
in hazelnuts led to additional recalls in 2012, 2013, 2015, and 2017 (Beuchat el al. 2013; Yada et al. 2019).
While Salmonella is responsible for the majority of recalls associated with biological contamination in tree nuts,
E. coli O157:H7 was responsible for recalls of hazelnuts and walnuts in 2001, and walnuts were also recalled
twice in 2014 for Listeria monocytogenes contamination (Yada et al. 2019). Increasing reports of foodborne
illness and outbreaks associated with the consumption of tree nuts underscore the need for effective
postharvest treatments that inactivate foodborne pathogens and a reevaluation of the efficacy of technologies
currently used for tree nut sanitation.
In response to outbreaks and recalls, the Almond Board of California approved a voluntary action plan
making pasteurization mandatory for almonds grown in California beginning in September 2007, with a final
rule published in 2009 (7 CFR Part 981). Under the rule, almonds grown in California and sold in North
America must be processed using a validated method that achieves a minimum 4-log reduction of Salmonella.
However, a 5-log reduction of Salmonella is the minimum process required for labeling almonds as pasteurized
(ABC 2007a). Several validated postharvest treatments for almonds are currently recognized by the Almond
Board of California, including propylene oxide (PPO) fumigation, steam, oil roasting, dry roasting, and hot
water blanching (ABC 2007b). Research conducted on almonds show promise for application to other nut
industries that may incorporate process elements into their own processing procedures. However,
postharvest treatments should be designed specifically for each type of nut product based on safety needs and
final product quality.
The Oregon Hazelnut industry produces 99 percent of the United States annual hazelnut crop. According to
the National Agricultural Statistics Service (NASS), in 2018, Oregon produced 51,000 tons of hazelnuts from
44,000 bearing acres for a total utilized production value of $91.8 million (NASS 2019). Historically, the
41
majority of Oregon hazelnuts (63 percent of total average yield in 2016-2017) (NASS 2019) are sold in-shell
and undergo minimal processing. However, hazelnuts sold in-shell only accounted for 38 percent of total
utilized production in 2018 (NASS 2019). Due to the increasing detection of Salmonella in hazelnuts and other
tree nuts, there is a need for postharvest treatments that not only effectively reduce levels of Salmonella with
minimal impact on final product quality, but are also cost-effective and flexible enough to incorporate into
existing processing lines.
Steam pasteurization is effective for reducing naturally occurring and pathogenic bacteria in foods (Nutsch et
al. 1998). The efficacy of steam pasteurization is largely due to the large amount of heat transferred to foods
when steam condenses, which rapidly increases the surface temperature (James et al. 2000). Steam has a
higher heat capacity than the same amount of water at a given temperature (James et al. 1997) and can
effectively penetrate small areas such as cracks and crevices on nuts that may protect microorganisms
(Morgan et al. 1996). There is a limited understanding of the efficacy of using steam as a postharvest
Salmonella treatment for in-shell hazelnuts and the effects of such treatments on final product quality.
The purpose of this study was to identify a steam treatment that would effectively pasteurize in-shell
hazelnuts (5-log reduction of Salmonella spp.) and would have minimal impact on nut quality. To achieve
these goals, a variety of steam treatments (time/temperature combinations) were evaluated for their ability to
reduce Salmonella spp. on in-shell hazelnuts and used to predict treatments that would achieve pasteurization.
Predicted time/temperature combinations that would achieve pasteurization were verified. Additional
hazelnuts were treated with pasteurizing steam treatments and sensory tests were conducted to evaluate
changes in consumer perception of the nuts.
42
4.3 Materials and Methods
4.3.1 Hazelnuts
Raw in-shell hazelnuts (washed, dried, graded, and packaged) were provided by the Oregon Hazelnut
Marketing Board (Aurora, OR). Barcelona hazelnuts (size large; 3 g/nut) in bulk 22.7 kg bags were stored at
ambient temperature for up to 6 months prior to use.
4.3.2 Preparation of Inoculum
Five strains of Salmonella enterica previously associated with tree nuts or peanuts were used in this study (Table
1). Stock cultures were stored at -80
0
C in Tryptic Soy Broth (TSB; Neogen, Lansing, MI) supplemented with
40% glycerol. Stock cultures were resuscitated by transferring to individual tubes containing TSB and
incubated at 37
C for 24 h. The resulting culture (1 ml) was spread onto several large format (150 x 15 mm)
petri dishes containing Tryptic Soy Agar (TSA, Neogen) and incubated at 37
C for 24-26 h to produce a lawn.
Bacterial lawns were harvested by adding 8.0 ml of 0.1% peptone water (Neogen) and loosening with a
disposable cell spreader. Cell suspensions for each strain were transferred into 50 ml sterile conical tubes and
thoroughly vortexed (1 min). Equivalent volumes of each harvest were combined to create a five strain
cocktail. For spot inoculation procedures, Tween 80 was added to the cocktail to achieve a final
concentration of 0.5% to reduce surface tension. Cell densities were determined by standard serial dilution
and plating on Hektoen Enteric agar (Neogen) plates and enumerated following incubation at 37
C for 24 h.
When necessary, the inoculum cocktail was diluted with 0.1% peptone water to achieve lower targeted
inoculation levels. The cocktail was held at 4
C for up to 2 weeks prior to use as hazelnut inoculum.
43
Table 1. Salmonella enterica strains included in the inoculation cocktail for this study.
Serotype Isolate Identifier Description Source
Salmonella Enteritidis
PT30
ATCC BAA-
1045
Almond isolate
American Type Culture Collection
(ATCC)
Salmonella Enteritidis
PT9c
RM4635
Clinical isolate from
almond outbreak
Rob Mandrell
USDA-ARS
Salmonella Montevideo GRC1 Pistachio isolate Food and Drug Administration
Salmonella Oranienburg MDD317 Pecan isolate
Michelle Danyluk
University of Florida
Salmonella Tennessee MDD319
Clinical isolate from
peanut butter
outbreak
Larry Beuchat
University of Georgia
4.3.3 Immersion Inoculation of Hazelnuts
In-shell hazelnuts were inoculated by immersion following previously published procedures for almond
kernels with minor modifications (Danyluk et al. 2005). Briefly, hazelnuts (400-2400 g) were transferred to a
1.5 L sterile sample bag (WhirlPak, Nasco, Salida, CA). The previously described inoculum was added to the
hazelnuts at a ratio of 1:6 (v/w) (25 ml inoculum per 400 g hazelnuts). The bag was closed and mixed by
hand shaking and inversion for 1 min to distribute inoculum evenly on the surface of the hazelnuts.
Inoculated hazelnuts were transferred to perforated stainless steel baskets (30 x 30 cm) in the biological safety
cabinet and air dried 18-24 hrs. Dried, inoculated hazelnuts were aseptically transferred into sterile sample
bags and held at 4
C for up to 1 month prior to steam treatment.
4.3.4 Spot Inoculation of Hazelnuts
Individual in-shell hazelnuts (n = 200) were arranged onto sterile baking sheets (33 cm x 45 cm) and
inoculated by dispensing 12.5 µl of the Salmonella cocktail onto the basal scar or middle shell surface of the
hazelnut. Inoculated hazelnuts were air dried for 16-18 hrs., transferred to sterile sample bags, and stored at
4
C for up to 2 weeks prior to steam treatment.
44
4.3.5 Steam Blancher
A pilot-scale steam blancher was designed and built by GEM Equipment of Oregon, Inc. (Mt. Angel, OR) for
this study (Figure 1). The blancher utilized all T304 stainless steel construction with overall blancher
treatment chamber dimensions being approximately 180 x 33 x 27.5 cm. The blancher was designed to
mimic continuous inline feed conveyor systems designed to treat hazelnuts prior to packaging. An inverter
(Sinamics V20 ; Siemens AG, Germany) was used to power and control the conveyor belt of the steam
blancher. The feed conveyor consisted of a continuous stainless steel belt used to transport stainless steel
hazelnut catches (30 x 30 x 15 cm tall with flat wire bottom; GEM Equipment) through a treatment vessel
containing upper and lower manual valve steam pipes above and below the continuous belt. Two control
timers (GT3A; IDEC, Sunnyvale, CA), a feed timer and dwell timer, were used to operate the feed conveyor
of the steam blancher. A separate automated steam control valve – regulated by a thermocouple that
measured the temperature of steam condensate inside the steam blancher – controlled the inlet of steam into
the blancher, helping to regulate the vessel’s temperature. Pressurized air connected to the automated steam
inlet valve and regulated at 20 psi was used to open the automated steam control valve. Temperature in the
steam blancher was regulated by a proportional-integral-derivative (PID) controller with a digital user
interface (EZ-ZONE PM Express; Watlow, Winona, MN), which is a control-loop feedback mechanism
commonly used in industrial control systems.
The manual valves on the upper and lower steam bars were used to influence the output and direction of
steam. A feed timer was used to load hazelnut catches into the blancher. When the conveyor was turned on,
hazelnut catches would move into the blancher based on the duration of this timer. We found a feed time of
10 s at maximum belt speed was optimum for quickly centering three baskets side-by-side in the blancher. A
second dwell timer determined the length of time that baskets remained in the blancher before the conveyor
restarted. A start/stop switch controlled the entire process. Turning on the selector switch engaged the
conveyor forward for the length of time set by the feed timer. The conveyor would then stop for the length
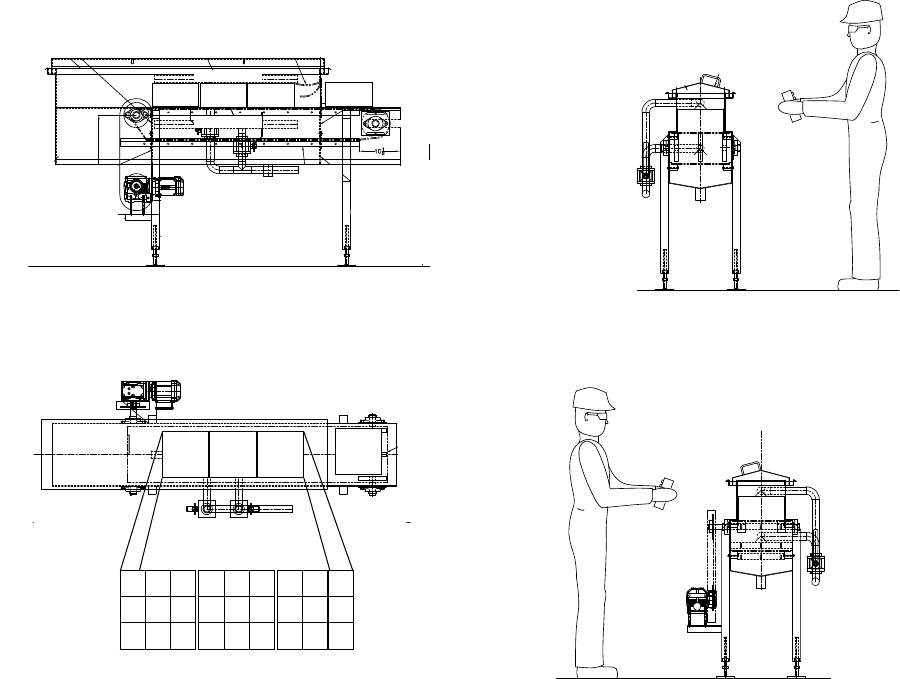
45
of time determined by the dwell timer (0-15 minutes), before restarting and moving baskets forward and
down an exit chute (outlet).
When the automated steam inlet valve opened, atmospheric steam (100
C) entered the treatment vessel and
would begin to condense, raising the temperature of the treatment vessel. In turn, when the automated steam
inlet valve closed, the temperature of the treatment vessel would drop. By monitoring the temperature and
controlling the input of steam, the steam blancher was designed to calibrate the internal temperature of the
treatment vessel, helping achieve targeted treatment temperatures.
Figure 1. Pilot-scale steam blancher and sample configuration for in-shell hazelnut treatments.
(Graphics courtesy of GEM Equipment of Oregon, Inc.)
NUTS
NUTS
NUTS
NUTS
NUTS
NUTS
TOP
VIEW
INLETOUTLET
NUTS
NUTS
1 2 3
4 5 6
7 8 9
10 11 12
13 14 15
16 17 18
19 20
22 23 24
25 26 27
21
SAMPLE
CONFIGURA TION
STEAM
BARS
FRONT
VIEW
INLET
OUTLET
NUTS
NUTS
NUTS
NUTS
NUTS
STEAM
BAR
STEAM
BAR
STEAM
BAR
OUT LET
VIEW
STEAM
BAR
STEAM
BAR
NUTS
CONDENSATE
INLET
VIEW
STEAM
BAR
STEAM
BAR
NUTS
46
4.3.6 Sample Preparation and Arrangement
Inoculated hazelnuts were removed from refrigerated storage at least 2 hours prior to steam treatment.
Immersion-inoculated hazelnuts (50 g) were aseptically transferred to nylon mesh produce bags (Royal, Santa
Fe Springs, CA). Spot-inoculated hazelnuts (12 g) were combined with uninoculated hazelnuts (38 g) in
produce bags. Samples were placed into stainless steel boxes (30 cm x 30 cm x 15 cm) with flat wire bottoms
divided into 9 compartments (9.85 cm x 9.85 cm x 15 cm) by four interlocking stainless steel plates (GEM
Equipment). This configuration provided 27 unique sample positions in the chamber for a single steam
treatment (Figure 2). For initial steam treatments, inoculated samples were assigned to six compartments
(positions 4, 9, 11, 18, 19, 24) with the remaining positions being filled with ~200 g of bulk uninoculated
hazelnuts. Later experiments had inoculated samples in all positions with a subset of samples being placed
between two layers of bulk, uninoculated hazelnuts (~200 g each) to assess the impact of bed-depth on
Salmonella cell survival. A temperature data logger equipped with a 559 mm stainless steel flexible probe
(OM-CP-HITEMP140-PT; Omega Engineering, Stanford, CT) was placed in the center compartment of the
middle basket (position 14) to record the temperature profile of the treatments. On a subset of treatments,
additional temperature data loggers equipped with 50 mm stainless steel rigid probes (OM-CP-HITEMP140)
or with a 131 mm flexible probe (OM-CP-HITEMP140-5.25) were placed in various positions to measure the
temperature variability throughout the chamber.
47
4.3.7 Steam Treatments
The steam blancher was set to a targeted calibrated temperature (79
C or 88
C) and allowed to self-calibrate
for at least 20 min prior to treating samples. Once calibrated, the first steam basket (positions 1-9) was placed
at the inlet of the steam vessel and the conveyor belt was initiated. The second and third baskets were placed
in quick succession on the conveyor to minimize air space between the baskets. Hazelnuts were steam
treated for 15 s to 15 min prior to exiting the steam blancher on the outlet end. Each time-temperature steam
treatment combination was performed in triplicate.
4.3.8 Microbiological Analysis
Baskets were immediately recovered from the outlet of the steam blancher. The entire contents of the
hazelnut samples (50 g) were aseptically transferred to sterile Whirl-Pak bags (250 ml; Nasco) and mixed with
50 ml of 0.1% peptone water. Samples were forcibly shaken by hand for 1 min. Serial dilutions were
Figure2.Samplelayoutblancher:Locationofcatchcompartmentsusedfortreatmentvalidation
Back
A BC
1
b
23 10
11
12
19
20 21
Exit
4
a
56 12
14
c
15 22 23
24
Entrance
78
9
16 17
18
25 26 27
Front
a
Hazelnutcatchcompartmentsusedforsampleplacementareshowninbold
b
Theother21compartmentsareblockedwithapprox.200g/eachofuninoculatedhazelnuts
c
Datalogger(OM‐CP‐HITEMP140‐PT;OmegaEngineering,Stanford,CT)
Hazelnutcatches(A‐C)
Orientationofhazelnutcatchesandcompartmentsintheblancher
48
prepared using 0.1% peptone water and plated in duplicate onto Hektoen Enteric agar (HE; Neogen) using a
spiral plater (Autoplate 4000; Advanced Instruments, Norwood MA). Plates were enumerated following
incubation at 37C for 24-48 hrs. Spot-inoculated samples were recovered similarly to immersion-inoculated
cells with the following exception. For sample treatments >3 min, the detection limit was improved by
dividing 1 ml samples in two, then spread plating using two HE plates (0.5 ml/ HE plate) and incubated as
described earlier. Samples were also enriched in lactose broth (200 ml) with incubation at 37
C for 24 h prior
to streaking onto HE plates to qualitatively determine the presence or absence of surviving Salmonella cells in
the entire sample. Colonies displaying typical Salmonella spp. morphology on HE (black, no acid production)
were considered confirmed. Inoculated, untreated hazelnuts (n = 3) were serially diluted and plated as
described above to determine initial populations (time = 0).
4.3.9 Sensory Analysis
Additional raw, in-shell hazelnuts were provided by the Hazelnut Marketing Board for sensory evaluation to
evaluate the ability of the consumer to detect differences and evaluate attributes between untreated and
steam-treated product. To protect consumers from potential cross-contamination from the laboratory,
uninoculated, in-shell hazelnuts were processed using a secondary pilot-scale steam blancher (GEM
Engineering) in the Oregon State University Food Science Pilot Plant. Hazelnuts were processed at 88
C for
8 and 15 minutes. A data logger (OM-CP-HITEMP) with a 175 mm flexible probe was used to verify
temperature and time exposure to steam. Processed hazelnuts were dried at room temperature for 72 h then
placed in unsealed cardboard boxes and transported to the Oregon State University Food Innovation Center
(Portland, OR).
The sensory tests were conducted by a consumer panel (n = 58) recruited from a database that indicated
them as tree nut consumers. A triangle test was conducted using two sets of samples: (i) untreated compared
to steam treatment (8 min) and (ii) untreated compared to steam treatment (15 min). In a second session,
49
panelists were asked to complete an attributes acceptability test consisting of a 20 question ballot to rank
attributes such as flavor, aroma, and color by level of acceptability.
4.3.10 Statistical Analysis
Triplicate data was used to plot survivor curves. Data were analyzed using GraphPad Prism software
(GraphPad Software Inc.; LaJolla, CA). Linear regression analyses of survivor curves with 95% confidence
intervals were used to predict D values and times required to achieve 5-log reductions of Salmonella on in-shell
hazelnuts.
4.4 Results
Preliminary data was gathered using only the bottom steam bar (valve fully open). Used alone, steam from
the bottom bar was inefficient at penetrating beds of hazelnuts up to 15 cm tall and results were variable
making it hard to consistently achieve a 5 log reduction of Salmonella. Using both the bottom and top steam
bars (valves fully opened) allowed steam to more effectively penetrate hazelnut bed layers from two sides and
the variability in results, while not eliminated, was reduced helping to better predict and achieve treatment
parameters (time and temperature) that achieve a minimum 5 log reduction of Salmonella. All subsequent
studies were used using both steam bars (valves fully opened) and any treatments mentioned in this study
should be assumed to use both steam bars unless otherwise specified.
4.4.1 Reduction of Salmonella spp. after Steam Treatment
Hazelnuts were initially inoculated with a Salmonella cocktail (~ 8.5 log CFU/g) following the inoculation
methods of Danyluk et al. with minor modifications, as previously described, and subjected to a combination
of treatment times and temperatures. At treatment temperatures of 79
C and 88
C, Salmonella population
levels decreased overtime on in-shell hazelnuts; however, considerable variability was observed between
steam treatment runs using the same operating parameters (time and temperature) (Figure 3 and Table 2).
Salmonella population levels varied by as many as 4 log CFU/g between sample locations after treatments
50
(Figure 3). At 88
C, the predicted treatment times required to achieve a 5 log reduction of Salmonella were
7.67 min and 6.23 min for inoculated samples in locations 9 and 11, respectively, and 4.11 min for location 24
(Table 2). At 79
C, predicted times to achieve a 5 log kill were longer, with estimates ranging from 8.99 min
and 14.90 min for locations 9 and 11, respectively, to 6.39 min for test location 24 (Table 2). Due to
treatments at 88
C achieving a 5- log reduction of Salmonella in a shorter amount of time, and with no
perceived negligible impact on final product quality (Tables 5 and 6), all further experiments were conducted
at 88
C.
51
Timerequiredfor5logreduction(min)D‐value:timerequiredfor1logreduction(min)
Samplelocation Samplelocation
Temperature
(
o
C)
Estimate 4 9 11 18 19 24 4 9 11 18 19 24
88 Bestfit 4.34 6.15 4.98 4.18 4.16 3.43 0.87 1.23 1.00 0.84 0.83 0.69
95%CI
a
5.26 7.67 6.23 5.50 4.99 4.11 1.05 1.53 1.25 1.10 1.00 0.82
79 Bestfit 7.07 7.38 8.51 6.47 7.81 5.65 1.41 1.48 1.70 1.29 1.56 1.13
95%CI
a
8.58 8.99 14.90 7.98 8.62 6.39
1.72 1.80 2.98 1.60 1.72 1.28
a
Upperlimitofconfidenceinterval
Table 2. Estimated times required to achieve 1 and 5 log reductions of Salmonella with steam treatment at 88
o
C and 79
o
C
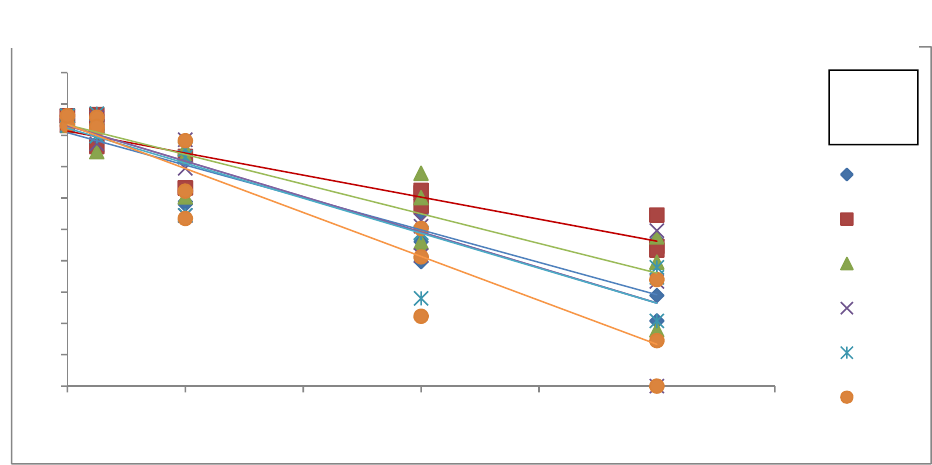
52
4.4.2 Temperature Profile of Steam Blancher
Temperature was not uniform throughout the steam blancher during steam treatments. Mapping the
temperature profiles of the six hazelnut catch compartments used to predict thermal death times with data
loggers did not help to establish a map of potential hot and cold locations within the blancher (data not
shown). Rather, temperatures in the blancher appeared to be dynamic and variable, varying by treatment run,
and making it challenging to create a temperature profile of different sample positions within the blancher.
Mapping the recovery frequency of Salmonella after enrichment of the 27 hazelnut catcher locations used for
process verification also failed to distinguish a reliable temperature profile pattern or hot/cold spots for the
steam blancher (Figure 4).
0
1
2
3
4
5
6
7
8
9
10
0.00 1.00 2.00 3.00 4.00 5.00 6.00
log(CFU/g)
Treatmenttime(min)
4
9
11
18
19
24
Sample
layout
position:
Figure 3. Survival of Salmonella spp. on immersion inoculated hazelnuts after exposure to steam at 88
°
C
53
Figure 4. Ratio of Salmonella positive samples after enrichment
a
by sample layout position and bed layer after 88
°
C steam treatment
4.4.3 Endpoint Determination – 5 Log Verification
4.4.3.1 Immersion Inoculated Hazelnuts
Hazelnuts were inoculated by immersion into inoculum, as previously described, at 5 log CFU/g to verify
results of plate counts and predicted times required to achieve 5-log reductions. Immersion inoculated
hazelnuts were unable to consistently inactivate all Salmonella cells within a sample and did not adhere to the
predicted reduction times derived using plate counts (Tables 2 and 3). At 88
C, a conservative time of 7.67
minutes (location 9) was required to achieve a 5-log reduction of Salmonella (Table 2). However, Salmonella
was recovered from 5/27 (18.5%) samples treated for 7.67 minutes and 13/54 (24.1%) samples treated for 15
minutes at 88
C (Table 3). We postulated that the variability in treatments and discordance with the
predicted times may have been caused by Salmonella cells penetrating split sutures (cracks) in the hazelnut
shells during inoculation, which in turn may have harbored and protected Salmonella cells from steam contact
during steam treatment. To test this, we began spot-inoculating hazelnuts.
0/1
b
0/3
c
0/3
0/1
0/1
1/3
1/1
2/3
1/1
2/3
2/3
0/1
1/4
0/0
0/3
1/1
0/1
3/3
0/1
c
1/1
d
0/1 1/1 0/1
1/1
c
0/1 0/1 0/1
1/2
0/2
0/4
0/0
0/1
0/3
1/2
2/2
0/1
2/3
0/0
3/4
1/1
2/3
0/3
1/1
1/3
1/1
0/1 1/1
1/1
c
0/1 0/1 0/1
1/1
c
0/1 1/1
1/4
0/0
1/3
0/1
0/3
0/1
0/0
1/4
0/2
2/2
0/1
3/3
0/2
1/2
1/3
1/1
0/1
3/3
0/1 0/1 0/1 0/1
1/1
c
0/1 1/1 1/1
0/1
c
1/1
0/3
0/3
1/1
0/1
0/3
0/1
0/3
0/1
1/3
0/3
0/1
0/4
0/0
0/3
0/1
0/1
1/3
0/1
c
0/1 0/1 0/1 0/1
0/1
c
1/1 0/1 1/1
0/2
1/2
0/4
0/0
0/1
0/3
0/2
0/2
0/1
2/3
0/0
1/4
0/1
1/3
1/3
1/1
0/3
0/1
0/1 1/1
0/1
c
0/1 0/1 0/1
1/1
c
0/1 0/1
0/4
0/0
0/3
0/1
1/3
0/1
0/0
1/4
0/2
0/2
0/1
1/3
0/2
0/2
0/3
1/1
0/1
0/3
0/1 0/1 0/1 0/1
0/1
c
0/1 0/1 0/1
1/1
c
0/1
0/3
0/3
0/1
0/1
0/3
0/1
0/3
0/1
0/3
1/3
0/1
0/4
0/0
0/3
0/1
0/1
0/3
0/1
c
0/1 0/1 0/1 0/1
0/1
c
1/1 0/1 0/1
0/2
0/2
0/4
0/0
0/1
0/3
1/2
0/2
0/1
0/3
0/0
0/4
0/1
1/3
0/3
0/1
0/3
0/1
0/1 1/1
0/1
c
0/1 0/1 0/1
0/1
c
0/1 0/1
0/4
0/0
0/3
0/1
1/3
0/1
0/0
1/4
0/2
0/2
0/1
0/3
0/2
0/2
0/3
0/1
0/1
0/3
0/1 0/1 0/1 0/1
0/1
c
0/1 0/1 0/1
1/1
c
0/1
c
1/2 1/2 0/0 1/2
0/1
c
1/2 0/0 0/0
0/1 2/2
0/1
c
0/0 0/0 1/2
0/1
c
0/0 0/0
0/0 1/2 0/0 1/2
0/1
c
1/2 1/2 1/2
1/2
c
b
Singlelayersamples
c
Centersamples‐15cmlaye
r
d
RedboxesindicateSalmonella positivesamples
No.ofpos itivesamplesbybasketcompartmentandbedlaye
r
Spotinoculatedsamples(~5logCFU/g) Immersioninoculatedsamples(~5logCFU/g)
a
Lactoseenrichment(37
o
C24h)
No.ofpositivesamplesbybasketcompartmentandbedlaye
r
3minutes
5minutes
10minutes
15minutes
10minutes
7.67minutes
6.23minutes
54
Table 3. Qualitative reduction of immersion-inoculated 5 log CFU/g of Salmonella spp. on in-shell hazelnuts
treated in the steam blancher.
Sample
Number of positive samples after steam treatment (88C set point)
6.2 min 7.7 min 10 min 15 min
Single Layer 6/21 (28.5%) 3/21 (14.3%) 2/21 (9.5%) 12/42 (28.6%)
Center Layer
b
3/6 (50%) 2/6 (33.3%) 1/6 (16.7%) 1/12 (8.3%)
Combined 9/27 (33.3%) 5/27 (18.5%) 3/27 (11.1%) 13/54 (24.1%)
b
Center layer samples were placed in the middle of two layers of uninoculated in-shell hazelnuts. Total depth
of the product was 15 cm.
4.4.3.2 Spot Inoculated Hazelnuts
Spot inoculated hazelnuts (~ 5 log CFU/g) were able to consistently achieve a 5-log inactivation of Salmonella
at 88
C for 10 minutes (Table 4). Spot inoculated hazelnuts showed a more consistent reduction of Salmonella
cells over time compared to immersion inoculated hazelnuts (Tables 3 and 4). At treatment durations of 3
and 5 minutes, hazelnut bed layer had a significant impact on the reduction levels of Salmonella after steam
treatment, with center layer samples (15 cm layer) exhibiting a higher recovery frequency of Salmonella
compared to single layer samples (Table 4). At 3, 5, and 10 min treatment durations, the frequency of
Salmonella recovery for single layer samples after lactose enrichment were 12/54 (22.2%), 3/54 (5.56%), and
3/54 (5.56%), respectively (Table 4). In contrast, Salmonella was recovered from center layer samples at a
frequency of 31/54 (57.4%), 12/54 (22.2%), and 3/54 (5.6%) for treatment times of 3, 5, and 10 min,
respectively (Table 4). At ten minutes, the hazelnut bed layer had no significant impact on the recovery of
Salmonella after steam treatment (Table 4).
55
Table 4. Qualitative reduction of spot-inoculated 5 log CFU/g of Salmonella spp. on in-shell hazelnuts treated in the steam blancher.
Sample
Placement
Number of hazelnut samples positive for Salmonella spp. after steam treatment (88C set point)
3 min 5 min 10 min
a
Basal
Scar
Shell Combined
Basal
Scar
Shell Combined
Basal
Scar
Shell Combined
Single
Layer
8/28
(28.6%)
4/26
(15.4%)
12/54
(22.2%)
1/28
(3.6%)
2/26
(7.7%)
3/54
(5.6%)
1/28
(3.6%)
2/26
(7.7%)
3/54
(5.6%)
Center
Layer
b
19/26
(73.1%)
12/28
(42.9%)
31/54
(57.4%)
8/26
(30.8%)
4/28
(14.3%)
12/54
(22.2%)
2/26
(7.7%)
0/26
(0%)
2/54
(3.7%)
Combined
27/54
(50%)
16/54
(29.6%)
43/108
(39.8%)
9/54
(16.7%)
6/54
(11.1%)
15/108
(13.9%)
3/54
(5.5%)
2/54
(3.7%)
5/108
(4.6%)
a
All positives for the 10-minute treatment were from the same treatment replicate.
b
Center layer samples were placed in the middle of two layers of uninoculated in-shell hazelnuts.
56
The inoculation surface (basal scar or shell) had a significant impact on the recovery frequency of Salmonella
after steam treatment for 3 min at 88
C. Salmonella was recovered from 27/54 (50.0%) of the basal scar
inoculated samples and 16/54 (29.6%) of the shell inoculated samples treated at 88
C for 3 min (Table 4). At
longer treatments of 5 and 10 min at 88
C, the inoculation surface did not have a significant impact on
Salmonella recovery (Table 4). Table 4 illustrates how the combination of inoculation surface and hazelnut
bed layer significantly impacted the recovery frequency of Salmonella for 3 min treatments at 88
C. At
treatment times of 3 min, center layer samples (15 cm layer) inoculated on the basal scar of in-shell hazelnuts
had a Salmonella recovery rate of 19/26 (73.1%); compared to a 4/26 (15.4%) recovery rate for single layer
samples that were spot inoculated on the shell surface (Table 4).
4.4.5 Sensory Analysis
Volunteer participants in a sensory test conducted by the Oregon Food Innovation Center (Portland, OR)
were unable to distinguish steam processed hazelnuts at 88
C for 8 and 15 minutes from unprocessed
hazelnuts. In a difference test (triangle), 45 out of 58 consumers (78%) could not tell a significant difference
(p-value 0.975) between hazelnuts treated at 88
o
C for 8 min and control hazelnuts (Table 5). However, only
60% of consumers could not tell a difference (p-value 0.188) between 15 min treated samples and controls
(Table 5). The attribute acceptability levels were similar for all treatments except for aroma and color based
on an attributes acceptability test consisting of a 20 question ballot (Table 6). Consumers favored the aroma
of steam treated samples for their “roasted” aroma and steam treated samples appeared somewhat darker in
color (Table 6).

57
Table 5. Ability of consumers to distinguish steam treated hazelnuts from
untreated control samples
Difference (Triangle) Test
Control vs. 8 minutes Control vs. 15 minutes
No. of judgments: 58
58
Incorrect: 45 35
Correct: 13 23
Confidence: 0.025 0.812
Significance
a
(p-
value):
0.975 0.188
Table 6. Sensory properties of hazelnuts treated by steam blanching at various holding times.
Values presented are the mean score (n = 100). Values within a row with different superscript
capital letters are significantly different (p <0.05).
Parameters
Steam treatment time (88C set point)
Untreated 8 min 15 min
Appearance
a
3.68 1.07 3.69 1.13 3.43 1.21
Color
b
3.06 0.47
A
3.12 0.56
AB
3.28 0.53
B
Crunchiness
e
2.64 0.72 2.84 0.73 2.72 0.71
Flavor Strength
c
2.51 0.76 2.52 0.72 2.59 0.77
Sweetness
d
2.47 0.69 2.49 0.70 2.41 0.73
Texture
f
2.81 0.65 2.85 0.56 2.93 0.71
Liking
g
:
Aroma
5.39 0.84
AB
5.33 0.82
B
5.47 0.87
A
Flavor
6.81 1.57 6.73 1.66 6.77 1.46
Texture
6.20 1.88 6.43 1.72 6.16 1.85
Overall
6.75 1.67 6.75 1.67 6.60 1.61
Purchase Intent
h
3.49 1.09 3.49 1.12 3.39 1.25
a
Appearance was evaluated on a scale from 1 (very unappealing) to 5 (very appealing).
b
Color was evaluated on a scale from 1 (much too light) to 5 (much too dark).
c
Flavor strength was evaluated on a scale from 1 (much too weak) to 5 (much to strong).
d
Sweetness was evaluated on a scale from 1 (not at all sweet enough) to 5 (much too sweet).
e
Crunchiness was evaluated on a scale from 1 (not at all crunchy enough) to 5 (much too crunchy).
f
Texture was evaluated on a scale from 1 (much too soft) to 5 (much too hard).
g
Liking was evaluated on a scale from 1 (dislike extremely) to 9 (like extremely).
h
Purchase intent was evaluated on a scale from 1 (would definitely not buy) to 5 (would definitely buy).
58
4.5 Discussion
4.5.1 Steam Treatment Efficacy – This Study Compared to Previous Studies
The primary objective of thermal pasteurization of foodstuffs is to inactivate pathogenic and spoilage
microorganisms to produce a shelf-stable product in which the original properties of the raw material are
retained as much as possible (Da Silva et al. 2009). In this study, we show that steam treatment at 88
C for
10 minutes achieves a 5-log reduction of Salmonella on in-shell hazelnuts with negligible and minimal impact
on final product quality.
Several similar studies have evaluated the efficacy of steam pasteurization at inactivating Salmonella Enteritidis
on the surface of inoculated raw almond kernels with conflicting results. Using the same variety of almonds
(Nonpareil) and same SE strains (S. Enteritidis 43353, ME-13, ME-14), Chang et al. (2010) observed a 5-log
reduction of Salmonella after 25-s exposure to steam at 143 kPa, whereas Lee et al. (2006) was unable to
achieve a 4-log reduction even after 35-s exposure to atmospheric steam. Chang et al. (2010) attributed the
disparity in results to differences in the condition of steam applied, noting that the effectiveness of steam
treatment on S. Enteritidis is dependent on the type of steam technology used for treatment, with
pressurized and atmospheric steam treatments yielding different results (Chang et al. 2010). Chang et al.
(2010) used a custom built steam pasteurizer with a pressurized steam treatment chamber, whereas Lee et al.
(2006) used conventional steaming by placing inoculated almonds directly above boiling water. The enhanced
reduction of S. Enteritidis observed by Chang et al. (2010) may be because of rapid increases in temperature
within the enclosed chamber compared to heat dissipation in open air with conventional steaming. Both
authors noted that prolonged exposure to steam (35 s) had negative impacts on the quality of almond kernels,
resulting in increased moisture content and loss in visual quality. In a separate study, a combination of
superheated steam (115
C) for 70 s followed by infrared heating for 70 s completely eliminated Salmonella
Enteritidis without resuscitation in enrichment (Bari el al. 2010) and with minimal impact on product quality.
Due to the dissipation of heat in an open system, steam blanching at atmospheric pressure typically requires
longer exposure times to steam to sufficiently inactivate Salmonella on tree nuts than pressurized or
59
superheated steam processes which require an enclosed treatment chamber. However, inline continuous
atmospheric steam blanching systems often are able to more efficiently treat tree nuts and have a greater
product throughput rate than batch style pasteurization systems that use enclosed chambers to administer
pressurized and superheated steam. In addition, prolonged atmospheric steam blanching of in-shell hazelnuts
does not appear to affect the final product quality.
There is a limited understanding of the heat resistance of Salmonella spp. on hazelnut shells. Salmonella
Oranienburg and S. Enteritidis PT30 were extremely resistant on dry (4% w/w moisture), crushed (1mm)
hazelnut and cocoa shells, with D
100
o
C
values ca. 2.5 min in crushed cocoa bean shells and 7-11 min in
crushed hazelnut shells, respectively. Addition of moisture to ca. 7% w/w markedly reduced D-values (D
80
o
C
of 2-4.5min) for both strains in the two matrices (Izurieta et al. 2012). Heating in hazelnut shells resulted in
significantly higher (p<0.05) D-values than in cocoa bean shells, possibly due to the matrix effect and
possibly cocoa shell components, such as polyphenols and flavonoids. However, it is worth noting that
crushed cocoa bean shells had significantly higher (p<0.05) z-values than in crushed hazelnut shells (Izurieta
et al. 2012). The heat resistance of S. Oranienburg and S. Enteritidis PT30 under dry conditions (4% w/w)
gives rise to concerns about the efficacy of mild or dry roasting conditions that may be insufficient to
eliminate Salmonella in nut processing (Izurieta et al. 2012). Inactivation of Salmonella using steam helps
decrease the heat resistance of Salmonella on low moisture foods by increasing the moisture content. During
preliminary work, we found the moisture content of in-shell hazelnuts to be ca. 10% w/w after exposure to
steam (88
C) for 1 min (data not shown).
4.5.2 Steam Treatment Efficacy – Variability at Pilot Scale
At shorter treatment times (1 to 3 min) the efficacy of the steam blancher at reducing Salmonella levels was
inconsistent, varying by steam run. This inconsistency is partly explained by temperature variations within the
steam blancher during steam treatment runs. In addition to temperature fluctuations, there is likely unequal
dispersal of steam condensate in the steam blancher. Jeong et al. (2009) found moisture status at the surface
60
of almonds rather than the humidity of the bulk air was the primary factor controlling the rate of Salmonella in
a moist-air convection heating process. Fluctuations of steam flow within the pilot-scale steam blancher may
have led to unequal dispersal of moisture among inoculated samples which would likely influence the
inactivation rates of Salmonella spp. While longer treatment times are able to reduce the inconsistences in
Salmonella inactivation, commercial level hazelnut steam blanchers should be better optimized to have more
consistent temperatures and even steam flow.
4.5.3 Predicted Model versus Verification Results
In this study, we were able to demonstrate that steam treatment at 88
C for10 minutes achieved a 5- log
reduction of Salmonella spp. on in-shell hazelnuts. However, the 10 min required to achieve a 5-log reduction
is longer than the predicted 7.67 minutes (Table 2), and considerably longer than seen in other studies using
steam to inactivate Salmonella on almonds (Chang et al. 2010; Lee et al. 2006; Bari et al. 2010). A number of
factors can affect the inactivation and recovery of Salmonella. The effectiveness of steam treatment is
dependent on the condition of steam applied (Chang et al. 2010), and factors such as inoculation method,
drying time, growth medium and treatment affect Salmonella recovery (Lang et al. 2004). Factors that affect
Salmonella inactivation and recovery should be carefully considered when designing thermal validation studies
and comparing validation processes.
4.5.4 Physical Characteristics of the Nut and Immersion Inoculation versus Spot Inoculation
Inoculation method, drying time and treatment affect Salmonella recovery (Lang et al. 2004). Two methods of
inoculating in-shell hazelnuts were used in this study – immersion and spot inoculation. Initially, we used an
immersion inoculation procedure designed for almond kernels to derive D-values, but later found that liquid
inoculum likely penetrated small cracks in the hazelnut shells, potentially protecting Salmonella cells from
exposure to steam and making results unpredictable and inconsistent. Spot inoculation was used for enriched
samples to verify inactivation of Salmonella after immersion inoculated samples. Studies suggest that
immersion inoculation provides the most rigorous test of chemical sanitizer efficacy. Salmonella inoculated on
61
egg shells was more resistant to chemicals when eggs were inoculated by immersion vs. spot inoculation or
fecal smear (Musgrove et al. 2010). Higher levels of E. coli were recovered on lettuce leaves using immersion
inoculation compared to spot inoculation (Singh et al. 2010). Lang et al. (2004) recommend spot inoculation
with a drying time of 24 h at 22
C for inoculation of tomatoes. They found significantly larger populations of
Salmonella were recovered from the surface of raw tomatoes subjected to water and chlorine washes that were
inoculated by immersion compared to spot and spray inoculated treatments. The observed differences were
attributed to the larger number of cells adhering to the surface of tomatoes during immersion inoculation
(Lang et al. 2004). Similar to these studies, we recovered significantly higher levels of Salmonella after
exposure to steam from immersion inoculated hazelnuts compared to spot inoculated hazelnuts (data not
shown).
4.5.5 Sublethally-Injured Foodborne Pathogens
Sublethally-injured foodborne pathogens pose a serious food safety risk. Selective media such as HE contain
agents that can inhibit heat-injured Salmonella from growing, whereas tryptic soy agar (TSA) does not. To
facilitate recovery of heat-injured Salmonella cells while also providing selectivity of isolation of Salmonella form
other bacteria, several methods use a combination of TSA and selective media. A traditional overlay method
(OV) involves pouring selective agar on top of resuscitated cells on TSA agar 3-4 h after incubation. A thin
agar layer (TAL) procedure consists of pouring nonselective media (TSA) onto a solidified selective (XLD)
medium in a petri dish. In both methods, heat-injured Salmonella cells are resuscitated with TSA and a
selective medium such as XLD or HE is used for the isolation of Salmonella.
In the current study, the selective medium HE was used for plate counts. Thus, heat-injured Salmonella cells
may have survived steam treatment and not been recovered on HE media plates. The inability to recover
heat injured Salmonella using HE may have led to artificially low plate counts and an underestimation of D-
values and time required to achieve a 5-log reduction of Salmonella. In addition, any Salmonella surviving
below the limit of detection, would also not be seen on HE, but would be resuscitated after enrichment in
62
lactose, helping further underestimate the predicted time required to achieve a 5- log reduction. Studies have
compared the use of selective and nonselective media on the recovery of Salmonella. Lee et al. (2006) found
no significant difference in recovery of Salmonella on OV XLD and XLD. Counts on BSA were consistently,
but not significantly lower than on TSA (Harris et al. 2012). In contrast, Bari et al. (2011) found significant
differences in plate counts on TSAR vs BSAR, suggesting superheated steam (115
C) may thermally injure
Salmonella. In the current study, sublethally injured Salmonella cells may have survived at shorter treatment
times and potentially skewed D-values. However, after a 10 minute steam treatment no survivors were found
in enrichment medium, suggesting Salmonella spp. cells were completely inactivated after 10 minutes.
4.6 Conclusion
Steam is an effective and practical alternative for inactivating Salmonella spp. on in-shell hazelnuts. A 5-log
reduction of Salmonella spp. can be achieved after 10 min at 88
C in a steam blancher without deteriorating
final product quality.
4.7 Acknowledgements
Funding for this study was provided by the Oregon Hazelnut Marketing Board. The pilot-scale steam
blancher was designed and built by GEM Equipment of Oregon, Inc. The authors would like to thank Dr.
Linda Harris at University of California, Davis for providing Salmonella strains used in this study. The authors
would like to additionally thank Alex Emch, Daniel Wright, and Joey Minarsich for media and sample
preparation.
63
4.8 References
[ABC] Almond Board of California. 2007a. Considerations for Proprietary Processes for Almond
Pasteurization and Treatment. Almond Board of California [Online]. Available from:
http://www.almonds.com/sites/default/files/content/attachments/proprietary-processes.pdf Accessed: Mar
27, 2020.
[ABC] Almond Board of California. 2007b. Pasteurization Treatments. Almond Board of California [Online].
Available from:https://www.almonds.com/processors/processing-safe-product/pasteurization. Accessed:
Apr 7, 2020.
Bari, M.L, Nei, D., Sotome, I., Nishina, I.Y., Hayakawa, F., Isobe, S., and Kawamoto, S. 2010. Effectiveness
of superheated steam and gas catalytic infrared heat treatments to inactivate Salmonella on raw almonds.
Foodborne Path. Dis. 7:845–850.
Beuchat, L.R., Komitopoulou, E., Beckers, H., Betts, R.P., Bourdichon, F., Fanning, S., Joosten, H.M., and
Ter Kuile, B.H. 2013. Low-water activity foods: increased concern as vehicles of foodborne pathogens. J.
Food Prot. 1:150–172.
Chang, S.S., Han, A.R., Reves-De-Corcuera, J.I., Powers, J.R., and Kang, D.H. 2010. Evaluation of steam
pasteurization in controlling Salmonella serotype Enteritidis on raw almond surfaces. Lett. Appl. Microbiol.
50:393–398.
[CFR] Code of Federal Regulations. 2008a. Almonds Grown in California. 7 CFR 981. Washington, D.C.:
U.S. Department of Agriculture, Office of the Federal Register.
Da Silva, F.V.M. and Gibbs, P.A. 2009. Principles of thermal processing: pasteurization. In Simpson (Ed.),
Engineering aspects of thermal food processing (pp. 13-49). Taylor & Francis, Boca Raton, FL.
Danyluk, M.D., Uesugi, A.R., and Harris, L.J. 2005. Survival of Salmonella Enteritidis PT 30 on inoculated
almonds after commercial fumigation with propylene oxide. J. Food Prot. 68:1613–1622.
Harris, L.J., Uesugi, A.R., Abd, S.J., and McCarthy, K.L. 2012. Survival of Salmonella Enteritidis PT 30 on
inoculated almond kernels in hot water treatments. Food Res. Int. 45:1093–1098.
Harris, L.J., Yada, S., Beuchat, L.R., and Danyluk, M.D. 2019. Outbreaks of foodborne illness associated with
the consumption of tree nuts, peanuts, and sesame seeds (version 2) [Table and references]. In Outbreaks
from tree nuts, peanuts, and sesame seeds. Available at:
https://ucfoodsafety.ucdavis.edu/low-moisture-foods/nuts-and-nut-pastes. Accessed: Apr 9, 2020.
Izurieta,W.P., Komitopoulou , E. 2012. Effect of moisture on Salmonella spp. heat resistance in cocoa and
hazelnut shells. Food Res Int. 45:1087-1092.
James, C. and James, S.J. 1997. Meat decontamination- the state of the art. MAFF advanced fellowship in
food process engineering. EU concerted action programmed CT94 1881, ISBN O 86292 460 X. Bristol, UK:
University of Bristol.
James, C., Goksoy, E.O., Corry, J.E.L., and James, S.J. 2000. Surface pasteurization of poultry meat using
steam at atmospheric pressure. J Food Process Eng. 45:111-117.
Jeong, S., Marks, B.P., and Orta-Ramirez, A. 2009. Thermal inactivation kinetics for Salmonella Enteriditis
PT30 on almonds subjected to moist-air convection heating. J. Food Prot. 72:1602–1609.
64
Lang, M.M., Harris, L.J., and Beuchat, L.R. 2004. Evaluation of inoculation method and inoculum drying time
for their effects on survival and efficiency of recovery of Eschericia coli O157:H7, Salmonella, and Listeria
monocytogenes inoculated on the surface of tomatoes. J. Food Prot. 4:732-741.
Lee, S.Y., Oh, S.W., Chung, H.J., Reyes-de-Corcuera, J.I., Powers, J.R., and Kang, D.H. 2006. Reduction of
Salmonella enterica serovar Enteritidis on the surface of raw shelled almonds by exposure to steam. J. Food Prot.
69:591–595.
Morgan, A. I., Goldberg, N., Radewonuk, E.R., and Scullen, O.J. 1996. Surface pasteurization of raw poultry
meat by steam. LWT- Food Science and Technology. 29: 447-451.
Musgrove, M.T., Cox, N.A., Berrang, M.E., Buhr, R.J., Richardson, L.J., and Mauldin, J.M. 2010. Effect of
inoculation and application methods on the performance of chemicals used to disinfect Salmonella
contaminated broiler hatching eggs. J App Poult. 19(4):387-392.
[NASS] National Agricultural Statistics Service. 2019. NW Noncitrus Fruits and Nuts 2018 Summary. USDA
National Agricultural Statistics Service. [Online]. Available from:
https://www.nass.usda.gov/Publications/Todays_Reports/reports/ncit0619.pdf . Accessed Apr 9, 2020.
Nutsch, A.L., Phebus, R.K., Riemann, M.J., Kotrola, J.S., Wilson, R.C., Boyer, J.E. Jr, and Brown, T.L. 1998.
Steam pasteurization of commercially slaughtered beef carcasses: evaluation of bacterial populations at five
anatomical locations. J Food Prot. 61:571-577.
Singh, N., Singh, R., Bhunia, A., and Stroshine, R. 2002. Effect of inoculation and washing methods on the
efficacy of different sanitizers against Escherichia coli O157: H7 on lettuce. Food Microbiol. 19(2-3):183-193.
Yada, S. and Harris, L.J. 2019. Recalls of tree nuts and peanuts in the U.S., 2001 to present (version 2) [Table
and references]. In U.S. recalls of nuts. Available at: http://ucfoodsafety.ucdavis.edu/Nuts_and_Nut_Pastes.
Accessed: Apr 9, 2020.
65
5. Overall Conclusion
The aim of this research was to help the Oregon hazelnut industry characterize the hazards of Salmonella
contamination associated with in-shell hazelnuts and to evaluate the efficacy of steam blanching as a
postharvest thermal inactivation step for Salmonella spp. on the surface of in-shell hazelnuts. Hardly any prior
studies have been conducted on hazelnuts to assess their biological safety and postharvest treatments used to
inactivate Salmonella and other foodborne pathogens. Therefore, this research consisted of a two-year
Salmonella prevalence survey, quantified contamination on in-shell Oregon hazelnuts, and determined a steam
blanching treatment process that would reduce contamination while maintaining quality of in-shell hazelnuts.
The average prevalence of Salmonella on in-shell hazelnuts (33.3%) over the 2013 and 2014 harvests is
dramatically higher than seen on other tree nuts (~1.0%). It is rather dramatic and disconcerting from an
industry standpoint to see such high prevalence levels of Salmonella on in-shell hazelnuts compared to other
tree nuts, and raises concerns about harvest and postharvest handling practices that may contribute to
Salmonella contamination. There are a number of points during the harvest and postharvest handling of
hazelnuts when Salmonella could feasibly be introduced, making it difficult to identify and mitigate sources of
contamination. However, it is our opinion that ineffective postharvest washing procedures likely contributed
to cross contamination of in-shell hazelnuts resulting in the high prevalence levels of Salmonella observed.
Effective washing procedures should be used to ensure hazelnuts are clean and of high quality prior to market
distribution, but washing should not be used as a standalone postharvest treatment for the inactivation of
Salmonella. In addition to washing, validated postharvest treatments such as steam treatment should be used
to ensure 5-log reductions of Salmonella on in-shell hazelnuts.
Steam treatment is a practical technology for the inactivation of Salmonella on raw, in-shell hazelnuts. We
were able to demonstrate that a continuous steam treatment at 88C for 10 min achieved a 5-log reduction of
Salmonella spp. on in-shell hazelnuts with minimal impact on final product quality. For many low-moisture
foods, including almond kernels, prolonged exposure to steam may have detrimental effects on product
66
quality, limiting the application of steam as a cost-effective postharvest thermal process. However, prolonged
exposure to steam had a negligible impact on the product quality of in-shell hazelnuts, with consumers
actually favoring the “roasted” flavor and aroma of in-shell hazelnuts treated with steam at 88C for up to 15
min in a consumer sensory test conducted by the Food Innovation Center (Portland, OR). The fact that in-
shell hazelnuts can be exposed to steam for prolonged periods without degradation of product quality
indicates that treatment parameters that achieve a 5-log reduction of Salmonella without affecting product
quality should be achievable at an industry level. In addition, inline continuous steam blanchers can be
incorporated into existing hazelnut processing lines. Steam treatment technology shows promise of
application to Oregon hazelnut processors in the form of a flexible technology for the inactivation of
Salmonella spp. and other foodborne pathogens on the surfaces of in-shell hazelnuts.
